Published online Jul 26, 2025. doi: 10.12998/wjcc.v13.i21.105816
Revised: March 4, 2025
Accepted: March 21, 2025
Published online: July 26, 2025
Processing time: 78 Days and 23.3 Hours
Simultaneous acute ischemic stroke (AIS) and myocardial infarction (cardio
We present the case of a 27-year-old Chinese man who simultaneously experie
Urgent thrombolysis followed by elective PCI was an appropriate strategy for the management of simultaneous CCI.
Core Tip: Simultaneous acute ischemic stroke and acute myocardial infarction [cardio-cerebral ischemic attack, concomitant cerebrocardiac infarction (CCI)] has been rarely reported in the literature. There are currently no clear evidence-based guidelines or clinical trials in order to address an optimal therapeutic strategy for patients with this dilemma. This report aimed to highlight the urgent thrombolysis followed elective percutaneous coronary intervention, that showed to be relatively an appropriate strategy for management of simultaneous CCI.
- Citation: Zheng WX, Liu LY. Urgent thrombolysis followed by percutaneous coronary intervention for the simultaneous acute cardio-cerebral ischemic attack: A case report. World J Clin Cases 2025; 13(21): 105816
- URL: https://www.wjgnet.com/2307-8960/full/v13/i21/105816.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i21.105816
Acute ischemic stroke (AIS) and acute myocardial infarction (AMI) are life-threatening medical emergencies associated with a poor prognosis in the absence of timely intervention. Notably, the concept of concomitant cerebrocardiac infarction (CCI), including synchronous (simultaneous infarction in the cerebral and coronary vascular territories) and metachronous (thrombosis of one vessel preceding the other) events, was initially proposed by Omar et al[1] to describe concomitant AIS and AMI in 2010. The incidence of CCI was previously reported to be 0.009%[2], with occurrences in the young population being particularly rare. The optimal approach for the immediate management of concurrent AIS and AMI, particularly ST-segment elevation MI (STEMI), remains elusive. To date, no well-established, evidence-based guidelines or comprehensive clinical studies have systematically addressed the most effective treatment strategies for this rare comorbidity. Herein, we report the case of a 27-year-old man with simultaneous acute CCI. The patient was successfully treated with elective percutaneous coronary intervention (PCI) after urgent systemic thrombolysis at a standard dose for AIS.
A 27-year-old Chinese man was admitted to the emergency department of our hospital 34 minutes after the sudden onset of unconsciousness, right hemiplegia, and dense aphasia.
At the time of admission, the patient had a pulse rate of 95 beats per minute, bilateral blood pressure levels of 147/91 and 142/90 mmHg, a respiratory rate of 20 times/minute, and a temperature of 36.5 °C. Neurological assessment revealed a National Institutes of Health Stroke Scale (NIHSS) score of 17 and a Glasgow Coma Scale (GCS) score of 9.
The patient had no history of hypertension, diabetes, or sleep apnea.
The patient reported no history of smoking and drinking.
The muscle strength levels were 2/5 on the right side and 5/5 on the left side. Tendon reflexes were diminished, and muscle tone was decreased on the right side. The cardiovascular examination demonstrated no murmurs and pericardial rubbing. The respiratory assessment yielded normal findings.
Electrocardiography (ECG) at admission showed sinus rhythm with typical ST-segment elevation in leads V1–V5, indicating anterior MI (Figure 1A). In addition to the abnormal ECG findings, blood tests conducted upon admission revealed a B-type natriuretic peptide level of 207.8 pg/mL (normal: < 100 pg/mL) and a C-reactive protein level of 11.12 mg/L (normal: < 10 mg/L). Troponin I (TnI) and creatine kinase-MB (CK-MB) levels were normal. The lipid profile showed a low-density lipoprotein cholesterol level of 2.10 mmol/L, a triglyceride level of 1.83 mmol/L, and a glucose level of 6.40 mmol/L. Coagulation studies indicated a D-dimer level of 0.43 mg/L. Hematological test results showed a white blood cell count of 6.67 × 109/L, a neutrophil count of 4.50 × 109/L, a lymphocyte count of 1.89 × 109/L, a hemoglobin count of 164 g/L, and a platelet count of 158 × 109/L. The electrolyte levels were within normal limits, with potassium at 4.34 mmol/L, sodium at 139.1 mmol/L, and chloride at 108.0 mmol/L. The anti-human immunodeficiency virus test yielded a negative result.
Initial computed tomography (CT) of the brain revealed no signs of acute ischemic lesions or hemorrhage.
Based on the clinical symptoms, ECG findings, and brain CT results, the patient was diagnosed with simultaneous acute transmural anterior MI and AIS.
Emergency thrombolysis therapy was initiated during the early hyperacute phase to address concurrent CCI. Primary PCI was not performed due to the delayed intravenous administration of recombinant tissue plasminogen activator (rt-PA) for stroke and the increased risk of hemorrhagic conversion associated with dual antiplatelet therapy (clopidogrel plus aspirin) and anticoagulation following coronary angioplasty. Therefore, alteplase (0.9 mg/kg, 7.0 mg bolus, and 63.0 mg for 1 h) was administered 48 min after symptom onset.
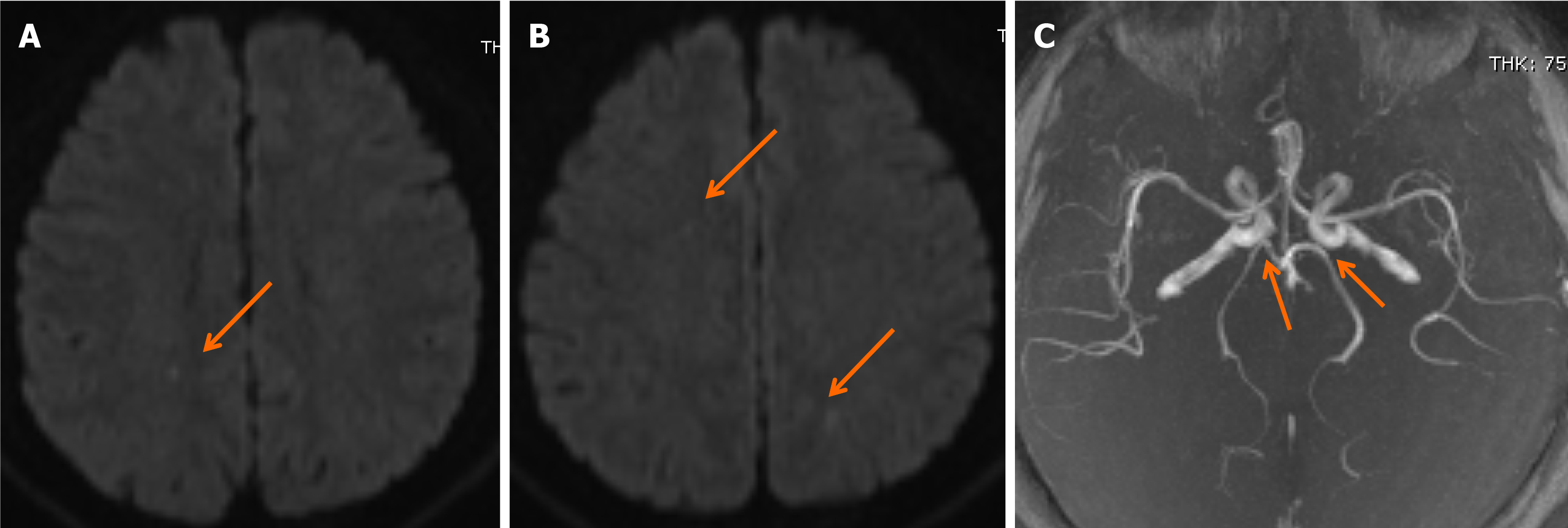
Following rt-PA infusion, the patient’s clinical condition gradually improved, with significant recovery of right limb strength and speech function. Upon regaining consciousness, he did not complain of chest discomfort in the preceding hour. Follow-up brain CT after thrombolysis showed normal results. Diffusion-weighted magnetic resonance (MR) imaging showed subtle ischemic changes in the right frontal lobe and both parietal lobes (Figure 2A and B), whereas MR angiography revealed an incomplete basilar artery ring and the absence of bilateral posterior communicating arteries (Figure 2C). Transthoracic echocardiography demonstrated normal wall motion, with no evidence of ventricular thrombus, heart valve vegetation, or pericardial effusion. Three hours after admission, a repeat ECG showed anterior STEMI, with no significant changes from the initial ECG results (Figure 1B), indicating unsuccessful recanalization of the culprit coronary artery. The patient was subsequently admitted to the intensive care unit, with an NIHSS score of 4 points and a GCS score of 15 points.
An acute increase in the levels of cardiac biomarkers was also observed. The follow-up CK-MB level increased to 56.0 ng/mL (normal value: ≤ 3.6 ng/mL), whereas the cardiac TnI increased to 0.140 ng/mL (normal value: ≤ 0.02 ng/mL), confirming the diagnosis of AMI. Thirty-six hours after admission, the peak cardiac TnI level reached 1.490 ng/mL. Bedside transthoracic echocardiography revealed normal wall motion without signs of cardiac tamponade or systolic dysfunction. No ventricular thrombus or heart valve vegetation was noted, and carotid or vertebral ultrasonography showed the absence of emboligenic foci. Based on the diagnosis of acute CCI, treatment with butylphthalide and atorvastatin was initiated. Given the potential risk of post-stroke bleeding and the patient’s suitability for dual antiplatelet therapy, aspirin and clopidogrel were prescribed on the 3rd day of hospitalization. On the 7th day of hospitalization, a follow-up transthoracic echocardiogram revealed mild left ventricular enlargement, with anteroposterior diameters of 52 mm (diastole) and 36 mm (systole), and superoinferior diameters of 72 mm (diastole) and 63 mm (systole). The cardiac TnI level had decreased to 0.100 ng/mL.
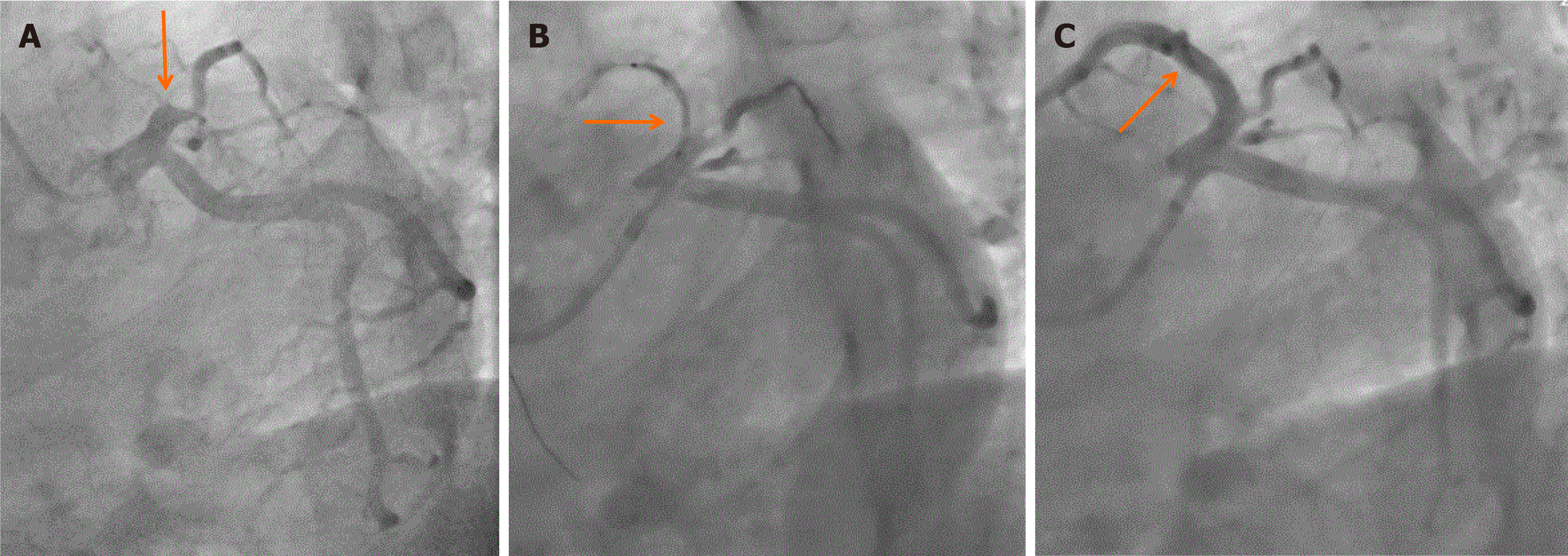
On the 8th day of hospitalization, coronary angiography revealed 100% occlusion of the mid-left anterior descending (LAD) artery (Figure 3A), with no significant stenotic lesions in the other coronary arteries. Percutaneous transluminal coronary angioplasty was performed on the LAD artery. However, due to a heavy thrombus burden, recanalization was unsuccessful. The residual diameter stenosis was 80%–90% (Figure 3B). Two drug-eluting stents (Excel, 3.5 mm × 36 mm, 3.5 mm × 14 mm; Jiwei Corp., Shandong, China) were deployed. Following stenting, coronary angiography demonstrated thrombolysis in MI grade 3 flow in the LAD artery (Figure 3C). The follow-up TnI level decreased to 0.080 ng/mL after coronary angioplasty.
The patient’s condition remained stable following the successful recanalization of the culprit coronary artery lesion. In addition to the pre-existing atorvastatin and dual antiplatelet therapy, losartan (an angiotensin receptor blocker) and metoprolol (a beta-blocker) were administered to reduce the risk of cardiac events. Two years after the simultaneous CCI, the patient resumed normal daily activities with only slight hypesthesia on the right side.
Several etiologies have been proposed for the pathogenesis of CCI based on rare cases and case series. I. These include atrial fibrillation, a cause of simultaneous CCI[3], and concurrent coronary and cerebral vasospasms due to electrical injury, an uncommon cause of simultaneous CCI[4]. A transient hypertensive crisis, triggered by emotional stress such as receiving bad news, can lead to a procoagulant state and plaque development, transiently occluding both coronary and cerebral arteries[5]. Other rare causes include the extension of an ascending aortic dissection to the ostium, along with the extension of the carotid or vertebral and basilar arteries[6,7]. Additionally, a pre-existing intracardiac thrombus, associated with reduced left ventricular ejection fraction[2], and deep vein thrombosis or thrombus formation in the right ventricle during acute right ventricular infarction with right ventricular failure can embolize both the coronary and cerebral arteries, especially when combined with a patent foramen ovale and elevated right-sided pressure[1].
In our patient, the fully occluded anterior descending artery was responsible for the acute anterior wall MI. However, the results of an emergency bedside transthoracic echocardiogram performed after thrombolysis showed normal wall motion with no evidence of ventricular thrombus, heart valve vegetation, or pericardial effusion. This finding was attributed to the early stage of AMI, during which the myocardium may not exhibit significant structural or functional changes, making it difficult for echocardiography to detect abnormalities. Additionally, the right coronary artery-dominant circulation may have contributed to this presentation. On the seventh day of hospitalization, follow-up transthoracic echocardiography revealed mild left ventricular enlargement.
Diffusion-weighted imaging (DWI) of the brain revealed scattered ischemic signals in the right frontal and bilateral parietal lobes. However, a discrepancy was observed between the NIHSS and GCS scores of 17 and 9, respectively, which were more severe than those observed in patients with subtle scattered ischemic lesions on DWI. The time from symptom onset to the completion of thrombolysis was approximately 2 h, significantly reducing the reperfusion time for the cerebral infarction lesions. In the hyperacute phase of cerebral infarction, particularly following thrombolysis, follow-up brain MR imaging (MRI) has limitations in detecting early ischemic lesions. This might explain the severe neurological deficits observed on admission (NIHSS score: 17) despite the small infarct on brain MRI. This also contributed to the absence of obvious acute stroke sequelae.
The patient had no history of sleep disorders, hypertension, diabetes mellitus, or neoplastic disease. Additionally, he had no history of smoking, alcohol abuse, or drug use. Laboratory tests for antinuclear antibodies, anti-DNA antibodies, antiphospholipid antibodies, immunoglobulins, and complement yielded negative results, thereby excluding vasculitis, connective tissue diseases, or immune disorders. Therefore, ascribing CCI to a single etiological factor remains challenging. Although neither carotid-vertebral ultrasonography nor echocardiography after thrombolysis revealed evidence of embolism, subtle and scattered ischemic foci were present in the right frontal lobe and bilateral parietal regions. Therefore, the possibility of a cardioembolic origin of CCI cannot be completely excluded. However, the development of concurrent CCI in young patients without underlying diseases and without obvious precipitating factors remains difficult to explain. No cases of CCI have been reported in young patients in the previous literature. Considering the lack of a clear cause and the uniqueness of this case, this type of CCI was diagnosed as “cardio-cerebral ischemia of unknown cause”.
The ideal management of simultaneous CCI involves a treatment strategy that benefits both infarcted cerebral and cardiac tissues. However, no established guidelines or clinical trials provide definitive management strategies for such cases. The therapeutic time window for restoring blood flow in simultaneous CCI and STEMI is narrow. The initial treatment of one infarcted territory may be delayed, potentially leading to permanent, irreversible morbidity; disability; and even death. Therefore, emergency thrombolysis therapy, which can be used to treat both conditions during the early stages of hyperacute injury, may be an ideal approach for this patient.
An obstacle to treatment with intravenous rt-PA is the different dosages and durations of thrombolytic therapy recommended for thrombosis in patients with acute STEMI and AIS. For example, if a patient weighs 60 kg, the American Stroke Association recommends a 54 mg infusion of intravenous alteplase for 60 min as treatment for AIS, with 10% of the total dose administered as an initial intravenous bolus for 1 min[8]. Studies examining on AIS have shown an increased risk of hemorrhagic transformation when intravenous rt-PA is administered at higher doses and over a longer infusion time compared with a standard dose[9]. Conversely, the administration of a thrombolytic dose lower than the recommended dose for AMI may be regarded as under-dosing[10].
By contrast, AMI within the previous 3 months is considered a relative or absolute contraindication for rt-PA in acute stroke. However, this recommendation (Class IIb; Level of Evidence, C) is not evidenced based[11]. Moreover, patients with AMI in the preceding 3 months differ from those with acute CCI. The Simplified Management of Acute Stroke using Revised Treatment and the Study of Safe Implementation of Thrombolysis in Stroke-Monitoring Study showed no significant differences in outcomes between patients with AIS who did not develop and who developed AMI treated with rt-PA[12,13]. Two large-scale studies suggested that pretreatment with rt-PA during the perioperative period of infarction did not compromise the coronary benefits of PCI[14,15].
In this case report, the patient exhibited stable hemodynamic status and the absence of chest pain. If primary PCI had been performed, the intravenous administration of rt-PA for stroke treatment would have been delayed. Conversely, antithrombotic regimens, including dual-antiplatelet therapy and anticoagulant agents typically used for STEMI interventions, significantly increase the risk of hemorrhagic transformation in thrombolysis-related AIS[16,17]. Therefore, urgent thrombolysis followed by elective PCI has proven to be a relatively appropriate strategy for managing simultaneous CCI. However, owing to existing limitations, physicians must initially decide whether to treat patients with AIS or AMI. In patients with a major artery occlusion, skipping the rt-PA infusion and opting for direct thrombectomy as an emergency cerebrovascular intervention, followed by PCI, is a viable treatment. Further studies are required to determine the optimal reperfusion therapeutic strategy to achieve favorable clinical outcomes in patients with synchronous acute CCI.
Urgent thrombolysis followed by elective PCI appears to be a relatively appropriate strategy for managing simultaneous CCI.
| 1. | Omar HR, Fathy A, Rashad R, Helal E. Concomitant acute right ventricular infarction and ischemic cerebrovascular stroke; possible explanations. Int Arch Med. 2010;3:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Yeo LLL, Andersson T, Yee KW, Tan BYQ, Paliwal P, Gopinathan A, Nadarajah M, Ting E, Teoh HL, Cherian R, Lundström E, Tay ELW, Sharma VK. Synchronous cardiocerebral infarction in the era of endovascular therapy: which to treat first? J Thromb Thrombolysis. 2017;44:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Tokuda K, Shindo S, Yamada K, Shirakawa M, Uchida K, Horimatsu T, Ishihara M, Yoshimura S. Acute Embolic Cerebral Infarction and Coronary Artery Embolism in a Patient with Atrial Fibrillation Caused by Similar Thrombi. J Stroke Cerebrovasc Dis. 2016;25:1797-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Verma GC, Jain G, Wahid A, Saurabh C, Sharma NK, Pathan AR, Ajmera D. Acute ischaemic stroke and acute myocardial infarction occurring together in domestic low-voltage (220-240V) electrical injury: a rare complication. J Assoc Physicians India. 2014;62:620-623. [PubMed] |
| 5. | Vassiliou V, Rana B, Goddard M, Braganza D. Acute myocardial infarction and acute stroke: between a rock and a hard place. Int J Cardiol. 2015;180:214-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Kawano H, Tomichi Y, Fukae S, Koide Y, Toda G, Yano K. Aortic dissection associated with acute myocardial infarction and stroke found at autopsy. Intern Med. 2006;45:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Nguyen TL, Rajaratnam R. Dissecting out the cause: a case of concurrent acute myocardial infarction and stroke. BMJ Case Rep. 2011;2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bushnell C, Kernan WN, Sharrief AZ, Chaturvedi S, Cole JW, Cornwell WK 3rd, Cosby-Gaither C, Doyle S, Goldstein LB, Lennon O, Levine DA, Love M, Miller E, Nguyen-Huynh M, Rasmussen-Winkler J, Rexrode KM, Rosendale N, Sarma S, Shimbo D, Simpkins AN, Spatz ES, Sun LR, Tangpricha V, Turnage D, Velazquez G, Whelton PK. 2024 Guideline for the Primary Prevention of Stroke: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2024;55:e344-e424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 9. | Nag DS, Swain A, Sahu S, Sen B, Vatsala, Parween S. Stroke: Evolution of newer treatment modalities for acute ischemic stroke. World J Clin Cases. 2024;12:6137-6147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Long J, Chen J, Huang G, Chen Z, Zhang H, Zhang Y, Duan Q, Wu B, He J. The differences of fibrinogen levels in various types of hemorrhagic transformations. Front Neurol. 2024;15:1364875. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344-e418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 4172] [Article Influence: 695.3] [Reference Citation Analysis (0)] |
| 12. | Patel MR, Meine TJ, Lindblad L, Griffin J, Granger CB, Becker RC, Van de Werf F, White H, Califf RM, Harrington RA. Cardiac tamponade in the fibrinolytic era: analysis of >100,000 patients with ST-segment elevation myocardial infarction. Am Heart J. 2006;151:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G; SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1562] [Cited by in RCA: 1670] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 14. | Morrison LJ, Verbeek PR, McDonald AC, Sawadsky BV, Cook DJ. Mortality and prehospital thrombolysis for acute myocardial infarction: A meta-analysis. JAMA. 2000;283:2686-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 329] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Bonnefoy E, Lapostolle F, Leizorovicz A, Steg G, McFadden EP, Dubien PY, Cattan S, Boullenger E, Machecourt J, Lacroute JM, Cassagnes J, Dissait F, Touboul P; Comparison of Angioplasty and Prehospital Thromboysis in Acute Myocardial Infarction study group. Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: a randomised study. Lancet. 2002;360:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 357] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Diener HC, Foerch C, Riess H, Röther J, Schroth G, Weber R. Treatment of acute ischaemic stroke with thrombolysis or thrombectomy in patients receiving anti-thrombotic treatment. Lancet Neurol. 2013;12:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Wang X, Ouyang M, Yang J, Song L, Yang M, Anderson CS. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2021;10:CD000024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |