Published online Jul 26, 2025. doi: 10.12998/wjcc.v13.i21.103841
Revised: March 19, 2025
Accepted: March 20, 2025
Published online: July 26, 2025
Processing time: 135 Days and 17.1 Hours
The microcystic, elongated, and fragmented (MELF) pattern of invasion in endometrioid endometrial carcinoma (EEC) is a special mode of myometrial invasion that has been recently recognized by the pathology community. Overexpression of CXC chemokine receptor 4 (CXCR4) in tumor cells contributes to tumor growth, invasion, angiogenesis, metastasis, and recurrence.
To explore the correlation between CXCR4 expression in EEC and MELF invasion and clinicopathological features.
A total of 205 EEC patients treated at Peking University People’s Hospital from June 2020 to December 2021 were selected (60 cases with MELF invasion, 145 cases without). The clinicopathological features of the two groups were compared, and expression of CXCR4 protein, estrogen receptor, and progesterone receptor was detected and compared by immunohistochemistry.
EEC with MELF invasion was significantly associated with low tumor grade, lymphovascular space invasion, deep myometrial invasion, cervical stromal involvement, and lymph node metastasis. There was a difference in CXCR4 expression between the two groups, with the MELF group having a significantly higher expression than the non-MELF group.
CXCR4 expression is significantly increased in EEC with MELF invasion and in the MELF invasion area, which may promote tumor invasion and metastasis and has some value for prognostic assessment.
Core Tip: Endometrioid endometrial carcinoma (EEC) with the microcystic, elongated, and fragmented (MELF) invasion is a special invasive pattern, and its risk of lymphovascular space invasion and lymph node metastasis is significantly higher than that of the non-MELF group. The presence of MELF infiltration in EEC is associated with a higher expression of CXC chemokine receptor 4, which may play a role in the progression of the disease.
- Citation: Wang JX, Shen DH, Zhang XB. CXCR4 expression and clinicopathological features of endometrial endometrioid carcinoma with the microcystic, elongated, and fragmented pattern. World J Clin Cases 2025; 13(21): 103841
- URL: https://www.wjgnet.com/2307-8960/full/v13/i21/103841.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i21.103841
Endometrioid endometrial carcinoma (EEC) is a common malignant tumor of the female reproductive system, and its overall prognosis is better than that of uterine serous carcinoma. However, there are also reports of recurrence and metastasis in a small number of low-grade EECs[1]. EEC is highly associated with estrogen levels. The microcystic, elongated, and fragmented (MELF) pattern of invasion in EEC is a special mode of myometrial invasion that has been recently recognized by the pathology community. The 5th edition of the World Health Organization (WHO) Classification of Tumors of the Female Reproductive System has officially proposed and named this pattern[2]. CXC chemokine receptor 4 (CXCR4) belonged to the G-protein-coupled receptor family and was one of the classic receptors for CXC ligand (CXCL) 12. CXCR4 is widely expressed in > 20 types of human cancers, including colorectal cancer, breast cancer, ovarian cancer, melanoma, and prostate cancer[3]. The epigenetic regulation of CXCR4 signaling might be responsible for the upregulation of CXCR4 in both primary and metastatic cancers. These mechanisms included regulation by noncoding RNAs, such as long noncoding RNAs, miRNAs, and circular RNAs[4]. Overexpression of CXCR4 in tumor cells contributes to tumor growth, invasion, angiogenesis, metastasis, and recurrence. CXCR4 has been shown to disrupt tumor–stromal interactions, which are crucial for support of cancer progression by the microenvironment. Targeting CXCR4 signaling is, therefore, a potential therapeutic strategy in various malignancies[5]. In this study, we compared the expression of CXCR4 in endometrial cancer with and without MELF infiltration, further exploring the mechanisms and related prognosis of endometrial cancer with special invasive patterns.
We selected clinicopathological data from 205 endometrial cancer patients treated at Peking University People’s Hospital from June 2020 to December 2021. All pathology slides were independently reviewed by two experienced gynecological pathologists (Zhang XB and Shen DH) following the 5th WHO Classification of Tumors of Female Reproductive Organs[2]. Those with MELF infiltration (60 cases) and without MELF infiltration (145 cases) were identified. The immunohistochemical EnVision two-step method of detection was performed on these cases, and their prognosis was followed up. This study was reviewed and approved by the hospital’s ethics committee.
The EnVision two-step method was used to detect expression of CXCR4 (GTX639064, HL2612, 1: 500, GeneTex) in MELF-infiltrated tissues. Paraffin sections of 3–5 μm were deparaffinized, hydrated, subjected to antigen retrieval, and incubated with primary antibodies, followed by secondary antibodies. 3,3'-Diaminobenzidine was used for color development, followed by counterstaining, dehydration, and mounting. The Ventana benchmark XT fully automatic immunohistochemistry stainer was used for detection.
Positive expression of estrogen receptor (ER) and progesterone receptor (PR) proteins were localized in the cell nuclei, with > 1% positive cells considered positive and > 50% positive cells was considered high expression. Positive signals of CXCR4 were localized in the cytoplasm and membrane, and the comprehensive score was derived from staining intensity and proportion of positive cells. Staining was scored as follows. Negative (score 0): < 1% of cells showed positive staining. Focal (score 1): Small clusters of cells with positive staining, but < 25% of cells were positive. Diffuse (score 2): ≥ 25% of the cells showed positive staining. Staining intensity was categorized as weak, moderate, or strong. Negative (score 0): No staining or < 1% of cells with any staining intensity. Positive (score 1): Focal or scattered staining, regardless of the intensity. Strong positive (score 2): Diffuse staining with strong intensity. The two scores were multiplied, and 0–2 was classified as low expression, and > 2 as high expression. Each sample is assigned a unique anonymous code. The two pathologists who will be conducting the scoring are trained to ensure they understand the scoring criteria. The pathologists engage exclusively with the coded samples, remaining utterly oblivious to the group affiliation of the samples and the experiment's underlying objective. Their scoring is conducted independently and solely in accordance with the predefined criteria. The third-party calculates the intraclass correlation coefficient for consistency check, and re-evaluates the data with low consistency.
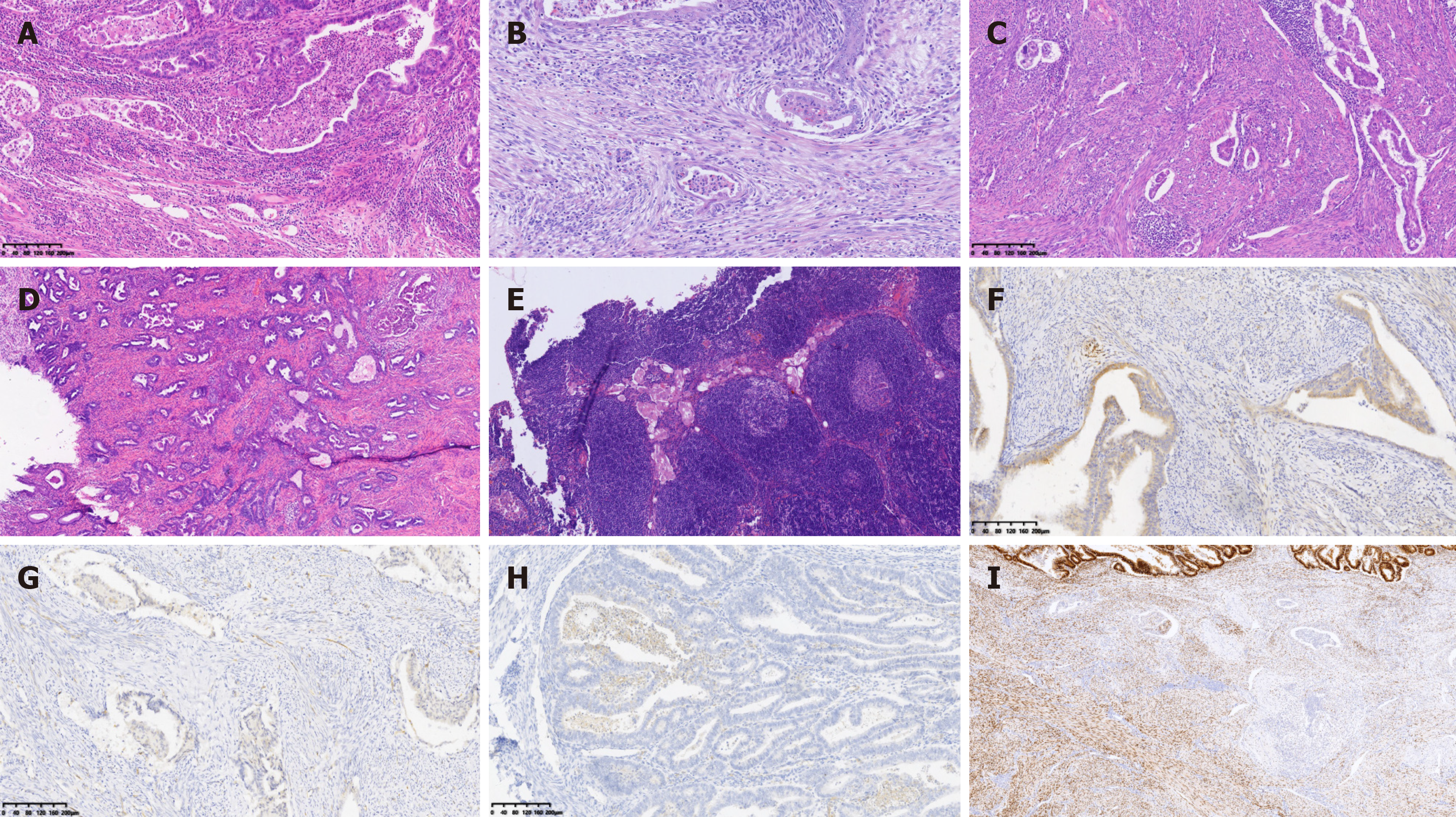
The diagnostic criteria for MELF infiltration were[6]: (1) Microcystic-type glandular structures: Glands showed cystic dilation, which may have been accompanied by a fibromyxoid stromal reaction; (2) Elongated glands: Glandular tubes were lined by flattened epithelium; and (3) Fragmented glands: Detached epithelial cells were visible within the glandular lumen, appearing in fragments (small clusters of cells). Meeting any one of the above three criteria was sufficient for a diagnosis of MELF infiltration.
SPSS 25.0 software was used for statistical analysis. Quantitative data that passed the normality test and followed a normal distribution were expressed as mean ± SD, and t tests were used; Pearson's correlation analysis was applied. The Kaplan–Meier Plotter curve was used to assess the risk of recurrence. P < 0.05 was considered to indicate statistical significance.
We compared the general clinicopathological features in 205 EEC patients with MELF infiltration (60 cases) and no MELF infiltration (145 cases). The median age of patients with MELF infiltration was 54 (45–73) years, and the median age of patients with no MELF infiltration was 51 (42–69) years. The patients in the MELF group were significantly older. The proportion of patients with tumor size, pathological grading, International Federation of Gynecology and Obstetrics (FIGO) staging, and deep muscle infiltration was significantly higher in the MELF infiltration group than in the no MELF infiltration group (P < 0.05). The risk of cervical stroma involvement, lymphovascular space invasion (LVSI), and lymph node metastasis (LNM) in the MELF infiltration group was significantly higher than in the no MELF infiltration group (P< 0.05) (Table 1).
| MELF EEC (n = 60) | No MELF EEC (n = 145) | P value | |
| Age | 0.002 | ||
| ≤ 50 | 9 (15) | 52 (35.86) | |
| > 50 | 51 (85) | 93 (64.14) | |
| Tumor size | 0.000 | ||
| ≤ 2 cm | 10 (16.67) | 67 (46.21) | |
| > 2 cm | 50 (83.33) | 78 (53.79) | |
| Tumor grade | |||
| 1 | 22 (36.67) | 89 (61.38) | 0.000 |
| 2 | 36 (60.00) | 39 (26.90) | |
| 3 | 2 (3.33) | 17 (11.72) | |
| 2023 FIGO staging | |||
| I | 33 (55.00) | 105 (72.41) | 0.011 |
| II | 16 (26.67) | 29 (20.00) | |
| III | 11 (18.33) | 8 (5.52) | |
| IV | 0 (0) | 3 (2.07) | |
| Myometrial invasion | 0.020 | ||
| < 1/2 | 33 (55.00) | 104 (71.72) | |
| ≥ 1/2 | 27 (45.00) | 41 (28.28) | |
| Cervical stromal involvement | |||
| Negative | 49 (81.67) | 127 (87.59) | 0.278 |
| Positive | 11 (18.33) | 18 (12.41) | |
| LNM | |||
| Negative | 47 (78.33) | 138 (95.17) | 0.000 |
| Positive | 13 (21.67) | 7 (4.83) | |
| Micrometastasis | 7 | 1 | |
| Macrometastasis | 6 | 6 | |
| LVSI | |||
| Absent | 31 (51.67) | 125 (91.04) | 0.000 |
| Present | 29 (48.33) | 20 (8.96) | |
| < 5/slice | 18 (30.00) | 14 (9.66) | |
| ≥ 5/slice | 11 (18.33) | 6 (4.14) | |
| ER | 0.988 | ||
| ≤ 50% | 22 (36.67) | 53 (36.55) | |
| > 50% | 38 (63.33) | 92 (63.45) | |
| PR | 0.204 | ||
| ≤ 50% | 18 (30.00) | 57 (39.31) | |
| > 50% | 42 (70.00) | 88 (60.69) | |
| CXCR4 | / | ||
| Negative | 0 | 145 (100.00) | |
| Positive | 60 (100.00) | 0 |
The positive expression of CXCR4 in the groups with MELF was 100%. Additionally, in the MELF group, there was a significant difference in CXCR4 expression between the main area of EEC and the MELF infiltration area (18.33%vs 81.67%) (Table 2 and Figure 1).
| Cases | High expression of CXCR4 | High expression of ER | High expression of PR | |
| EEC main area | 60 | 11 (18.33) | 38 (63.33) | 42 (70.00) |
| MELF invasion area | 60 | 49 (81.67) | 22 (36.67) | 18 (30.00) |
| P value | 0.000 | 0.000 |
In the MELF infiltration and no MELF infiltration groups, ER and PR showed positive expression, and there was no significant difference in expression between the two groups. However, in the MELF group, expression of ER and PR was inconsistent between the main area and the MELF infiltration area of EEC. Expression in the main area of EEC was significantly higher than in the MELF infiltration area. There was a significant difference in ER and PR expression between the main area of EEC and the MELF infiltration area (P< 0.05) (Table 2).
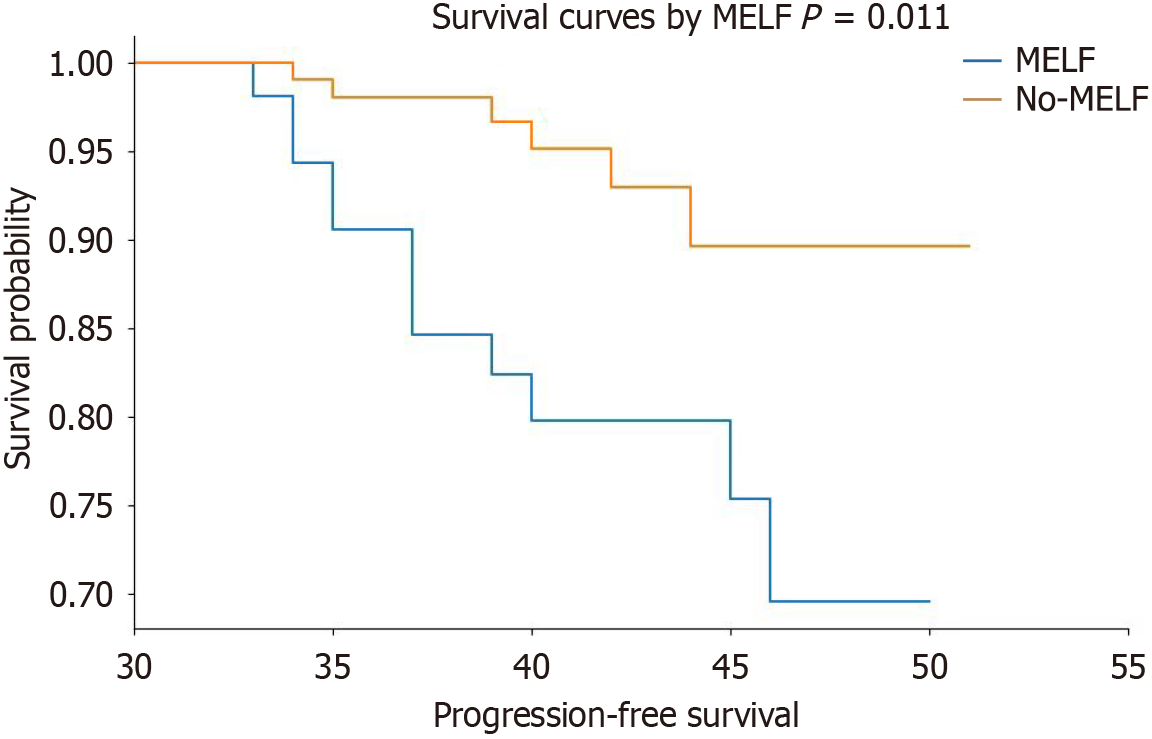
During follow-up of 33–51 mo, 19 patients had tumor recurrence, and none died due to EEC. The Kaplan-Meier survival analysis revealed that patients with higher expression levels of CXCR4, that is, the MELF group, have a higher risk of recurrence (Figure 2).
EEC is the most common type of endometrial cancer, and has five patterns of invasion, including classic infiltrating type, expansive infiltrating type, malignant adenoma type, adenomyosis-like type, and MELF type, with the classic infiltrating type being most common[7,8]. In 1994, Lee et al[9] found that cancer cells infiltrating the myometrium became slender and elongated, resembling endothelial cells in shape and surrounding each other to form lumens, within which cancer cell clusters were seen. In 2003, Murray et al[10] analyzed the invasion patterns of > 100 cases of EEC, providing a detailed description of EEC with MELF invasion. This pattern was characterized by microcystic, elongated glands, and fragmented structures resembling histiocytes, with the surrounding stroma often showing fibro myxoid changes and inflammatory cell infiltration, primarily neutrophils, and occasionally eosinophils[10]. Further research is necessary to fully elucidate the clinical significance of MELF invasion in EEC.
The association of the MELF pattern with an increased risk of LVSI and LNM underscores the importance of recognizing this pattern in pathological assessment. It may indicate a more aggressive biological behavior and could influence treatment decisions, such as the use of adjuvant therapies, more intensive surveillance, or a more conservative approach to fertility-sparing surgery. Further research is needed to clarify the clinical implications of the MELF pattern and to determine its role in personalized treatment strategies for endometrial cancer.
The mechanism behind the development of the MELF pattern is not yet fully understood. Initially, Murray and colleagues reported that the MELF invasion pattern might be a morphological degenerative change[10]. Later, Stewart et al[11] suggested that it might be related to epithelial–mesenchymal transition (EMT). EMT is a process in which tumor cells lose polarity, detach from the basement membrane, and gain the potential to transform into mesenchymal cells through certain chemokines. This leads to reduced adhesion and increased migratory ability in tumor cells, and their capacity to degrade the extracellular matrix, and promoted infiltration and metastasis into surrounding tissues.
The incidence of MELF invasion varied from 10%-20%. The low prevalence might be due to a previous lack of recognition among pathologists, often associated with low-grade endometrioid carcinoma. In this study, only two cases were high-grade, and the majority were low-grade (58/60). Additionally, there were significant differences in tumor size, deep myometrial invasion, and cervical stromal invasion between the two groups. In EEC with MELF invasion, the proportion infiltrating the deep myometrium was higher, and the deep myometrial layer contained many lymphatics and blood vessels, making LVSI more likely. In this study, the risk of LVSI and LNM in the MELF infiltration group was significantly higher than in the non-MELF infiltration group, consistent with previous studies[12]. LVSI is one of the independent risk factors that can adversely affect the prognosis of endometrial cancer[13]. The presence of LVSI indicates that cancer cells have gained the ability to enter and travel through the lymphatic or blood vessels, which can lead to distant metastases and worsen the patient's outcome. According to the FIGO 2023 staging system for endometrial cancer, the presence of LVSI was ≥ five per slide could be a criterion for upgrading the stage of the cancer from stage I to II[14]. This staging system was crucial for determining the appropriate treatment strategy and estimating the prognosis for patients with endometrial cancer. The upstaging due to LVSI highlighted the importance of meticulous pathological examination of the surgical specimen. It also emphasized the need for careful evaluation of LVSI in the context of the overall tumor characteristics, including the presence of special invasion patterns like MELF, which, as our study suggested, might be associated with a higher risk of LVSI and LNM. This information could be critical in planning comprehensive treatment approaches, which might include surgery, radiotherapy, chemotherapy, or a combination of these modalities, to optimize the chances of a favorable outcome for the patient.
LNM was indeed one of the most common routes of spread for endometrial cancer and is also considered a significant risk factor that could have a negative impact on prognosis. LNM indicates that the cancer has spread beyond the uterus, which is a critical factor in determining the stage of the cancer and the subsequent treatment approach. The 5-year survival rate for endometrial cancer patients with pelvic LNM is lower compared to those without LNM, with rates ranging from 44% to 52%[15].
CXCR4, a member of the chemokine receptor family, is a G protein-coupled receptor (GPCR) that holds a prominent position as one of the key receptors for stromal-cell-derived factor 1. Also known as CXCL12, CXCR4 is expressed on the cell membrane in various organs, encompassing as a monomer, dimer, and oligomer, and it is a seven-transmembrane domain GPCR[16]. CXCR4 conducts a pivotal role in the migration of cells during embryogenesis, such as the movement of neural crest cells and the formation of blood vessels. The receptor is involved in mediating the behavior of recipient cells and regulated the proliferation, migration, and invasion of cancer cells. This over-expression is associated with poorer prognosis and reduced survival rates in patients. Epigenetic regulation of CXCR4 signaling might contribute to up-regulation of CXCR4 in primary and metastatic cancers. This comprises regulation by a diverse array of non-coding RNAs, which, although not coding for proteins, conducts crucial roles in gene regulation[4]. This has given rise to the hypothesis that CXCR4 also promotes tumor growth, migration, and invasion in endometrial cancer.
As already demonstrated by research, CXCR4 is abnormally expressed in various types of cancer, comprising breast, colorectal, and liver cancer, and conducts a role in tumorigenesis and cancer progression[17]. Wu et al[18] used quantitative reverse transcription polymerase chain reaction to detect the mRNA expression of CXCL12, CXCR4, and CXCR7 in 115 cases of primary breast cancer and regional lymph node specimens. As suggested by their research findings, the expression level of CXCR4 in breast cancer tissues was dramatically higher than that in adjacent normal tissues. Aside from that, Kaplan-Meier survival analysis illustrated that patients with high CXCR4 expression had a shorter survival period in contrast to those with low expression. This demonstrates that the mRNA expression of CXCL12, CXCR4, and CXCR7 exert paramount influence on the progression and metastasis of breast cancer. As a consequence, they may serve as a pivotal prognostic predictor. In this study, there was a conspicuous discrepancy in CXCR4 expression between the main area of EEC and the MELF infiltration area. In the MELF infiltration area, CXCR4 expression was higher than in the non-MELF area of EEC, which supported the hypothesis that the MELF infiltration regions might behave differently at a biological level. The higher expression of CXCR4 in the MELF infiltration areas could be attributed to a more aggressive phenotype, encompassing increased invasiveness and potential for metastasis. This phenomenon reveal that CXCR4 conducts a more irreplaceable role in the MELF group, potentially on account of EMT-like changes in the MELF infiltration areas, which could be induced by chemokines to reinforce CXCR4 activity, ultimately giving rise to expression.
In MELF EEC, high-invasive activity was dependent on the CXCL14/CXCL12/CXCR4 axis, which was consistent with the literature[19]. Aside from that, this study also holds a standpoint that the MELF pattern may acquire its invasiveness via EMT as well as the activation of the CXCL14-CXCR4 and CXCL12-CXCR4 axes. The higher positive expression of CXCR4 in the MELF infiltration area in comparison with the area without MELF infiltration suggested that MELF infiltration might confer higher invasiveness through this chemokine axis. Hu et al[20] have brought forth a viewpoint that CXCR4-promoted CRC progression and EMT were regulated by the Wnt/β-catenin signaling pathway. Under such circumstance, it is essential to conduct further research to dig into the mechanistic link between MELF infiltration, CXCR4 expression, and the CXCL14/CXCL12/CXCR4 axis, and to evaluate the clinical utility of targeting this axis in endometrial cancer. For this reason, understanding these interactions could contribute significantly to the development of more effective personalized treatment strategies for patients suffering from endometrial cancer with the MELF pattern.
In EEC, ER and PR typically showed positive expression, which was consistent with the hormone-dependent nature of this type of cancer. There was no significant difference in ER and PR expression between the MELF and non-MELF groups, suggesting that the presence of the MELF pattern itself might not directly alter hormonal receptor status. However, within the CXCR4-positive MELF group, there was a discrepancy in ER and PR expression levels between the main tumor area and MELF infiltration area, with higher expression in the main tumor area, indicating a significant difference. This finding aligns with the study by Zhang et al[21], which noted a significant decrease in ER and PR expression in MELF infiltration foci. EEC was associated with high estrogen levels, and low-grade endometrioid carcinoma often exhibited higher ER and PR expression. As the tumor grade increases and differentiation decreases, the expression of these receptors also tends to decrease. This observation was inconsistent with the typical mechanism of endometrioid carcinoma development and further supports the possibility of EMT, reflected in reversible changes in tumor cell activity during tumor progression. There might be some antagonism between ER, PR, and CXCR4 in the process of EMT.
The fibromyxoid stromal reaction surrounding MELF infiltration foci, along with inflammation predominantly composed of neutrophils and eosinophils, may be one of the significant factors promoting cancer cell proliferation and migration within the infiltrated focus. This local environment could create a unique immunological microenvironment that facilitates tumor invasion. By enhancing the activity of certain transformation factors, such as CXCR4, this microenvironment may promote the migration and invasion of tumor cells[22]. The interaction between the tumor cells and the surrounding stroma, along with the immune cell infiltration, could play a crucial role in the biology of EEC with the MELF pattern. Targeting the CXCR4 pathway or other factors within this microenvironment could be a potential therapeutic strategy to disrupt the supportive niche for cancer cells and inhibit tumor progression.
Chemokines and their receptors are recognized for their significant contributions to the tumorigenesis of numerous malignancies. The chemokine CXCL12 as well as its receptors CXCR4 and CXCR7 were suggested to be involved in cancer invasion and metastasis. Goto et al[23] conducted IHC on 172 patients. As their research findings demonstrated, high expression of CXCR4 in the cytoplasm and nucleus is bound up with unfavorable cause-specific survival (CSS). On top of that, in comparison with normal esophageal mucosa, the expression level of CXCR4 messenger RNA (mRNA) in tumors was conspicuously heightened. In addition, the expression level of CXCR4 mRNA was associated with relapse-free survival and CSS differences. This study employed the Kaplan–Meier survival analysis chart database to demonstrate that ndividuals exhibiting high levels of CXCR4 expression are at a heightened risk of recurrence. These findings underscore the crucial role of CXCR4 expression in assessing the prognosis of endometrioid carcinoma patients.
Our study also has several limitations. To start with, this research is anchored on existing patient data sourced solely from one institution, thereby constituting a monolithic data source and limiting its generalizability. To mitigate bias, future investigations will encompass a more heterogeneous patient cohort. On top of that, other potential biomarkers or signaling pathways associated with MELF invasion have not been explored. In subsequent research, we will further study other markers connected with EMT and the immune microenvironment. Last but not least, recent research indicates the CXCL12–CXCR4/CXCR7 axis is considered a promising target for therapeutic intervention[16]. Our study is confined to the examination of CXCR4’s influence on patient prognosis, with anticipation for future endeavors to delve deeper into the ramifications of targeted therapy on patients.
To sum up, the reinforced expression of CXCR4 in the MELF infiltration of EEC was negatively correlated with expression of ER, suggesting a potential link with EMT. This association might contribute to the elevated proliferation and migration of tumor cells. In such case, it is imperative to conduct more in-depth research to probe into how CXCR4 associates with other chemokines, as well as its role in the prognosis and personalized treatment of EEC. By enhancing our comprehension of the role CXCR4 and its associated pathways play in EEC, we can strive to enhance treatment outcomes and offer more tailored care to patients suffering from this condition.
| 1. | Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, Angioli R, Tateo S, Mangili G, Katsaros D, Garozzo G, Campagnutta E, Donadello N, Greggi S, Melpignano M, Raspagliesi F, Ragni N, Cormio G, Grassi R, Franchi M, Giannarelli D, Fossati R, Torri V, Amoroso M, Crocè C, Mangioni C. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1047] [Cited by in RCA: 1096] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 2. | Hertel JD, Huettner PC, Pfeifer JD. Lymphovascular space invasion in microcystic elongated and fragmented (MELF)-pattern well-differentiated endometrioid adenocarcinoma is associated with a higher rate of lymph node metastasis. Int J Gynecol Pathol. 2014;33:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Samarendra H, Jones K, Petrinic T, Silva MA, Reddy S, Soonawalla Z, Gordon-Weeks A. A meta-analysis of CXCL12 expression for cancer prognosis. Br J Cancer. 2017;117:124-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Dong J, Xia R, Zhang Z, Xu C. lncRNA MEG3 aggravated neuropathic pain and astrocyte overaction through mediating miR-130a-5p/CXCL12/CXCR4 axis. Aging (Albany NY). 2021;13:23004-23019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Chatterjee S, Behnam Azad B, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014;124:31-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 492] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 6. | Stewart CJ, Little L. Immunophenotypic features of MELF pattern invasion in endometrial adenocarcinoma: evidence for epithelial-mesenchymal transition. Histopathology. 2009;55:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Rabe KM, Klein ME, Ghatak S, Stout I, Schefter A, Erickson BK, Khalifa MA. Sentinel Nodal Metastasis Detection in Endometrial Carcinoma With Microcystic, Elongated, and Fragmented (MELF) Pattern by Cytokeratin Immunostaining. Am J Clin Pathol. 2021;156:846-852. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Hu CF, Li LL, Li LY, Du Q, Zhang Y, Wang KP, Song Y. [Clinicopathological features and prognostic impact of MELF pattern in 512 endometrioid adenocarcinoma]. Zhonghua Zhong Liu Za Zhi. 2021;43:968-972. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Lee KR, Vacek PM, Belinson JL. Traditional and nontraditional histopathologic predictors of recurrence in uterine endometrioid adenocarcinoma. Gynecol Oncol. 1994;54:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Murray SK, Young RH, Scully RE. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: a study of their frequency, associated diagnostic problems, and prognostic significance. Int J Gynecol Pathol. 2003;22:324-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Stewart CJ, Crook ML, Lacey J, Louwen K. Cytokeratin 19 expression in normal endometrium and in low-grade endometrioid adenocarcinoma of the endometrium. Int J Gynecol Pathol. 2011;30:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Sanci M, Güngördük K, Gülseren V, Karadeniz T, Kocaer M, Gungorduk O, Özdemir İA. MELF Pattern for Predicting Lymph Node Involvement and Survival in Grade I-II Endometrioid-type Endometrial Cancer. Int J Gynecol Pathol. 2018;37:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Kihara A, Yoshida H, Watanabe R, Takahashi K, Kato T, Ino Y, Kitagawa M, Hiraoka N. Clinicopathologic Association and Prognostic Value of Microcystic, Elongated, and Fragmented (MELF) Pattern in Endometrial Endometrioid Carcinoma. Am J Surg Pathol. 2017;41:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, Lindemann K, Mutch D, Concin N; Endometrial Cancer Staging Subcommittee, FIGO Women's Cancer Committee. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 462] [Article Influence: 231.0] [Reference Citation Analysis (0)] |
| 15. | Pavlakis K, Messini I, Vrekoussis T, Panoskaltsis T, Chrysanthakis D, Yiannou P, Voulgaris Z. MELF invasion in endometrial cancer as a risk factor for lymph node metastasis. Histopathology. 2011;58:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Zhou W, Guo S, Liu M, Burow ME, Wang G. Targeting CXCL12/CXCR4 Axis in Tumor Immunotherapy. Curr Med Chem. 2019;26:3026-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 17. | Sengupta S, Mondal M, Prasasvi KR, Mukherjee A, Magod P, Urbach S, Friedmann-Morvinski D, Marin P, Somasundaram K. Differentiated glioma cell-derived fibromodulin activates integrin-dependent Notch signaling in endothelial cells to promote tumor angiogenesis and growth. Elife. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Wu W, Qian L, Chen X, Ding B. Prognostic significance of CXCL12, CXCR4, and CXCR7 in patients with breast cancer. Int J Clin Exp Pathol. 2015;8:13217-13224. [PubMed] |
| 19. | Kojiro-Sanada S, Yasuda K, Nishio S, Ogasawara S, Akiba J, Ushijima K, Yano H. CXCL14-CXCR4 and CXCL12-CXCR4 Axes May Play Important Roles in the Unique Invasion Process of Endometrioid Carcinoma With MELF-Pattern Myoinvasion. Int J Gynecol Pathol. 2017;36:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Hu TH, Yao Y, Yu S, Han LL, Wang WJ, Guo H, Tian T, Ruan ZP, Kang XM, Wang J, Wang SH, Nan KJ. SDF-1/CXCR4 promotes epithelial-mesenchymal transition and progression of colorectal cancer by activation of the Wnt/β-catenin signaling pathway. Cancer Lett. 2014;354:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Zhang XB, Zhao CL, Qi XL, Qin Y, Wang Y, Shen DH. [Microcystic, elongated and fragmented invasion pattern in endometrial carcinoma: the clinicopathology analysis]. Zhonghua Fu Chan Ke Za Zhi. 2018;53:811-815. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol Res. 2016;4:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 23. | Goto M, Yoshida T, Yamamoto Y, Furukita Y, Inoue S, Fujiwara S, Kawakita N, Nishino T, Minato T, Yuasa Y, Yamai H, Takechi H, Seike J, Bando Y, Tangoku A. CXCR4 Expression is Associated with Poor Prognosis in Patients with Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2017;24:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |