Published online Jun 26, 2025. doi: 10.12998/wjcc.v13.i18.99749
Revised: November 5, 2024
Accepted: February 14, 2025
Published online: June 26, 2025
Processing time: 212 Days and 19.9 Hours
Incisional hernia is one of the known complications of renal transplant surgery, with a reported incidence between 1.1% to 3.8%. Depending on the site and extent of incisional hernia, it may require surgery particularly if it contains the trans
We report four cases of post-renal transplant incisional hernia with a median time of 27 months post-surgery. The median size of the defect was 15 cm long. There was no post-operative complication. One patient required renal transplant biopsy after mesh repair, which was easily performed compared with polypropylene meshes repaired hernias in the past.
The OviTex 1S mesh provides benefits in hernial repairs pKTx, but cost is an issue, and their long-term viability is unclear. Continued use and reporting will help build a more informed picture.
Core Tip: An incisional hernia is one of the known complications of renal transplant surgery and, depending on the site and extent of the hernia, may require surgery. The repair of incisional hernias post kidney transplantation (pRTx) has traditionally used synthetic mesh. However, they are associated with complications. Hybrid meshes, such as OviTex 1S, are a new type of mesh combining both synthetic and biological mesh properties in an attempt to improve post-operative outcomes. This case series reports 4 cases where OviTex 1S permanent mesh is used in the repair of pRTx incisional hernias.
- Citation: Abed M, Bradley A, Ghazanfar A. Use of OviTex 1S, reinforced tissue matrix, for the repair of post renal transplant incisional hernias: Four case reports. World J Clin Cases 2025; 13(18): 99749
- URL: https://www.wjgnet.com/2307-8960/full/v13/i18/99749.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i18.99749
Incisional hernia is one of the known complications of renal transplant surgery, with the reported incidence between 1.1% to 3.8%[1]. It commonly presents within 3 months of surgery[1]. Post kidney transplantation (pRTx) incisional hernia has multi-factorial risk factors. Amongst others, these include incision type, recipient age, smoking history, body mass index (BMI), and wound infection[2]. Obesity has been found to be a strong predictor for incisional hernias[3]. This is due to the increased intra-abdominal pressure on the incision, causing mechanical stress. Immunosuppressive regime, long-term dialysis and associated co-morbidities such as adult polycystic kidney disease are other risk factors[3,4]. Depending on the site and extent of the incisional hernia, it may require surgery if it contains the transplanted kidney either partially or completely.
The current common clinical practice is to repair incisional hernias using polypropylene meshes. Although affordable and strong, these are associated with complications, including increased risk of infection, chronic inflammation, fistula formation and bowel obstruction[5]. They can also become brittle, reduce in size over time and can be difficult to remove[5]. Biological meshes made from human or animal-derived connective tissue are also in use. They have the advantage of reduced inflammatory response and can be left in situ during infection[6]. To date, their use has been limited due to high costs and some reports of high recurrence rates and increased laxity[7]. Recently, hybrid meshes have been developed. These are composed of both biological and synthetic products. One such example is OviTex 1S permanent, which is a sterile reinforced tissue matrix composed of ovine (sheep) derived extracellular matrix and monofilament polypropylene. It has been shown to produce a reduced inflammatory response, promote better wound healing and reduce hernia recurrence[8,9]. To date, there is limited data available for the use of OviTex 1S in pRTx incisional hernia repairs.
In this current case series, we report 4 cases where we used OviTex 1S permanent mesh for the repair of pRTx incisional hernias.
Case 1: A 67-year-old male patient with end-stage renal failure (ESRF) secondary to hypertensive nephropathy received a living donor renal transplant in 2021. His relevant history included a BMI of 25.6, more than 2 years of haemodialysis and a 10-year history of smoking. As per our unit’s protocol, he received 20 mg of Basiliximab at induction and day 4 post renal transplant. He was started on triple immunosuppression therapy, including Tacrolimus, Mycophenolate Mofetil and Prednisolone 20 mg. Prednisolone was stopped after day 7 due to primary graft function.
His renal allograft functions stabilised with a mean estimated glomerular filtration rate (eGFR) of 47 to 50 mL/min/1.73 m2 at 1-year post-transplant. He was maintained on Tacrolimus and Mycophenolate Mofetil. Fourteen months following his renal transplant, he noticed a swelling over the upper part of his right iliac fossa (RIF) incision. A screening Ultrasound (US) confirmed a fascial defect, and a computer tomography (CT) scan confirmed an approximately 16cm to 8cm wide incisional hernia. The hernia contained small bowel loops and the upper 2/3 of the transplanted kidney. The hernial defect was repaired with OviTex 1S mesh using sublay pre-peritoneal technique. The surgery was uneventful, with no post-operative complications. He was discharged three days post-operatively. A repeat CT confirmed intact repair after 3 months. The patient required an US of the kidney transplant after 6 months of surgery due to raised creatinine and later had a renal transplant biopsy. The US images had better depth and quality as compared with polypropylene mesh. The biopsy was also more controlled and less forceful as compared to polypropylene mesh repairs. There has been no recurrence until a recent follow-up 18 months after hernia repair.
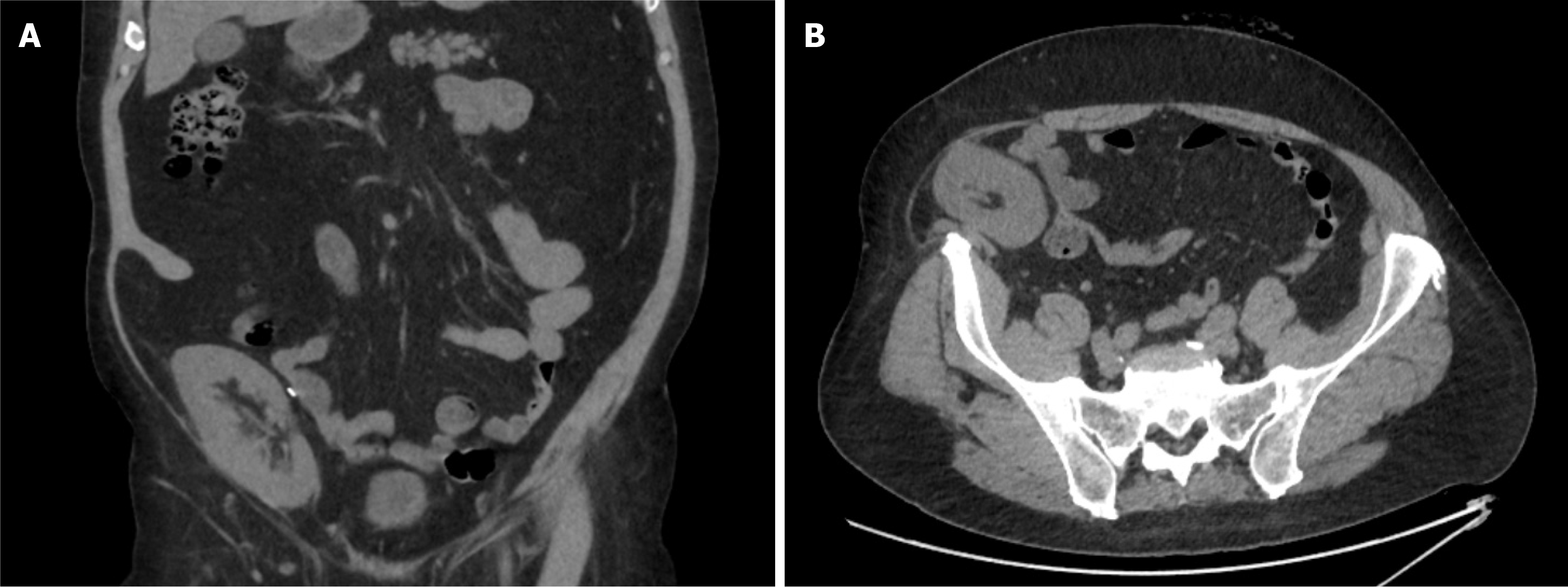
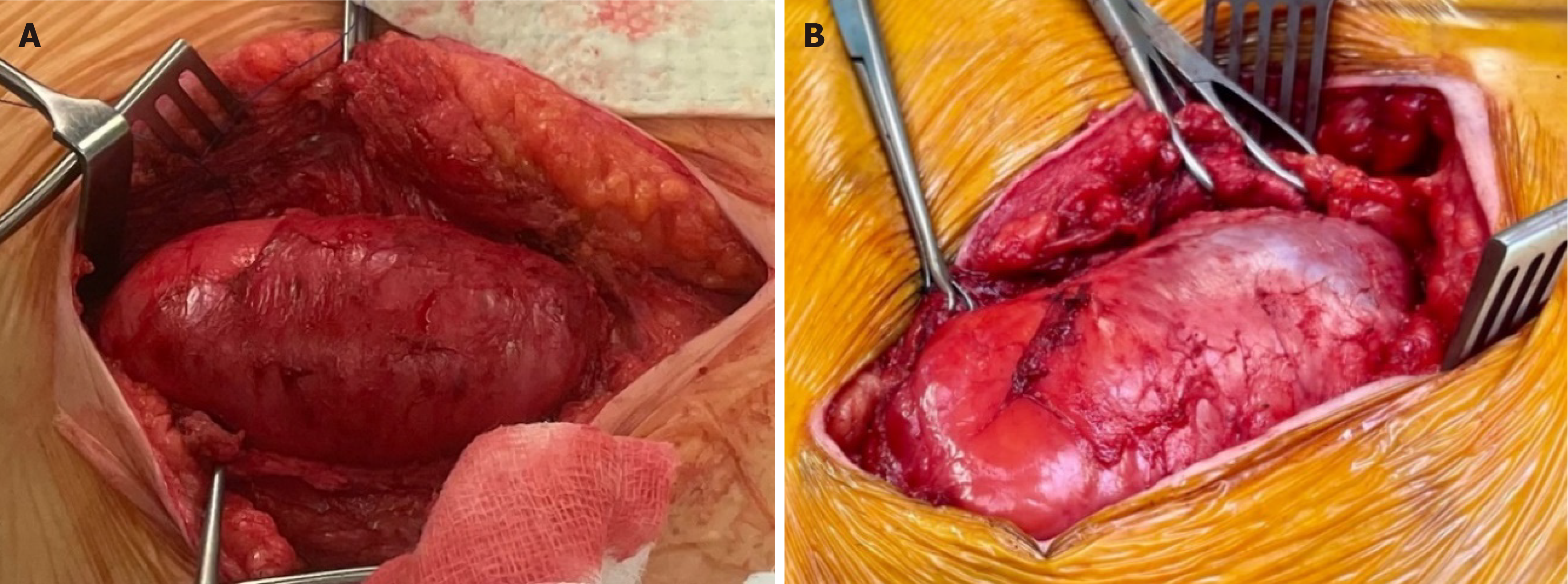
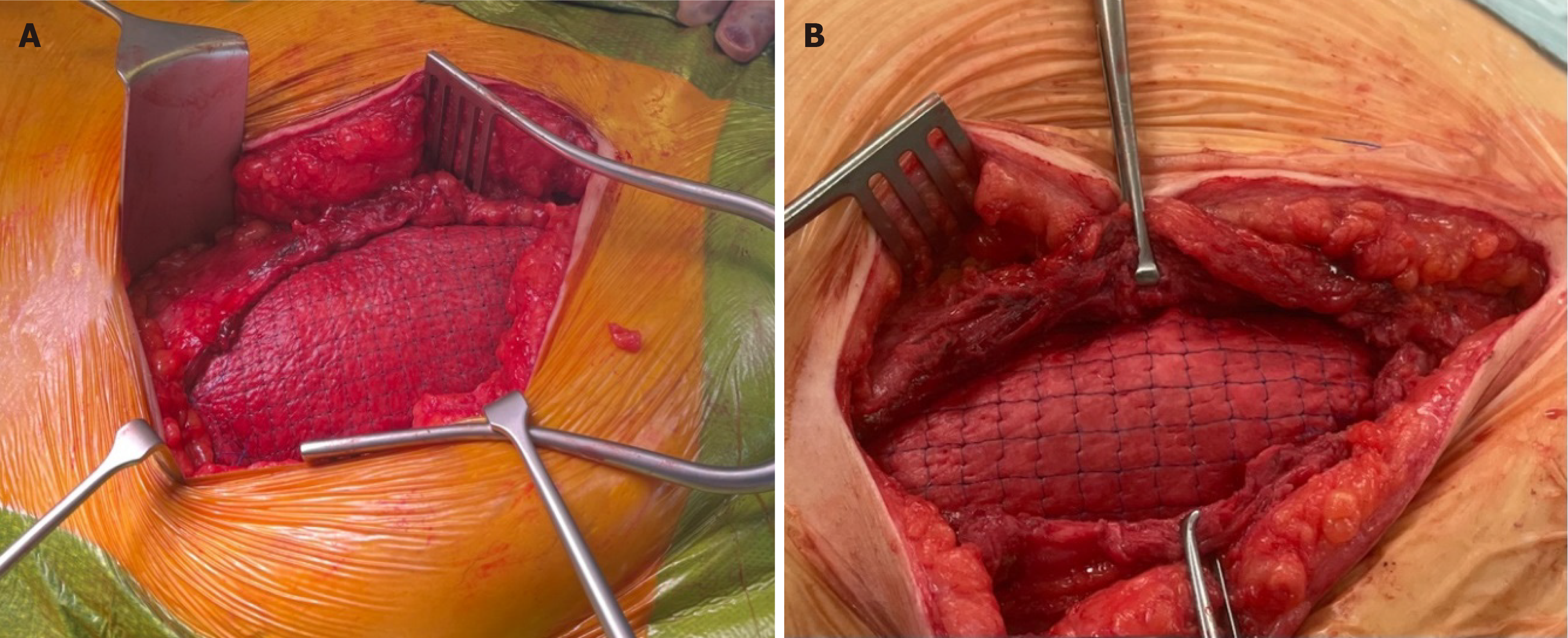
Case 2: A 45-year-old male patient with ESRF secondary to an unknown cause received a donation after a circulatory death (DCD) renal transplant in 2015. He had a past medical history of ulcerative colitis and thyroid cancer, was a non-smoker and had a BMI of 34. As per our unit’s protocol, he received 20 mg of Basiliximab at induction and day 4 post renal transplant. He was started on triple immunosuppression therapy, including Tacrolimus, Mycophenolate Mofetil and Prednisolone 20 mg. The patient was reviewed in the surgical clinic 7 years following the transplant with a failed transplant and an associated long-standing reducible incisional hernia. The cause of allograft failure was chronic allograft nephropathy on renal transplant biopsy. At the time of presentation for incisional hernia, he was on low-dose Tacrolimus and Mycophenolate Mofetil. The CT scan confirmed a significant defect, approximately 14 cm to 7 cm (Figure 1). The patient underwent simultaneous transplant nephrectomy and incisional hernia repair with OviTex 1S using sublay technique (Figures 2 and 3) and was discharged 4 days later. The operation and initial post-operative period proceeded without complication. The most recent follow-up, 14 months after surgery, did not show any recurrence.
Case 3: A 55-year-old female patient with ESRF secondary to polycystic kidney disease received a DCD renal transplant in 2014. As per our unit’s protocol, she received 20 mg of Basiliximab at induction and day 4 post renal transplant. She was started on triple immunosuppression therapy, including Tacrolimus, Mycophenolate Mofetil and Prednisolone 20 mg.
She developed an incisional hernia three years after transplant surgery, which was repaired using polypropylene mesh. She presented with recurrent incisional hernia in 2021, with tenderness over the RIF surgical site. At the time of presentation for incisional hernia, she was on Tacrolimus and Mycophenolate Mofetil. She had good renal allograft function with eGFR of 48 mL/min/1.73 m2. CT scan confirmed a loop of bowel herniating into the right inguinal canal and involving the lower half of the transplant incision. She had a BMI of 24.5 and was a non-smoker. The recurrent dual inguinal incisional hernia was repaired with OviTex 1S mesh using sublay technique. There were no intra-operative or post-operative complications. She was discharged after 1 day and has had no reported re-occurrence 13 months following the surgery.
Case 4: A 62-year-old male patient with ESRF secondary to focal segmental glomerulosclerosis (FSGS). He had a previous history of significant diverticular disease with multiple surgeries in the past, resulting in colectomy and ileostomy. His BMI was 32 at the time of surgery. He received an end-of-chain live donor kidney transplant in 2022. As per our unit’s protocol, he received 20 mg of Basiliximab at induction and day 4 post renal transplant. He was started on triple immunosuppression therapy, including Tacrolimus, Mycophenolate Mofetil and Prednisolone 20 mg. He had good renal allograft function with eGFR of 63 mL/min/1.73 m2. The patient developed a post-transplant incisional hernia six months after his transplant. At the time of presentation for incisional hernia, he was Tacrolimus and Mycophenolate Mofetil. His hernia was repaired with OviTex1S mesh using sublay technique. He had an uneventful recovery and was discharged 24 hours after his surgery. No recurrence was reported in a recent follow-up 6 months after surgery.
With regards to most of the cases, the patients presented with a non-tender swelling over the surgical incisional site. In case 3, the patient presented with tenderness over the RIF surgical site where she had a previous incisional hernia repair following her renal transplant.
The patients in this case report developed ESRF due to a variety of reasons. Case 1 developed hypertensive nephropathy, case 3 had a history of polycystic kidney disease, and case 4 developed FSGS. The cause for case 2 is unknown.
With regard to developing recurrent incisional hernia, there was no relevant family or personal history.
Examination revealed non-obstructed reducible swelling in all cases.
The eGFR in cases 1, 3 and 4 were 47, 48 and 63 mL/min/1.73 m2, respectively. Case 2 was on established haemodialysis.
All cases had a US scan as screening text followed by a CT scan to confirm and measure the defect size for surgical planning.
As with all our patients, they were thoroughly investigated, and CT imaging confirmed post-renal transplant incisional hernia with defects as large as 16 cm × 8 cm. In case 3, a loop of large bowel was found to be herniated through the incision site. However, there were no signs of strangulation or incarceration.
With a confirmed diagnosis of a recurrent incisional hernia, the patients underwent incisional hernia repair with OviTex1S mesh using a sublay technique. Post-operative recovery was uneventful, and patients were discharged within a few days of hospital admission.
The patients have been regularly followed up in the clinic following their repairs, and there has been no recurrence with follow-up up to 18 months post-operative. They will continue to be followed up regularly.
The above-described cases provide useful information for the use of OviTex 1S meshes in the repair of hernias pKTx.
A clear conundrum exists between synthetic and biological meshes; synthetics provide good strength, structure, and affordability but they do not integrate into the host tissue as well as biologics and are associated with a higher infection risk. Biologics, on the other hand, have been shown to lose their structural integrity quickly and are more expensive. Hybrid meshes such as the OviTex 1S have been developed with the aim of preserving the benefits of both components whilst negating their downsides. Benefits include greater strength and better integration into the host, as well as a reduced inflammatory response, improved wound healing, and reduced hernia recurrence[6]. In contrast to synthetic meshes, which create a pro-inflammatory response in the host, OviTex 1S has been shown to have limited foreign body response with earlier infiltration, earlier functional vasculature establishment and a decreased risk of bacterial colo
Kidney transplant patients are a unique group who require close follow-up. Throughout the life of an allograft, it is not uncommon to have an US and transplant biopsy due to allograft dysfunction. The type of mesh used and how it integrates into the host tissue can influence the effectiveness of these investigations. The cases showed that OviTex 1S produced better depth and quality US images when compared to polypropylene mesh and allowed for a more controlled, less forceful transplant biopsy. This provides more accurate imaging results, reduces the risk of damage to the kidney and improves precision in biopsy. Other potential benefits may include an easier nephrectomy following the use of OviTex 1S mesh. Reduced scar tissue production and better integration into the host provide an easier operating field.
There has been no reported hernial recurrence in the cases presented above, and all patients made a quick recovery with no associated complications. The current reported 12-month recurrence rate of hernias repaired with OviTex 1S use is approximately 2.6%[6]. This is significantly lower than the re-occurrence rate in synthetic meshes of approximately 10%[10].
The OviTex 1S mesh provides benefits in hernial repairs pKTx, but cost is an issue, and the long-term viability of them is unclear. Continued use and reporting will help build a more informed picture.
| 1. | Ooms LS, Verhelst J, Jeekel J, Ijzermans JN, Lange JF, Terkivatan T. Incidence, risk factors, and treatment of incisional hernia after kidney transplantation: An analysis of 1,564 consecutive patients. Surgery. 2016;159:1407-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Mazzucchi E, Nahas WC, Antonopoulos I, Ianhez LE, Arap S. Incisional hernia and its repair with polypropylene mesh in renal transplant recipients. J Urol. 2001;166:816-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Simson N, Parker S, Stonier T, Halligan S, Windsor A. Incisional Hernia in Renal Transplant Recipients: A Systematic Review. Am Surg. 2018;84:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Costa L, Martin D, Zingg T, Venetz JP, Demartines N, Golshayan D, Matter M. Incidence, Risk Factors, and Management of Incisional Hernias After Kidney Transplant: A 20-Year Single Center Experience. Transplant Proc. 2023;55:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578-83; discussion 583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1052] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 6. | DeNoto G 3rd, Ceppa EP, Pacella SJ, Sawyer M, Slayden G, Takata M, Tuma G, Yunis J. A Prospective, Single Arm, Multi-Center Study Evaluating the Clinical Outcomes of Ventral Hernias Treated with OviTex(®) 1S Permanent Reinforced Tissue Matrix: The BRAVO Study 12-Month Analysis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Cavallaro A, Lo Menzo E, Di Vita M, Zanghì A, Cavallaro V, Veroux PF, Cappellani A. Use of biological meshes for abdominal wall reconstruction in highly contaminated fields. World J Gastroenterol. 2010;16:1928-1933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Klinge U, Klosterhalfen B, Müller M, Schumpelick V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg. 1999;165:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 264] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Overbeck N, Nagvajara GM, Ferzoco S, May BCH, Beierschmitt A, Qi S. In-vivo evaluation of a reinforced ovine biologic: a comparative study to available hernia mesh repair materials. Hernia. 2020;24:1293-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Langer C, Neufang T, Kley C, Liersch T, Becker H. Central mesh recurrence after incisional hernia repair with Marlex--are the meshes strong enough? Hernia. 2001;5:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |