Published online Jun 6, 2025. doi: 10.12998/wjcc.v13.i16.102866
Revised: December 24, 2024
Accepted: January 9, 2025
Published online: June 6, 2025
Processing time: 101 Days and 0 Hours
Plasma concentration monitoring is crucial for optimizing vancomycin use, particularly in patients in the intensive care unit (ICU). However, the reference interval for vancomycin plasma concentration remains undetermined.
To evaluate the correlations of area under the curve (AUC0-24) and trough concentration (Cmin) with efficacy and nephrotoxicity in patients in the ICU.
A total of 103 patients treated with vancomycin for methicillin-resistant Staphylococcus aureus infections were analyzed in this study. The associations of clinicode
Cmin over 9.4 μg/mL and AUC0-24 exceeding 359.6 μg × hour/mL indicated good efficacy against infection. Cmin below 14.0 μg/mL predicted no significant nephrotoxicity.
In this study, the effective and safe concentration interval for vancomycin in patients in the ICU was Cmin 9.4-14.0 μg/mL. Close attention should be paid to adverse effects and renal function during vancomycin treatment.
Core Tip: Plasma concentration monitoring is essential for optimizing vancomycin therapy. This study investigated the relationship between clinical characteristics, pharmacokinetic parameters of vancomycin, specifically the trough concentration and area under the curve, and its efficacy and nephrotoxicity in Chinese patients in the intensive care unit to inform personalized vancomycin administration. The results showed that the effective and safe concentration interval for vancomycin for patients in the intensive care unit was a trough concentration of 9.4-14.0 μg/mL.
- Citation: Guo T, Du LY, Liu MF, Zhou XJ, Chen XR. Correlations of vancomycin trough concentration and its efficacy and toxicity in patients in the intensive care unit. World J Clin Cases 2025; 13(16): 102866
- URL: https://www.wjgnet.com/2307-8960/full/v13/i16/102866.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i16.102866
Vancomycin has always been the first-line drug treatment for methicillin-resistant Staphylococcus aureus (MRSA), especially in patients in the intensive care unit (ICU)[1]. However, the optimal vancomycin plasma concentration range remains a challenge to define. The Infectious Diseases Society of America recommends an area under the curve (AUC0-24)/minimum inhibitory concentration (MIC) ratio above 400 for MRSA infection with MIC not exceeding 1 mg/L, while the monitoring of trough concentration (Cmin) with a target of 15-20 μg/mL is no longer recommended[2]. Nevertheless, different AUC0-24/MIC breakpoints reported in different studies have cast doubt on the target of 400[3,4].
In China’s vancomycin consensus guideline, a Cmin of 10-15 μg/mL in common infections and a Cmin of 10-20 μg/mL in serious MRSA infections is recommended[5]. However, Shen et al[6] reported no significant correlation between pharmacokinetic indices and clinical or microbiological efficacy of vancomycin. Liang et al[7] found that vancomycin Cmin was not statistically correlated with clinical efficacy but was an indicator of nephrotoxicity. The cutoff point of Cmin on nephrotoxicity was 13 μg/mL. Van Hal et al[8] found that Cmin above 15-20 μg/mL increased renal function impairment. Therefore, the threshold for vancomycin-induced nephrotoxicity remains undefined[9]. Clinical factors may contribute to these different study findings.
This study aimed to collect data from 103 patients in the ICU who were treated with vancomycin in a Chinese hospital, monitor the entire treatment process, and analyze the relationship between vancomycin dose, Cmin, AUC0-24, and AUC0-24/MIC with efficacy and adverse reactions. We also examined the influence of clinical physiological and pathological parameters on treatment outcomes to support individualized treatment and rational use of vancomycin.
The study enrolled patients admitted to the ICU at the Fourth Hospital of Hebei Medical University between October 2021 and June 2024. This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University, No. 2021KS031. All study participants, or their legal guardian, provided informed written consent prior to study enrollment. Patients were included if they had MRSA infections. Infection sites included bloodstream, lung, chest cavity, and abdominal cavity. Many patients had comorbidities, including hypertension, diabetes, tumors, coronary disease, anemia, and hypoproteinemia. Bacterial culture and drug-sensitivity experiments were performed. The bacterial culture experiment was conducted once every 2-3 days. Each experiment lasted 3-7 days to obtain the result. The MIC experiment was conducted at or before vancomycin application. The MIC was determined using the broth microdilution method[10]. If no MRSA bacteria were found or vancomycin insensitivity was indicated, vancomycin treatment was discontinued, and the case was excluded in the study.
Vancomycin dosage regimens varied among patients. The dose included 0.5 g, 0.75 g, 1.0 g, or 1.25 g per dose. The dosing interval included 8 hours, 12 hours, or 24 hours. Each intravenous vancomycin infusion lasted 1 h. The study for each patient began with vancomycin administration and ended with its cessation. The application of vancomycin in every enrolled case started during the ICU treatment. Even if the patient was transferred from the ICU to the general ward after vancomycin application, the study for the case would continue until vancomycin cessation.
Inclusion criteria included: (1) MRSA infection; (2) Intravenous vancomycin injection for over 3 days; (3) Monitoring vancomycin Cmin; and (4) For all patients undergoing surgical treatment source control was addressed. Exclusion criteria included: (1) Not MRSA infection; (2) No Cmin tested; (3) Source uncontrolled; and (4) Pediatric, pregnant, or lactating patients.
Ultra-performance liquid chromatography with tandem mass spectrometry method was established for quantifying vancomycin in human plasma[11]. The results of vancomycin detection by this method passed the consistency assessment of the Beijing Clinical Laboratory Center. Plasma samples were collected 0.5 h before the fourth or subsequent vancomycin dose, with detection concentration serving as Cmin. In this study, 214 Cmin from 103 patients were examined. Among them, 51 cases were tested for Cmin once, 21 cases were tested 2 times, 15 cases were tested 3 times, 10 cases were tested 4 times, 4 cases were tested 5 times, 1 case was tested 6 times, and 1 case was tested 10 times.
Bacterial culture results were used for efficacy evaluation. Effective was defined as the disappearance or full recovery of all symptoms and signs, recovery of all non-microbial indicators such as imaging and laboratory tests, and no MRSA pathogenic bacteria cultured from the infection site. Ineffective was defined as persistent or worsening symptoms or signs after treatment, new symptoms or signs of the disease, or other antimicrobial treatments for this disease with the original infected pathogenic bacteria tested in the specimens from the infection site. Vancomycin-related adverse effects were judged by the clinical symptoms and laboratory test results. Nephrotoxicity was defined as an increase in serum creatinine (Scr) ≥ 26.5 μmol/L within 48 h or Scr increase ≥ 1.5 times the baseline within 7 days[12].
Real-time vancomycin concentration time curves were simulated using VCM-TDM software based on the vancomycin population pharmacokinetic model, Bayesian feedback method, and Cmin detection values. Patient information, dosing regimen, and Cmin detection values were input to the process to obtain the concentration values and concentration time curves for AUC calculation.
Statistical analyses were performed by GraphPad Prism 9.0. Clinicodemographic characteristics were analyzed as descriptive parameters. The Mann-Whitney U test was applied to compare continuous data. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic accuracy and specificity of pharmacokinetic/pharmacodynamic parameters. The value corresponding to the maximum of “sensitivity + specificity-1” was the optimal diagnostic cutoff point. Univariate and multivariate logistic regression analyses were performed to identify inde
A total of 103 patients were included in this study. Among these patients, 71 were male and 32 were female. The age range was 18-98 years, with a median age of 62.3 years. The average weight was 65.6 kg. The main etiologies of ICU cases included postoperative infection (80.6%), respiratory failure (12.6%), shock (3.9%), and severe injury (2.9%). A total of 91 patients (88.3%) had comorbidities. The infection sites included abdominal cavity (21.4%), lung (34.0%), chest cavity (9.7%), and bloodstream (35.0%). Disease status was recorded with the acute physiological chronic health evaluation II (APACHE II) score. The scoring range was 0-71. Of these, 54 cases (52.4%) were ≤ 35, and 49 cases (47.6%) were > 35. Seventy-four cases (71.8%) received mechanical ventilation, and 29 patients (28.2%) did not.
According to the effective evaluation criteria, among the 103 cases, 77 were effective, and 26 were ineffective, yielding an effective rate of 74.8%. The mean daily dose was not significantly different between the two groups (P = 0.4689) (Table 1). However, Cmin, AUC0-24, and AUC0-24/MIC of the effective group were significantly higher than those of the ineffective group (P < 0.001). Adverse reactions were also observed during the vancomycin treatment, including 2 cases of skin rashes, 3 cases of liver function impairment, and 27 cases of nephrotoxicity. Thus, nephrotoxicity was the primary adverse effect of vancomycin treatment, with a nephrotoxic rate of 26.2%. No significant differences were shown in the mean daily dose, Cmin, AUC0-24 and AUC0-24/MIC between the nephrotoxic and non-nephrotoxic groups (P = 0.1371, 0.5211, 0.2931, and 0.9458, respectively) (Table 2).
| Characteristics | Ineffective (n = 26) | Effective (n = 77) | P value |
| Mean daily dose (g) | 1.7 ± 0.5 | 1.8± 0.5 | 0.4689 |
| Cmin (μg/mL) | 6.9 ± 3.3 | 14.5 ± 6.2 | < 0.0001 |
| AUC0-24 | 342.8 ± 119.1 | 546.8 ± 171.8 | < 0.0001 |
| AUC0-24/MIC | 467.0 ± 248.1 | 696.2 ± 327.1 | 0.0015 |
| Characteristics | Non-nephrotoxic (n = 76) | Nephrotoxic (n = 27) | P value |
| Mean daily dose (g) | 1.7 ± 0.5 | 1.9 ± 0.4 | 0.1371 |
| Cmin (μg/mL) | 12.4 ± 6.3 | 13.3 ± 6.9 | 0.5211 |
| AUC0-24 | 484.9 ± 154.4 | 528.1 ± 245.6 | 0.2931 |
| AUC0-24/MIC | 640.6 ± 322.0 | 635.7 ± 332.5 | 0.9458 |
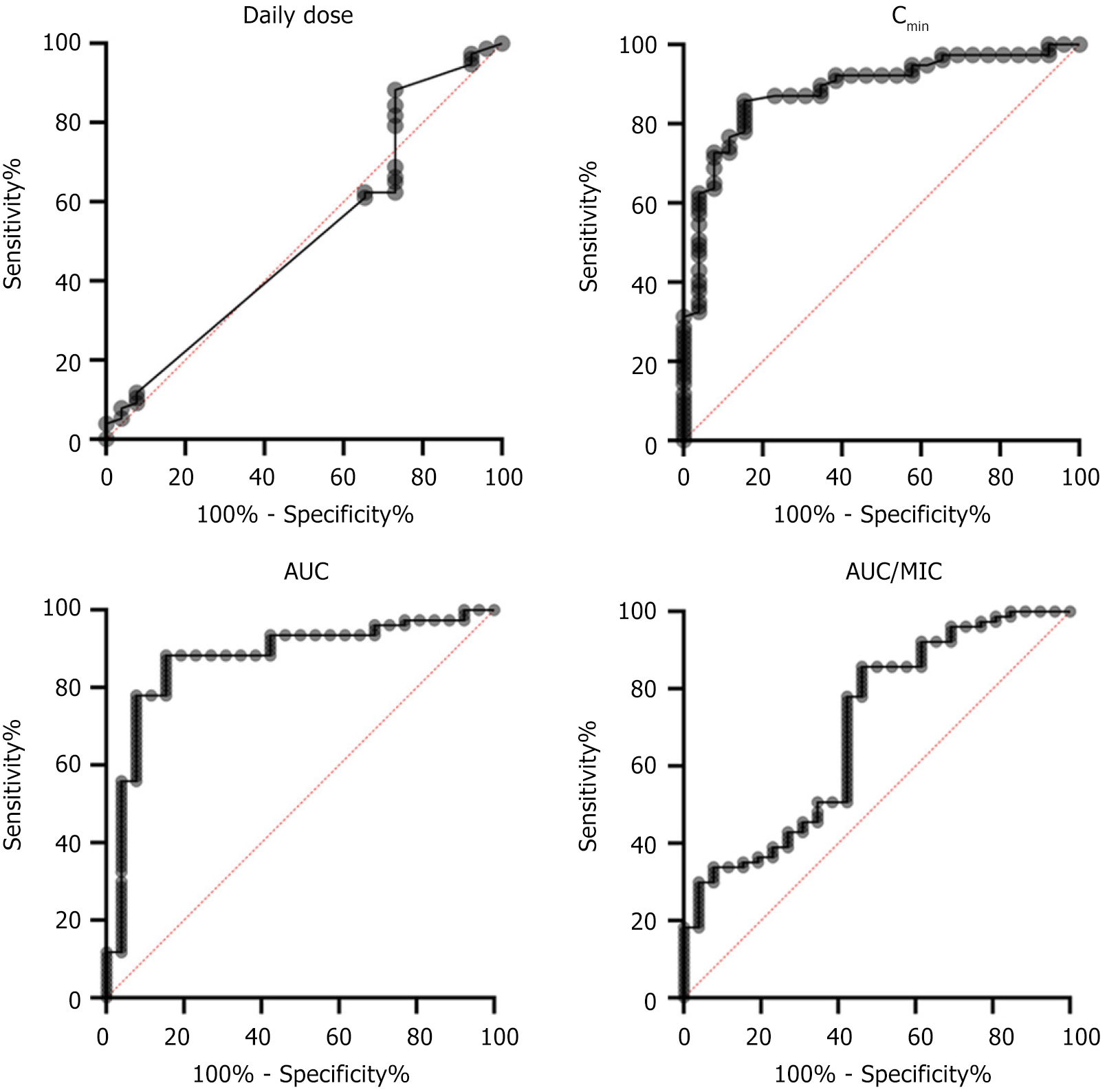
ROC curves were used to evaluate the accuracy and specificity of pharmacokinetics/efficacy (Figure 1). The AUCROC was 0.5147 with a 95% confidence interval of 0.3831 to 0.6464, 0.8854 (0.8145, 0.9562), 0.8761 (0.7945, 0.9578), and 0.6958 (0.5742, 0.8174) for mean daily dose, Cmin, AUC0-24, and AUC0-24/MIC, respectively, with P = 0.8228, P < 0.0001, P < 0.0001, and P = 0.0029. The cutoff point of daily dose was 1.125 μg/mL with 88.31% sensitivity and 26.92% specificity. The cutoff point of Cmin was 9.4 μg/mL with 85.71% sensitivity and 84.62% specificity. The cutoff point of AUC0-24 was 359.6 μg × hour/mL with 93.51% sensitivity and 53.85% specificity. The cutoff point of AUC0-24/MIC was 359.6 μg/mL with 92.21% sensitivity and 38.46% specificity.
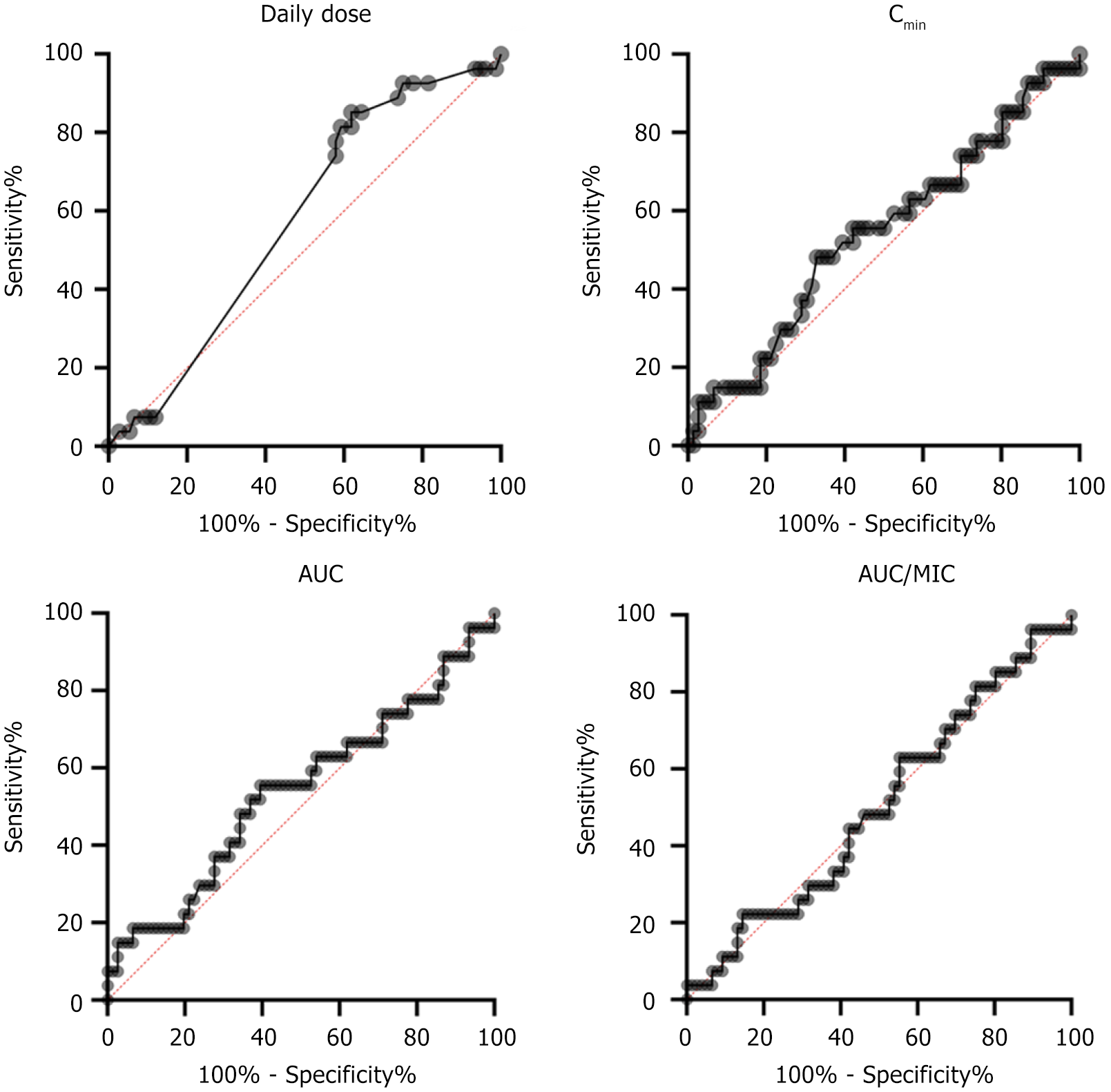
ROC curves were also used to evaluate the diagnostic accuracy and specificity of pharmacokinetic/nephrotoxicity (Figure 2). The AUCROC was 0.5765 with a 95% confidence interval of 0.4594 to 0.6936, 0.5436 (0.4143, 0.6729), 0.5402 (0.4060, 0.6744), and 0.5041 (0.3783, 0.6300) for daily dose, Cmin, AUC0-24, and AUC0-24/MIC, respectively, with P = 0.2391, P = 0.5021, P = 0.5362, and P = 0.9492. The cutoff point of daily dose was 1.65 μg/mL with 85.19% sensitivity and 38.16% specificity. The cutoff point of Cmin was 14.00 μg/mL with 48.15% sensitivity and 67.11% specificity. The cutoff point of AUC0-24 was 500.5 μg × hour/mL with 55.56% sensitivity and 60.53% specificity. The cutoff point of AUC0-24/MIC was 522.1 μg/mL with 62.96% sensitivity and 44.74% specificity.
The associations of clinical characteristics and pharmacokinetic indices on efficacy were analyzed by univariate logistic regression analysis. No significant differences were found in the baseline clinical data between the effective and ineffective cases, including sex, age, weight, main etiology, comorbidity, and infectious site, APACHE II score, and mechanical ventilation (Table 3). The results suggested that Cmin, AUC0-24, and AUC0-24/MIC were all significantly associated with clinical efficacy, with odds ratios (OR) of 33.0, 19.64, and 7.396, respectively (P < 0.001). In addition, there was a certain trend of correlation between daily dose and clinical efficacy, with an OR of 2.784 (P = 0.0709).
| Variable | Ineffective (n = 26) | Effective (n = 77) | OR | 95%CI | P value |
| Sex | 0.9815 | 0.360-2.517 | 0.9696 | ||
| Female | 8 (30.8) | 24 (31.2) | - | - | - |
| Male | 18 (69.2) | 53 (68.8) | - | - | - |
| Age (years) | 0.8780 | 0.358-2.150 | 0.7745 | ||
| ≤ 65 | 13 (50.0) | 41 (53.2) | - | - | - |
| > 65 | 13 (50.0) | 36 (46.8) | - | - | - |
| Weight (kg) | 0.6029 | 0.231-1.492 | 0.2830 | ||
| ≤ 60 | 9 (34.6) | 36 (46.8) | - | - | - |
| > 60 | 17 (65.4) | 41 (53.2) | - | - | - |
| Main etiologies of ICU cases | 0.5000 | 0.183-1.421 | 0.1805 | ||
| Postoperative infection | 18 (69.2) | 63 (81.8) | - | - | - |
| Other | 8 (30.7) | 14 (18.2) | - | - | - |
| Comorbidities | 0.5583 | 0.082-2.315 | 0.4720 | ||
| No | 2 (7.7) | 10 (13.0) | - | - | - |
| Yes | 24 (92.3) | 67 (87.0) | - | - | - |
| Infectious site | 0.7960 | 0.504-1.257 | 0.3235 | ||
| Abdominal cavity | 5 (19.2) | 17 (22.1) | - | - | - |
| Lung | 9 (34.6) | 26 (33.8) | - | - | - |
| Blood | 7 (26.9) | 29 (37.7) | - | - | - |
| Chest cavity | 5 (19.2) | 5 (6.5) | - | - | - |
| Daily dose (g) | 2.7840 | 0.892-8.484 | 0.0709 | ||
| ≤ 1.125 | 7 (26.9) | 9 (77.0) | - | - | - |
| > 1.125 | 19 (73.1) | 68 (88.3) | - | - | - |
| APACHE II score | 0.7143 | 0.289-1.743 | 0.4597 | ||
| ≤ 35 | 12 (46.2) | 42 (54.5) | - | - | - |
| > 35 | 14 (53.8) | 35 (45.5) | - | - | - |
| Mechanical ventilation | 1.1850 | 0.431-3.076 | 0.7320 | ||
| No | 8 (30.8) | 21 (27.3) | - | - | - |
| Yes | 18 (69.2) | 56 (72.7) | - | - | - |
| Cmin | 33.0000 | 10.440-130.700 | < 0.0001 | ||
| ≤ 9.4 | 22 (84.6) | 11 (14.3) | - | - | - |
| > 9.4 | 4 (15.4) | 66 (85.7) | - | - | - |
| AUC0-24 | 19.6400 | 6.321-71.150 | < 0.0001 | ||
| ≤ 359.6 | 15 (57.7) | 5 (6.5) | - | - | - |
| > 359.6 | 11 (42.3) | 72 (93.5) | - | - | - |
| AUC0-24/MIC | 7.3960 | 2.405-24.680 | 0.0006 | ||
| ≤ 359.6 | 10 (38.5) | 6 (7.8) | - | - | - |
| > 359.6 | 16 (61.5) | 71 (92.2) | - | - | - |
The associations of clinical characteristics and pharmacokinetic indices on nephrotoxicity were also analyzed by univariate logistic regression analysis. The results demonstrated that no significant differences were found in the baseline clinical data between the non-nephrotoxic and nephrotoxic cases, including sex, age, weight, main etiology, comorbidity, infectious site, APACHE II score, and mechanical ventilation (Table 4). The results showed that daily dose, Cmin, AUC0-24, and AUC0-24/MIC were not significantly associated with clinical nephrotoxicity, with ORs of 1.465, 1.894, 1.917, and 1.376 and P = 0.4289, P = 0.1612, P = 0.1508, and P = 0.4880, respectively.
| Variable | Non-nephrotoxic (n = 76) | Nephrotoxic (n = 27) | OR | 95%CI | P value |
| Sex | 1.0960 | 0.430-2.977 | 0.8509 | ||
| Female | 24 (31.6) | 8 (29.6) | - | - | - |
| Male | 52 (68.4) | 19 (70.4) | - | - | - |
| Age (years) | 1.2620 | 0.522-3.068 | 0.6046 | ||
| ≤ 65 | 41 (53.9) | 13 (46.4) | - | - | - |
| > 65 | 35 (46.1) | 14 (53.6) | - | - | - |
| Weight (kg) | 1.1770 | 0.486-2.927 | 0.7193 | ||
| ≤ 60 | 34 (44.7) | 11 (40.7) | - | - | - |
| > 60 | 42 (55.3) | 16 (59.3) | - | - | - |
| Main etiologies of ICU cases | 1.0710 | 0.347-2.994 | 0.8987 | ||
| Postoperative infection | 60 (78.9) | 21 (77.8) | - | - | - |
| Other | 16 (21.1) | 6 (22.2) | - | - | - |
| Comorbidities | 1.0750 | 0.292-5.141 | 0.9190 | ||
| No | 9 (11.8) | 3 (11.1) | - | - | - |
| Yes | 67 (88.2) | 24 (88.9) | - | - | - |
| Infectious site | 0.7187 | 0.432-1.147 | 0.1808 | ||
| Abdominal cavity | 18 (23.7) | 4 (14.8) | - | - | - |
| Lung | 23 (30.2) | 12 (44.4) | - | - | - |
| Blood | 27 (35.5) | 9 (33.3) | - | - | - |
| Chest cavity | 7 (9.2) | 3 (11.1) | - | - | - |
| Daily dose (g) | 1.4650 | 0.582-3.947 | 0.4289 | ||
| ≤ 1.65 | 29 (38.2) | 8 (29.6) | - | - | - |
| > 1.65 | 47 (61.8) | 19 (70.4) | - | - | - |
| APACHE II score | 0.5580 | 0.220-1.356 | 0.2047 | ||
| ≤ 35 | 37 (48.7) | 17 (63.0) | - | - | - |
| > 35 | 39 (51.3) | 10 (37.0) | - | - | - |
| Mechanical ventilation | 0.4510 | 0.177-1.160 | 0.0945 | ||
| No | 18 (23.7) | 11 (40.7) | - | - | - |
| Yes | 58 (76.3) | 16 (38.2) | - | - | - |
| Cmin (μg/mL) | 1.8940 | 0.771-4.663 | 0.1612 | ||
| ≤ 14.0 | 51 (67.1) | 14 (51.9) | - | - | - |
| > 14.0 | 25 (32.9) | 13 (48.1) | - | - | - |
| AUC0-24 | 1.9170 | 0.792-4.731 | 0.1508 | ||
| ≤ 500.5 | 46 (60.5) | 12 (44.4) | - | - | - |
| > 500.5 | 30 (39.5) | 15 (55.5) | - | - | - |
| AUC0-24/MIC | 1.3760 | 0.565-3.485 | 0.4880 | ||
| ≤ 522.1 | 34 (44.7) | 10 (37.0) | - | - | - |
| > 522.1 | 42 (55.3) | 17 (63.0) | - | - | - |
The association of clinical characteristics and pharmacokinetic indices with clinical efficacy and nephrotoxicity were performed by multivariate logistic regression analysis. Compared with AUC0-24/MIC, daily dose, Cmin, and AUC0-24 were significantly correlated with efficacy, with P values of 0.0178, 0.0003, and 0.0353, respectively (Table 5). Relative to other clinical characteristics and pharmacokinetic indices, Cmin emerged as an independent influencing factor affecting vancomycin nephrotoxicity (P = 0.0429) (Table 6). Collectively, Cmin and AUC0-24 were significantly associated with efficacy and nephrotoxicity. Cmin over 9.4 μg/mL and AUC0-24 over 359.6 μg × hour/mL indicated good efficacy of vancomycin against infection. Cmin below 14.0 μg/mL predicted no significant nephrotoxicity. Therefore, in this study, Cmin over 9.4 μg/mL and 14.0 μg/mL predicted good efficacy without significant nephrotoxicity.
| Variables | OR | 95%CI | Wald χ2 | P value |
| Intercept | 0.3617 | 0.011-8.888 | 0.6165 | 0.5375 |
| Sex | 0.5565 | 0.079-3.229 | 0.6345 | 0.5257 |
| Age | 0.3449 | 0.030-2.656 | 0.9746 | 0.3298 |
| Weight | 0.5051 | 0.089-2.624 | 0.8085 | 0.4188 |
| Main etiologies of ICU cases | 0.5206 | 0.076-3.206 | 0.7011 | 0.4833 |
| Comorbidities | 0.1924 | 0.016-1.535 | 1.4520 | 0.1464 |
| Infectious site | 0.9723 | 0.405-2.311 | 0.0651 | 0.9481 |
| Daily dose | 11.0400 | 1.752-109.200 | 2.3700 | 0.0178 |
| Cmin | 75.0000 | 9.551-1201.000 | 3.6430 | 0.0003 |
| AUC0-24 | 28.7700 | 1.710-2026.000 | 1.9320 | 0.0353 |
| AUC0-24/MIC | 0.1049 | 0.002-1.789 | 1.3610 | 0.1736 |
| APACHE II score | 0.5606 | 0.100-2.929 | 0.6910 | 0.4895 |
| Mechanical ventilation | 2.5520 | 0.363-22.680 | 0.9161 | 0.3596 |
| Variables | OR | 95%CI | Wald χ2 | P value |
| Intercept | 0.2826 | 0.026-2.565 | 1.0970 | 0.2727 |
| Sex | 0.8383 | 0.255-2.805 | 0.2919 | 0.7704 |
| Age | 1.1870 | 0.449-3.148 | 0.3484 | 0.7275 |
| Weight | 1.1540 | 0.403-3.393 | 0.2666 | 0.7898 |
| Main etiologies of ICU cases | 1.1600 | 0.338-3.700 | 0.2468 | 0.8051 |
| Comorbidities | 1.0740 | 0.247-5.781 | 0.0913 | 0.9272 |
| Infectious site | 0.6946 | 0.395-1.164 | 1.3400 | 0.1803 |
| Daily dose | 2.5480 | 0.502-17.650 | 1.0620 | 0.2882 |
| Cmin | 2.9900 | 1.003-10.470 | 1.8600 | 0.0429 |
| AUC0-24 | 1.0810 | 0.151-5.831 | 0.0872 | 0.9305 |
| AUC0-24/MIC | 0.8763 | 0.258-2.906 | 0.2160 | 0.8290 |
| APACHE II score | 0.6485 | 0.212-1.960 | 0.7714 | 0.4404 |
| Mechanical ventilation | 0.5025 | 0.156-1.592 | 1.1730 | 0.2407 |
There are two primary approaches for calculating vancomycin AUC0-24 with test plasma concentration. The first is the Bayesian method used in this study, which employs Cmin to generate accurate and reliable estimates of the AUC0-24 using a software program embedded with a population pharmacokinetic model and Bayesian feedback method[13]. Such software is readily available for bedside use to identify optimal vancomycin dosage[14]. In China, two Bayesian dose-optimizing software programs are commonly used: Smart dose application on Wechat; and the VCM-TDM on the web (http://www.pharmado.net/). Other software based on vancomycin population pharmacokinetics and Bayesian theory can achieve an equal results[15]. The second method is the equation method, which determines the AUC0-24 value with Cmax, Cmin, and a simple first-order pharmacokinetic formula[14,15]. The approach also has reasonable precision with low bias and has been recently validated for AUC0-24 calculation[13-15]. Both methods are based on vancomycin pharmacokinetic formulas.
Vancomycin, one of the glycopeptide antibiotics, primarily inhibits peptidoglycan synthesis by combining D-alanyl-D-alanine dipeptide and the precursor lipid II ends of peptidoglycan synthesis[16]. Vancomycin can inhibit cell wall synthesis of gram positive bacteria but is ineffective against gram negative bacteria. Smirnova et al[17] conducted a pharmacodynamic study of vancomycin and found a correlation between the minimum bacterial survival number small control growth curve and time bactericidal curve between the area and AUC0-24/MIC. A pharmacodynamic study of vancomycin treatment in 108 patients with a respiratory tract infection showed that AUC/MIC greater than 400 could achieve good clinical efficacy, and pathogen eradication was poor when AUC0-24/MIC was less than 400[18]. In the latest study, the starting dose of vancomycin should be calculated based on actual body weight, including patients with obesity, with dose adjustments to reach the target therapeutic concentration based on the actual serum concentration[2]. Continuous infusion administration is not considered more effective than brief administration but has a better safety profile[19].
In this study, the efficacy evaluation of vancomycin on MRSA infection, through U test, ROC evaluation, univariate logistic analysis, and multivariate logistic analysis showed that Cmin and AUC0-24 were significantly associated with efficacy. Cmin exceeding 9.4 μg/mL and AUC0-24 over 359.6 suggested better clinical efficacy. In addition, the daily dose and AUC0-24/MIC were also correlated with efficacy but was weaker than Cmin and AUC0-24. These results suggest that good clinical benefit may be obtained by adjusting Cmin above 9.4 μg/mL or AUC0-24 above 356.9. The results were generally consistent with the above reports, but the Cmin and AUC0-24 were slightly lower than the reference values. The subtle differences possibly arise from ethnic differences. AUC0-24/MIC has a slightly lower correlation with efficacy, and one possible reason is detection method or detection error.
The exact cause of acute kidney injury from vancomycin is not yet definite, but the biopsies show two possible reasons[20]. On one hand, vancomycin induces proximal renal tubular epithelial cells to produce oxidative stress, causing cell damage and apoptosis. One the other hand, vancomycin causes obstructive thrombosis, damaging renal tubular epithelial cells and then develops into acute kidney injury. It has been proven that vancomycin can increase the production of superoxide, destroy the mitochondrial structure, and then cause apoptosis. This damage positively correlated with vancomycin dose and action time[21].
In this study, the nephrotoxicity evaluation of vancomycin on MRSA infection through U test, ROC evaluation, and univariate logistic analysis showed that daily dose, Cmin, and AUC0-24 were not significantly associated with nephrotoxicity significance. However, in the multivariate logistic analysis, Cmin was a factor affecting nephrotoxicity, with Cmin exceeding 14 μg/mL, suggesting potential nephrotoxicity. These results suggest that higher Cmin of vancomycin may lead to increased nephrotoxicity in patients in the ICU. However, Cmin did not show a significant correlation with nephrotoxicity in the univariate logic analysis, suggesting that the effect of vancomycin Cmin on nephrotoxicity may be influenced by other factors.
The thresholds of the Cmin and AUC0-24 causing nephrotoxicity vary in different studies, with some concluding that Cmin and AUC0-24 are not associated with nephrotoxicity. This difference may come from two causes. The difference may come from the different patient characteristics, including the varying bases of renal function in different patients and the physiological and pathological factor status of the patients. The cases in this study came from the ICU and had complex clinical conditions and reduced compensatory capacities of renal functions, which increases the vulnerability to renal dysfunction. In addition, the extent of renal impairment was defined differently by different studies. Some reports have shown that an AUC0-24 greater than 1300 increases acute renal injury by 2.5 times[22,23]. We selected an Scr increase of 26.5 μmol/L within 48 h or a 1.5-fold increase as the definition of acute renal injury.
There were some limitations in this study. First, this was a single-center observational study with a small sample size and a long study duration, primarily due to the relatively small number of patients with MRSA infection. This study also excluded patients with combined effective antibiotics against gram positive bacteria, which may disturb the efficacy of vancomycin. These factors cause difficulties in patient collection and lengthen the experiment. Second, there was a lack of comprehensive baseline treatment data as a control. Vancomycin is currently the first-line treatment for MRSA infections, making it difficult to find patients with MRSA infection who have not received vancomycin treatment. Additionally, vancomycin plasma concentration monitoring is commonly used in the clinic, and clinicians will monitor vancomycin plasma concentration during treatment to avoid risks. Therefore, it is also difficult to find patients who have not received concentration monitoring as controls. The lack of baseline data may bias the findings. These limitations may limit the accuracy and applicability of the study findings.
It is beneficial to monitor Cmin and AUC0-24 of vancomycin during the entire treatment process. Cmin exceeding 9.4 μg/mL and AUC0-24 over 359.6 suggested better clinical efficacy. Additionally, Cmin exceeding 14 μg/mL suggests potential nephrotoxicity. A Cmin range of 9.4-14.0 μg/mL is suggested as an effective and safe concentration interval. Moreover, during vancomycin treatment of MRSA, close attention should be paid to adverse effects and renal function, as different patients may respond differently to vancomycin.
VCM-TDM software program used in this study was developed by the Preparation Team of “China Vancomycin Treatment Drug Monitoring Guidelines” and Development Team of “PharmVAN” of the Individualized Drug Delivery Platform of Vancomycin in the Third Hospital of Beijing University. We are grateful for the free use of the software.
| 1. | Burns AN, Goldman JL. A Moving Target-Vancomycin Therapeutic Monitoring. J Pediatric Infect Dis Soc. 2020;9:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77:835-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 721] [Article Influence: 180.3] [Reference Citation Analysis (0)] |
| 3. | Castañeda X, García-de-la-Mària C, Gasch O, Pericas JM, Armero Y, Soy D, García-González J, Falces C, Ninot S, Almela M, Ambrosioni J, Quintana E, Vidal B, Fuster D, Llopis J, Soto S, Moreno A, Marco F, Miró JM; Hospital Clinic Endocarditis Study Group. AUC/MIC Pharmacodynamic Target Is Not a Good Predictor of Vancomycin Efficacy in Methicillin-Resistant Staphylococcus aureus Experimental Endocarditis. Antimicrob Agents Chemother. 2017;61:e02486-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Gawronski KM, Goff DA, Brown J, Khadem TM, Bauer KA. A stewardship program's retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. Clin Ther. 2013;35:772-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | He N, Su S, Ye Z, Du G, He B, Li D, Liu Y, Yang K, Zhang X, Zhang Y, Chen X, Chen Y, Chen Z, Dong Y, Du G, Gu J, Guo D, Guo R, Hu X, Jiao Z, Li H, Liu G, Li Z, Lv Y, Lu W, Miao L, Qu J, Sun T, Tong R, Wang L, Wang M, Wang R, Wen A, Wu J, Wu X, Xu Y, Yang Y, Yang F, Zhan S, Zhang B, Zhang C, Zhang H, Zhang J, Zhang J, Zhang J, Zhang W, Zhao L, Zhao L, Zhao R, Zhao W, Zhao Z, Zhou W, Zeng XT, Zhai S. Evidence-based Guideline for Therapeutic Drug Monitoring of Vancomycin: 2020 Update by the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Clin Infect Dis. 2020;71:S363-S371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 6. | Shen K, Yang M, Fan Y, Liang X, Chen Y, Wu J, Yu J, Zhang H, Wang R, Zhang F, Hang J, Wen X, Li H, Shen L, Zhang Z, Wu S, Shen B, Huang W, Chang C, Shen Y, Ren H, Yuan Q, Song X, Luo X, Zhang H, Yang W, Yang J, Zhang J. Model-based Evaluation of the Clinical and Microbiological Efficacy of Vancomycin: A Prospective Study of Chinese Adult In-house Patients. Clin Infect Dis. 2018;67:S256-S262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Liang X, Fan Y, Yang M, Zhang J, Wu J, Yu J, Tao J, Lu G, Zhang H, Wang R, Wen X, Li H, Zhang F, Hang J, Shen L, Zhang Z, Lin Q, Fu F, Wu S, Shen B, Huang W, Chang C, Zhang H, Huang Q, Shi Y, Ren H, Yuan Q, Song X, Luo X, Zhang H. A Prospective Multicenter Clinical Observational Study on Vancomycin Efficiency and Safety With Therapeutic Drug Monitoring. Clin Infect Dis. 2018;67:S249-S255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 471] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 9. | Neely MN, Kato L, Youn G, Kraler L, Bayard D, van Guilder M, Schumitzky A, Yamada W, Jones B, Minejima E. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62:e02042-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 10. | Koeth LM, DiFranco-Fisher JM, McCurdy S. A Reference Broth Microdilution Method for Dalbavancin In Vitro Susceptibility Testing of Bacteria that Grow Aerobically. J Vis Exp. 2015;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Chen X, Du L, Liu M. Development, validation, and application of an UPLC-MS/MS method for vancomycin, norvancomycin, methotrexate, paclitaxel, and imatinib analysis in human plasma. Ann Clin Biochem. 2022;59:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Oprea AD, Del Rio JM, Cooter M, Green CL, Karhausen JA, Nailer P, Guinn NR, Podgoreanu MV, Stafford-Smith M, Schroder JN, Fontes ML, Kertai MD. Pre- and postoperative anemia, acute kidney injury, and mortality after coronary artery bypass grafting surgery: a retrospective observational study. Can J Anaesth. 2018;65:46-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Aubron C, Corallo CE, Nunn MO, Dooley MJ, Cheng AC. Evaluation of the accuracy of a pharmacokinetic dosing program in predicting serum vancomycin concentrations in critically ill patients. Ann Pharmacother. 2011;45:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Turner RB, Kojiro K, Shephard EA, Won R, Chang E, Chan D, Elbarbry F. Review and Validation of Bayesian Dose-Optimizing Software and Equations for Calculation of the Vancomycin Area Under the Curve in Critically Ill Patients. Pharmacotherapy. 2018;38:1174-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58:3162-3167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Barna JC, Williams DH. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol. 1984;38:339-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 350] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Smirnova MV, Lubenko IY, Portnoy YA, Zinner SH, Firsov AA. Concentration-response relationships as a basis for choice of the optimal endpoints of the antimicrobial effect: daptomycin and vancomycin pharmacodynamics with staphylococci in an in vitro dynamic model. Int J Antimicrob Agents. 2007;29:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 576] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 19. | Hao JJ, Chen H, Zhou JX. Continuous versus intermittent infusion of vancomycin in adult patients: A systematic review and meta-analysis. Int J Antimicrob Agents. 2016;47:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Luque Y, Louis K, Jouanneau C, Placier S, Esteve E, Bazin D, Rondeau E, Letavernier E, Wolfromm A, Gosset C, Boueilh A, Burbach M, Frère P, Verpont MC, Vandermeersch S, Langui D, Daudon M, Frochot V, Mesnard L. Vancomycin-Associated Cast Nephropathy. J Am Soc Nephrol. 2017;28:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Arimura Y, Yano T, Hirano M, Sakamoto Y, Egashira N, Oishi R. Mitochondrial superoxide production contributes to vancomycin-induced renal tubular cell apoptosis. Free Radic Biol Med. 2012;52:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Chuma M, Makishima M, Imai T, Tochikura N, Suzuki S, Kuwana T, Sawada N, Komatsu T, Sakaue T, Kikuchi N, Yoshida Y, Kinoshita K. Relationship Between Initial Vancomycin Trough Levels and Early-Onset Vancomycin-Associated Nephrotoxicity in Critically Ill Patients. Ther Drug Monit. 2018;40:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Zasowski EJ, Murray KP, Trinh TD, Finch NA, Pogue JM, Mynatt RP, Rybak MJ. Identification of Vancomycin Exposure-Toxicity Thresholds in Hospitalized Patients Receiving Intravenous Vancomycin. Antimicrob Agents Chemother. 2018;62:e01684-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |