Published online Jun 6, 2025. doi: 10.12998/wjcc.v13.i16.102740
Revised: December 8, 2024
Accepted: January 7, 2025
Published online: June 6, 2025
Processing time: 105 Days and 19.4 Hours
Kidney transplantation is one of the most effective treatments for patients with end-stage renal disease. However, many regions face low deceased donor rates and limited ABO-compatible transplant availability, which increases reliance on living donors. These regional challenges necessitate the implementation of kidney paired donation (KPD) programs to overcome incompatibilities such as ABO mismatch or positive cross-matching, even when suitable and willing donors are available.
To evaluate the effectiveness of a single-center domino KPD model in both operational planning and clinical management processes and to assess its impact on clinical outcomes.
Between April 2020 and January 2024, we retrospectively evaluated patients enrolled in our center’s domino kidney transplantation program. Donor-recipient pairs unable to proceed due to ABO incompatibility or positive cross-matching with their own living donors were included. Donors and recipients were assessed based on blood group compatibility, HLA tissue typing, and negative cross-match results. A specialized computer algorithm grouped patients into three-way, four-way, and five-way chains. All surgical procedures were performed on the same day at a single center.
A total of 169 kidney transplants were performed, forming 52 domino chains. These domino KPD transplants accounted for a notable proportion of our center’s overall transplant activity, which included both living donor kidney transplants and deceased donor transplants. Among these chains, the primary reasons for participation were ABO incompatibility (74%), positive cross-matching (10%), and the desire to improve HLA mismatch (16%). Improved HLA mismatch profiles and high graft survival (96% at 1 year, 92% at 3 years) and patient survival (98% at 1 year, 94% at 3 years) rates were observed, as well as low acute rejection episodes.
The single-center domino KPD model enhanced transplant opportunities for incompatible donor-recipient pairs while maintaining excellent clinical outcomes. By providing a framework that addresses regional challenges, improves operational efficiency, and optimizes clinical management, this model offers actionable insights to reduce waiting lists and improve patient outcomes.
Core Tip: This retrospective study demonstrated that a single-center domino kidney paired donation program significantly increased transplant opportunities for incompatible donor-recipient pairs while achieving excellent clinical outcomes. By strategically utilizing altruistic living non-directed donors and centralizing all procedures within one center, the program enhanced operational efficiency and reduced ischemia times. Expanding this model could play a crucial role in decreasing transplant waiting lists and improving patient outcomes.
- Citation: Huseynov A, Kuşlu Çicek SN, Tuncer M. Advantages of the single-center model in domino transplant processes: Operational planning and management experience. World J Clin Cases 2025; 13(16): 102740
- URL: https://www.wjgnet.com/2307-8960/full/v13/i16/102740.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i16.102740
Kidney transplantation stands as one of the most successful therapeutic options for patients with end-stage renal disease[1]. However, limited availability of organs from deceased donors, the better long-term outcomes achieved with grafts obtained from living donors, and the widespread adoption of surgical techniques such as the use of laparoscopic donor nephrectomy have increased the reliance on living donors[2,3]. Nevertheless, incompatibilities between donor-recipient pairs (DRPs), such as ABO incompatibility or positive cross-matching, prevent transplantation involving willing and suitable donors[4]. At this point, kidney paired donation (KPD) programs implemented in many countries emerge as an effective method developed to overcome these challenges[5].
In many regions, low deceased donor organ donation rates and the scarcity of ABO-compatible transplants have further complicated access to suitable grafts, intensifying the need for alternative strategies[1]. Moreover, KPD programs have shown cost-effectiveness by reducing prolonged dialysis dependence and associated healthcare expenses[4,5]. Our center established its KPD program several years ago and over time has expanded its scope and complexity, allowing for an in-depth evaluation of both operational and clinical outcomes.
Matching DRPs through cross-transplantation in KPD programs becomes increasingly complex as the number of participating centers and pairs grows[6]. Therefore, forming the optimal matching sets using computer algorithms enhances the success of these programs[7]. However, despite the effectiveness of these systems, a significant portion of incompatible pairs may remain unmatched even after being included in the programs[8].
Domino kidney transplantation offers a solution to overcome such incompatibilities[9]. This model operates by initiating the process with an altruistic living non-directed donor (LND)[10]. The LND can donate a kidney to a patient with priority on the waiting list for deceased donor organs or can be included in the pool of incompatible pairs in the KPD program to start chain transplants[11]. In this way, recipients with willing but incompatible donors also have the chance for transplantation, allowing more patients to access kidney transplants[12].
Despite the growing interest in KPD, there is limited literature addressing the operational challenges associated with implementing and scaling such programs, especially those involving complex chain formations. By focusing on both operational planning and clinical management, our study aimed to highlight the dual benefits of a single-center domino KPD model. This combined perspective is crucial for understanding how to optimize chain transplantation processes, enhance communication, reduce ischemic times, and ultimately improve patient outcomes[13]. In light of our 4 years of experience, the purpose of this study was to reveal the operational and clinical benefits of domino transplantation.
This study encompassed patients enrolled in our single-center domino kidney transplantation program between April 2020 and January 2024, including DRPs who could not undergo transplantation due to ABO incompatibility or positive cross-matching with their own living donors. We maintained a dedicated KPD/incompatible transplant registry to track such cases. Patients under 18 years of age were excluded to ensure informed consent and autonomous decision-making capacity as well as to maintain ethical and legal standards.
Donors and recipients were evaluated based on blood group compatibility, HLA tissue typing, and negative cross-match results. A specialized computer algorithm was employed to optimize donor-recipient matching. This algorithm incorporated multiple priority factors, including sensitivity levels (panel-reactive antibodies), age differences, and clinical parameters. Recipients were scored using a comprehensive system that factored in blood group compatibility, HLA mismatches, and the presence of donor-specific antibodies (DSAs). The algorithm also prioritized disadvantaged recipients, such as those with blood group O or those receiving from AB donors, patients on prolonged dialysis, pediatric recipients, individuals with extended waiting times, and those lacking family organ donation history. Unacceptable incompatibilities (e.g., high-titer ABO incompatibility, strong positive cross-matches, or critical DSAs above an established threshold) were predefined to ensure no allocation to pairs at high immunologic risk. Primary allocation criteria focused on maximizing the number of successful transplants, minimizing immunologic risk, and improving recipient outcomes.
All surgical procedures were performed on the same day at a single center. Donor nephrectomies were carried out laparoscopically, while recipient surgeries followed standard surgical protocols. To achieve maximal operational efficiency, we allocated an adequate number of surgeons, anesthesiologists, and nursing staff specifically trained for simultaneous procedures. Multiple operating rooms (typically 2-4) were utilized concurrently to accommodate simultaneous surgeries. The schedule was organized to minimize cold ischemia time (CIT) and keep warm ischemia time (WIT) as short as possible. Surgical teams were carefully scheduled to ensure manageable workloads, with each surgeon performing a limited number of procedures per day. Contingency plans were in place for emergencies or unexpected donor or recipient cancellations, including backup recipients or delayed chain activation protocols.
Patients’ demographic data, and perioperative and postoperative clinical outcomes were obtained from medical records. Collected data included age, gender, blood group, HLA mismatches, operation times, ischemia times, graft function, complications, and patient and graft survival rates. We organized these data into comprehensive tables. For each donor and recipient pair, we detailed blood group (noting the high prevalence of blood group O donors), HLA profiles, WIT, CIT, ABO and HLA mismatch rates, and thresholds for acceptable DSAs. Religious backgrounds, socioeconomic status, and motivational factors were recorded for altruistic LNDs, who generally had flexible schedules and frequently possessed blood group O, facilitating broader matching potential.
Exclusion criteria were patients under 18 years of age, those with incomplete or inaccurate medical records, and patients participating in multicenter transplant programs. The age exclusion criterion ensured that all individuals could provide informed consent independently, aligning with ethical and legal standards.
Statistical analysis was performed using dedicated statistical software (e.g., SPSS version 25.0). Continuous variables were reported as mean ± standard deviation, while categorical variables were summarized as frequencies and percentages. Suitable statistical tests (e.g., t-test, χ2 test) were employed to compare groups. Kaplan-Meier survival analysis was used to assess patient and graft survival rates, with differences across chain types evaluated via the log-rank test. A P value below 0.05 was deemed statistically significant.
The KPD program was partially supported by institutional grants and external funding dedicated to improving organ allocation strategies. This financial support facilitated algorithm development, staff training, and resource expansion within the transplant center.
This study was approved by the relevant Biruni University Ethics Committee (ID = 2024-BİAEK/04-02), and written informed consent was obtained from all participants. All procedures adhered to the principles of the Declaration of Helsinki, and no additional risks were imposed on donors or recipients.
Between April 2020 and January 2024, a total of 169 kidney transplants were performed at our center, organized into 52 domino chains. During the same period, our center performed a total of 835 living donor kidney transplants and 16 deceased donor kidney transplants, indicating that these KPD procedures accounted for approximately 19.8% of all kidney transplants performed. The chains were categorized as follows: 41 three-way chains involving 123 patients, 9 four-way chains involving 36 patients, and 2 five-way chains involving 10 patients. All surgeries were conducted on the same day and at a single center to optimize operational efficiency and minimize CITs.
The primary reasons for enrollment in the KPD program were ABO incompatibility (74%), positive cross-match (10%), and desire to improve HLA mismatch (16%). Among the recipients, 13% had significant HLA mismatches, and the algorithm incorporated sensitivity (defined as panel-reactive antibody levels and the presence of DSAs) to prioritize recipients with higher immunologic risk. The demographic characteristics of the donors and recipients are summarized in Table 1.
| Characteristic | Donors (n = 169) | Recipients (n = 169) |
| Sex (M/F) | 80/89 (47%/53%) | 95/74 (56%/44%) |
| Mean age (years) | 42.8 ± 8.5 | 43.2 ± 8.1 |
Among donors, the majority were spouses (48%), followed by other relatives (32%), and altruistic LNDs (20%). The proportion of female donors was slightly higher than male donors (53% vs 47%), whereas males were more prevalent (56% vs 44%) among recipients. The mean age of donors was 42.8 ± 8.5 years, and the mean age of recipients was 43.2 ± 8.1 years. Blood group O prevalence among donors (especially LNDs) was 40%, which is notably higher than the general population prevalence of approximately 30%-35%, thus broadening the pool of compatible recipients and reducing waiting times (Table 2).
| Blood type | Donors | Recipients |
| A | 55 (32.5) | 61 (36.1) |
| B | 32 (18.9) | 34 (20.1) |
| AB | 14 (8.3) | 17 (10.0) |
| O | 68 (40.2) | 57 (33.7) |
| Total | 169 (100) | 169 (100) |
All surgical procedures were performed simultaneously using 2-4 operating rooms, with an average of 4-6 surgeries taking place concurrently, depending on chain complexity. Approximately 4 surgeons, 5 anesthesiologists, and 16 nursing/support staff were involved in each multichain surgery day. Total operating room usage per chain typically ranged from 4-15 h, depending on chain length and complexity. Contingency plans for unexpected donor or recipient cancellations included designated backup recipients and delayed chain activation protocols.
In pairs enrolled to improve HLA mismatch, the number of HLA mismatches significantly decreased in transplanted pairs compared to their intended pairs (3.2 ± 0.8 vs 4.7 ± 1.1, P < 0.001). Specifically, DR mismatches were reduced from 1.6 ± 0.5 to 0.9 ± 0.4 (P < 0.001) (Table 3). All surgical procedures were completed successfully without intraoperative complications. The mean CIT was 46 ± 9 min, and the mean WIT was 4 ± 1 min. There were no significant differences in ischemia times among different chain types.
| Characteristic | Intended pairs (mean ± SD) | Transplanted pairs (mean ± SD) | P value |
| Total HLA mismatches | 4.7 ± 1.1 | 3.2 ± 0.8 | < 0.001 |
| DR mismatches | 1.6 ± 0.5 | 0.9 ± 0.4 | < 0.001 |
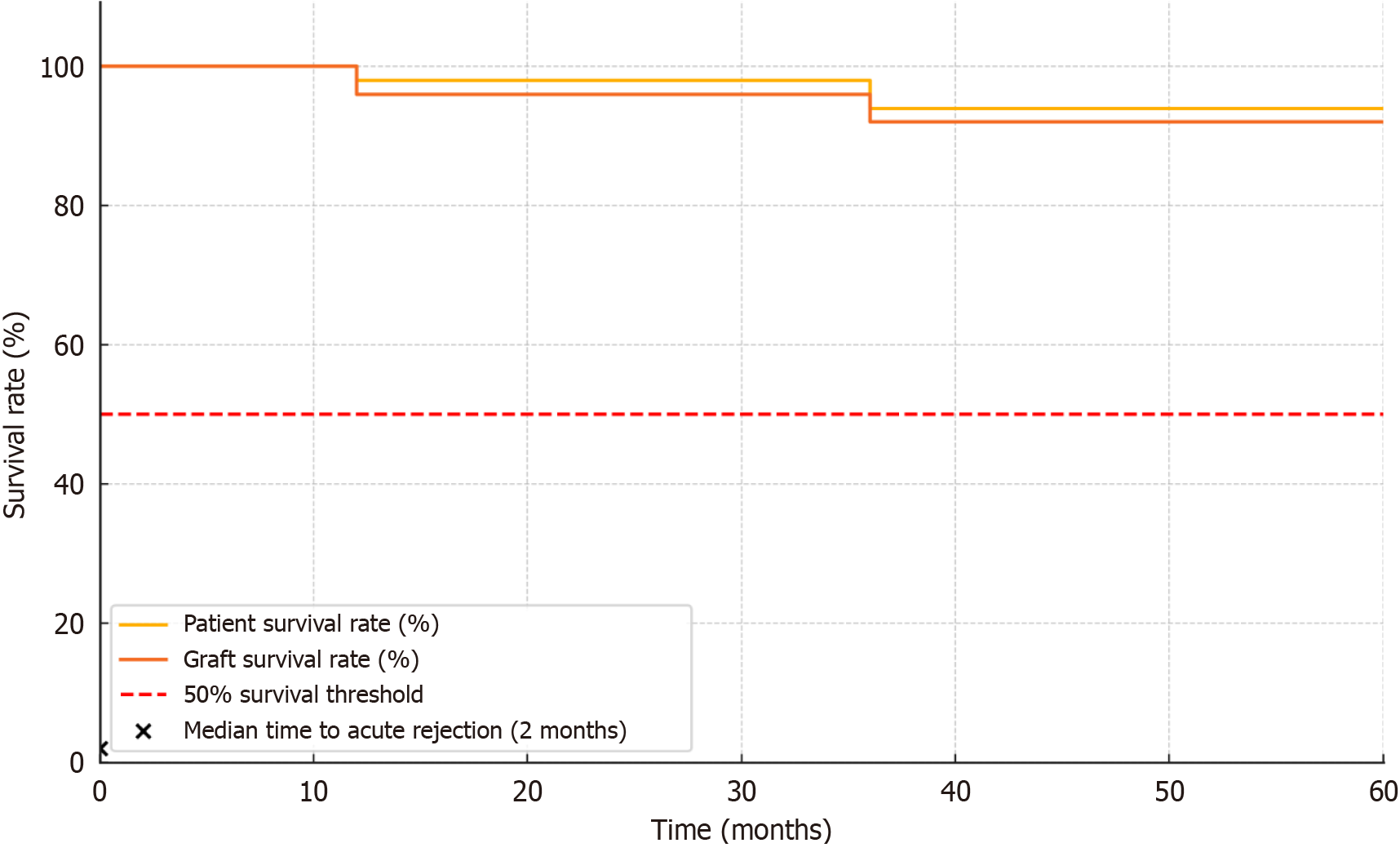
The graft survival rates at 1 year and 3 years were 96% and 92%, respectively, with a median follow-up of 36 months (ranging from 12-60 months). The 1-year and 3-year patient survival rates were 98% and 94%, respectively (Table 4). Kaplan-Meier survival analysis indicated no significant differences in patient or graft survival among different chain types (P > 0.05). A Kaplan-Meier survival curve illustrating patient and graft survival over the follow-up period is presented in Figure 1. The median time to acute rejection episodes was approximately 2 months post-transplant, and rejection rates and timing did not differ significantly among chain types.
| Characteristic | 1-year survival (%) | 3-year survival (%) | Median follow-up |
| Graft survival | 96 | 92 | |
| Patient survival | 98 | 94 | 36 months (range: 12-60 months) |
| Differences among chain types | - | - | No significant difference (P > 0.05) |
Postoperative complications occurred in 15% of recipients. The most common complications were acute rejection episodes (8%), delayed graft function (5%), and infections such as urinary tract and wound infections (2%). There were no significant differences in complication rates among the different chain types (P > 0.05). The low loss-to-follow-up rate
All donors recovered uneventfully from surgery, with no significant postoperative complications reported. The mean hospital stay for donors was 4.5 ± 1.2 days. There were no instances of donor mortality or significant morbidity. The altruistic LNDs had a mean age of 43.5 ± 8.7 years. Occupations varied, with a higher representation of individuals from religious professions (30%) and self-employed individuals with flexible schedules (25%). Blood type O was more prevalent among LNDs (40%), which facilitated matching with a broader range of recipients.
The median duration between patient enrollment and transplantation was 2.5 months (interquartile range: 1.0-6.0 months) (Table 5). Recipients with blood type O or sensitized patients had slightly longer waiting times, but the difference was not statistically significant.
| Parameter | Value/range |
| PRA level (mean ± SD) | 20% ± 8% |
| DSA threshold (MFI cutoff) | > 3000 MFI |
| ABO/HLA mismatch frequency | 90% |
| Cold ischemia time, min | 46 ± 9 |
| Warm ischemia time, min | 4 ± 1 |
| Mean OR time per chain, h | 4-6 |
| Total surgeries per multichain session | 4-6 |
| Surgeries/day per surgeon | 1-2 |
| Median waiting time (months, IQR) | 2.5 (1.0-6.0) |
All recipients received a standardized immunosuppressive regimen consisting of basiliximab induction, tacrolimus, mycophenolate mofetil, and low-dose steroids, tailored according to immunologic risk profiles and monitored closely throughout the follow-up period.
This study evaluated the clinical outcomes of our single-center domino KPD program over the period between April 2020 and January 2024. Our findings indicated that the domino KPD model significantly enhanced the number of successful kidney transplants among incompatible DRPs while maintaining excellent patient and graft survival rates.
The 1-year and 3-year graft survival rates in our program were 96% and 92%, respectively, with patient survival rates of 98% and 94%, respectively. These outcomes are comparable to, or even exceed, those reported in conventional living unrelated donor kidney transplants and other KPD programs[14,15]. This suggests that the domino KPD approach is not only effective in increasing transplant numbers but also ensures high-quality clinical results.
A notable advantage of the domino KPD model is its ability to maximize the impact of altruistic LNDs. By initiating transplant chains with LNDs, we were able to facilitate longer transplant chains-up to five pairs in our study, which are often challenging to coordinate in traditional KPD due to logistical constraints[16]. Performing all 169 surgeries on the same day and within a single center minimized CITs (mean 46 ± 9 min) and reduced the risk of donor withdrawal, contributing to the favorable outcomes observed[17].
Our program particularly benefited disadvantaged DRPs, including those with blood type O recipients or AB donors, who traditionally face greater difficulty in finding compatible matches[18]. In our study, 100% of transplanted DRPs included such disadvantaged pairs. This highlights the effectiveness of the domino KPD model in overcoming blood type incompatibility issues that are less readily addressed in conventional KPD systems[19].
Additionally, the use of a specialized computer algorithm for matching allowed us to optimize donor-recipient compatibility beyond basic ABO and cross-match considerations. This included improvements in HLA mismatch profiles, with the number of mismatches significantly decreasing from 4.7 ± 1.1 in intended pairs to 3.2 ± 0.8 in transplanted pairs
Despite these positive outcomes, our study had limitations. The retrospective nature and single-center scope of the study may restrict the generalizability of the findings[21]. Moreover, while simultaneous surgeries reduce the risk of donor reneging, unforeseen events can still disrupt transplant chains. Based on our experience, only a single donor withdrew due to medical reasons that precluded donation. This underscores the importance of having contingency plans in place to manage such scenarios[22].
Furthermore, the single-center approach in our domino KPD program offered several operational advantages that contributed to its success. Centralizing all activities within a single institution simplifies coordination, scheduling, and resource allocation[23]. This centralization reduces logistical complexities and potential delays associated with multicenter programs, where varying protocols and communication barriers can impede efficiency[24]. By streamlining processes, we were able to minimize CITs and ensure that all surgical teams were synchronized, which is critical for preserving graft function and viability.
Our findings also underscored the importance of meticulous operational planning and management in executing complex transplant chains. The success of longer chains, such as those involving five pairs, hinges on precise timing and coordination[25]. Implementing standardized protocols and utilizing specialized software for matching and scheduling were instrumental in the ability of our program to handle such complexity effectively.
Notably, when comparing these outcomes to our previous experience with ABO-incompatible transplants outside of a domino KPD framework, the domino approach provided more consistent access to suitable donors and improved overall transplant rates[26]. This suggests that the operational refinements and enhanced donor-recipient matching strategies of the domino KPD model may be superior to traditional methods of managing ABO-incompatible pairs.
Despite the advantages, the single-center model may face challenges in terms of scalability and resource requirements. Performing multiple simultaneous surgeries demands significant surgical capacity, including operating rooms, surgical teams, and postoperative care facilities[27]. Therefore, institutions considering this model must assess their capabilities and possibly invest in expanding their resources.
For centers seeking to implement a similar domino KPD model, our results highlighted several key recommendations: (1) Invest in advanced matching algorithms that incorporate both ABO and HLA factors; (2) Develop standardized protocols for scheduling and coordinating multichain transplants; and (3) Ensure adequate resource allocation of operating rooms, trained personnel, and backup plans to handle the logistical complexities of performing multiple surgeries simultaneously. By doing so, other centers may replicate our success, improve their transplant rates, and offer better outcomes for patients facing immunologic challenges.
Our study demonstrated that a single-center domino KPD model not only increased transplantation opportunities for incompatible DRPs but also delivered strong clinical outcomes by combining operational efficiency with strategic donor-recipient matching. This dual focus on clinical excellence and operational management can serve as a roadmap for other transplant centers, particularly those facing ABO incompatibility and limited donor availability.
Our single-center domino KPD program yielded tangible improvements in transplant opportunities for previously incompatible DRPs, as evidenced by favorable graft and patient survival rates, reduced waiting times, and minimized donor withdrawal events. Operational strategies such as centralizing all surgical procedures, incorporating LNDs to initiate longer transplant chains, and employing specialized algorithms to refine donor-recipient matching directly contributed to these outcomes. These specific measures, grounded in the observed results, demonstrated that an approach focused on targeted operational enhancements and strategic clinical management can optimize the effectiveness of the domino KPD model in a defined institutional setting.
| 1. | Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, Friedewald JJ, Hays R, Howard A, Jones E, Leichtman AB, Merion RM, Metzger RA, Pradel F, Schweitzer EJ, Velez RL, Gaston RS. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol. 2008;3:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 420] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 2. | Vernadakis S, Marinaki S, Darema M, Soukouli I, Michelakis IE, Beletsioti C, Zavvos G, Bokos I, Boletis IN. The Evolution of Living Donor Nephrectomy Program at A Hellenic Transplant Center. Laparoscopic vs. Open Donor Nephrectomy: Single-Center Experience. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Ghods AJ. Living kidney donation: the outcomes for donors. Int J Organ Transplant Med. 2010;1:63-71. [PubMed] |
| 4. | de Klerk M, Keizer KM, Claas FH, Witvliet M, Haase-Kromwijk BJ, Weimar W. The Dutch national living donor kidney exchange program. Am J Transplant. 2005;5:2302-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Anderson R, Ashlagi I, Gamarnik D, Roth AE. Finding long chains in kidney exchange using the traveling salesman problem. Proc Natl Acad Sci U S A. 2015;112:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Cheng Y, Yang Z. Efficient Kidney Exchange with Dichotomous Preferences. J Health Econ. 2021;80:102536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wang W, Leichtman AB, Rees MA, Song PX, Ashby VB, Shearon T, Kalbfleisch JD. Kidney Paired Donation Chains Initiated by Deceased Donors. Kidney Int Rep. 2022;7:1278-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Huh KH, Kim MS, Ju MK, Chang HK, Ahn HJ, Lee SH, Lee JH, Kim SI, Kim YS, Park K. Exchange living-donor kidney transplantation: merits and limitations. Transplantation. 2008;86:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Blumberg JM, Gritsch H, Veale JL. Kidney paired donation: advancements and future directions. Curr Opin Organ Transplant. 2011;16:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Dew MA, Jacobs CL, Jowsey SG, Hanto R, Miller C, Delmonico FL; United Network for Organ Sharing (UNOS); American Society of Transplant Surgeons; American Society of Transplantation. Guidelines for the psychosocial evaluation of living unrelated kidney donors in the United States. Am J Transplant. 2007;7:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Melcher ML, Leeser DB, Gritsch HA, Milner J, Kapur S, Busque S, Roberts JP, Katznelson S, Bry W, Yang H, Lu A, Mulgaonkar S, Danovitch GM, Hil G, Veale JL. Chain transplantation: initial experience of a large multicenter program. Am J Transplant. 2012;12:2429-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Nassiri N, Kwan L, Bolagani A, Thomas AG, Sinacore J, Ronin M, Cooper M, Segev DL, Cecka JM, Veale JL. The "oldest and coldest" shipped living donor kidneys transplanted through kidney paired donation. Am J Transplant. 2020;20:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Abrol N, Bentall A, Torres VE, Prieto M. Simultaneous bilateral laparoscopic nephrectomy with kidney transplantation in patients with ESRD due to ADPKD: A single-center experience. Am J Transplant. 2021;21:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Cozzi M, Donato P, Ugolini G, Nguefouet Momo RE, Nacchia F, Ballarini Z, Piccoli P, Cantini M, Caletti C, Andreola S, Gandini G, Gambaro G, Boschiero L. Outcomes in AB0 Incompatible Living Donor Kidney Transplantation: A Case - Control Study. Front Med (Lausanne). 2022;9:932171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Chandra Shrestha P, Bhandari TR, Adhikari R, Baral H, Verma RK, Shrestha KK. Living donor kidney paired exchange: An observational study. Ann Med Surg (Lond). 2022;78:103761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Kute VB, Patel HV, Shah PR, Modi PR, Shah VR, Rizvi SJ, Pal BC, Modi MP, Shah PS, Varyani UT, Wakhare PS, Shinde SG, Ghodela VA, Patel MH, Trivedi VB, Trivedi HL. Past, present and future of kidney paired donation transplantation in India. World J Transplant. 2017;7:134-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Ahmad SB, Inouye B, Phelan MS, Kramer AC, Sulek J, Weir MR, Barth RN, LaMattina JC, Schweitzer EJ, Leeser DB, Niederhaus SV, Bartlett ST, Bromberg JS. Live Donor Renal Transplant With Simultaneous Bilateral Nephrectomy for Autosomal Dominant Polycystic Kidney Disease Is Feasible and Satisfactory at Long-term Follow-up. Transplantation. 2016;100:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Uchida J, Kosoku A, Naganuma T, Tanaka T, Nakatani T. Latest insights on ABO-incompatible living-donor renal transplantation. Int J Urol. 2020;27:30-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Verma U, Rangaraj N, Billa V, Usulumarty D. Long term simulation analysis of deceased donor initiated chains in kidney exchange programs. Health Syst (Basingstoke). 2024;13:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Morris T, Maple H, Norton S, Chilcot J, Burnapp L, Draper H, Mamode N, McCrone P. Challenges and Opportunities in the Supply of Living Kidney Donation in the UK National Health Service: An Economic Perspective. Transplantation. 2022;106:2137-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Villa S, Patrone F. Incentive compatibility in kidney exchange problems. Health Care Manag Sci. 2009;12:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Osbun N, Thomas AG, Ronin M, Cooper M, Flechner SM, Segev DL, Veale JL. The benefit to waitlist patients in a national paired kidney exchange program: Exploring characteristics of chain end living donor transplants. Am J Transplant. 2022;22:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Weng FL, Grogan T, Patel AM, Mulgaonkar S, Morgievich MM. Characteristics of compatible pair participants in kidney paired donation at a single center. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Zhou S, Massie AB, Luo X, Ruck JM, Chow EKH, Bowring MG, Bae S, Segev DL, Gentry SE. Geographic disparity in kidney transplantation under KAS. Am J Transplant. 2018;18:1415-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Holscher CM, Jackson K, Chow EKH, Thomas AG, Haugen CE, DiBrito SR, Purcell C, Ronin M, Waterman AD, Garonzik Wang J, Massie AB, Gentry SE, Segev DL. Kidney exchange match rates in a large multicenter clearinghouse. Am J Transplant. 2018;18:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Lee YJ, Lee SU, Chung SY, Cho BH, Kwak JY, Kang CM, Park JT, Han DJ, Kim DJ. Clinical outcomes of multicenter domino kidney paired donation. Am J Transplant. 2009;9:2424-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Smeulders B, Mankowski MA, van de Klundert J. Kidney Exchange Program Reporting Standards: Evidence-Based Consensus From Europe. Front Public Health. 2021;9:623966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |