Published online May 16, 2025. doi: 10.12998/wjcc.v13.i14.102534
Revised: December 15, 2024
Accepted: January 3, 2025
Published online: May 16, 2025
Processing time: 86 Days and 2.9 Hours
At present, the conventional methods for diagnosing cerebral edema in clinical practice are computed tomography (CT) and magnetic resonance imaging (MRI), which can evaluate the location and degree of peripheral cerebral edema, but cannot realize quantification. When patients have symptoms of diffuse cerebral edema or high cranial pressure, CT or MRI often suggests that cerebral edema is lagging and cannot be dynamically monitored in real time. Intracranial pressure monitoring is the gold standard, but it is an invasive operation with high cost and complications. For clinical purposes, the ideal cerebral edema monitoring should be non-invasive, real-time, bedside, and continuous dynamic monitoring. The dis
To offer a promising new approach for non-invasive adjuvant therapy in cerebral edema treatment.
A total of 160 patients with hypertensive cerebral hemorrhage admitted to the Department of Neurosurgery, Second Affiliated Hospital of Xi’an Medical University from September 2018 to September 2019 were recruited. The patients were randomly divided into a control group (n = 80) and an experimental group (n = 80). Patients in the control group received conventional empirical treatment, while those in the experimental group were treated with mannitol dehydration under the guidance of DC. Subsequently, we compared the two groups with regards to the total dosage of mannitol, the total course of treatment, the incidence of complications, and prognosis.
The mean daily consumption of mannitol, the total course of treatment, and the mean hospitalization days were 362.7 ± 117.7 mL, 14.8 ± 5.2 days, and 29.4 ± 7.9 in the control group and 283.1 ± 93.6 mL, 11.8 ± 4.2 days, and 23.9 ± 8.3 in the experimental group (P < 0.05). In the control group, there were 20 patients with pulmonary infection (25%), 30 with electrolyte disturbance (37.5%), 20 with renal impairment (25%), and 16 with stress ulcer (20%). In the experimental group, pulmonary infection occurred in 18 patients (22.5%), electrolyte disturbance in 6 (7.5%), renal impairment in 2 (2.5%), and stress ulcers in 15 (18.8%) (P < 0.05). According to the Glasgow coma scale score 6 months after discharge, the prognosis of the control group was good in 20 patients (25%), fair in 26 (32.5%), and poor in 34 (42.5%); the prognosis of the experimental group was good in 32 (40%), fair in 36 (45%), and poor in 12 (15%) (P < 0.05).
Using DC for non-invasive dynamic monitoring of cerebral edema demonstrates considerable clinical potential. It reduces mannitol dosage, treatment duration, complication rates, and hospital stays, ultimately lowering hospitalization costs. Additionally, it improves overall patient prognosis, offering a promising new approach for non-invasive adjuvant therapy in cerebral edema treatment.
Core Tip: The disturbance coefficient for non-invasive dynamic monitoring of cerebral edema possesses certain guiding significance for the treatment of cerebral edema, which can not only reduce the total dosage of mannitol and the incidence of complications, but also shorten hospital stays, reduce the total cost of hospitalization, and improve the overall prognosis rate of patients. Therefore, it can be used as a new method of clinical non-invasive adjuvant therapy.
- Citation: Gao WW, Jiang XB, Chen P, Zhang L, Yang L, Yuan ZH, Wei Y, Li XQ, Tang XL, Wang FL, Wu H, Zhao HK. Role of disturbance coefficient in monitoring and treatment of cerebral edema in patients with cerebral hemorrhage. World J Clin Cases 2025; 13(14): 102534
- URL: https://www.wjgnet.com/2307-8960/full/v13/i14/102534.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i14.102534
Intracerebral hemorrhage (ICH) is one of the most common diseases in neurosurgery, with a fatality rate of 30%-40% in the acute phase[1]. Hypertensive ICH (HICH) is the most common hemorrhage, with an incidence of 20%-30% in north
This study involved human subjects and was reviewed and approved by the Ethics Review Committee of the Second Affiliated Hospital of Xi’an Medical University (approval No. 2020 LP01).
The inclusion criteria were as follows: (1) ≥ 18 years of age without gender limitation; (2) A history of hypertension; (3) CT/MRI confirmed a diagnosis of supratentorial acute hemorrhagic stroke; (4) The time from onset to enrolment was < 24 hours; and (5) Patients who signed an informed consent form.
The exclusion criteria were as follows: (1) After admission, the Glasgow coma scale (GCS) score was 3, with dilated and fixed pupils and no spontaneous breathing; (2) Pregnant and lactating patients; (3) Patients with severe heart, lung, liver, and kidney dysfunction; (4) Cerebral hemorrhage caused by brain trauma, aneurysm, vascular malformation, tumor, and coagulation dysfunction; and (5) Patients who refused to provide signed and informed consent.
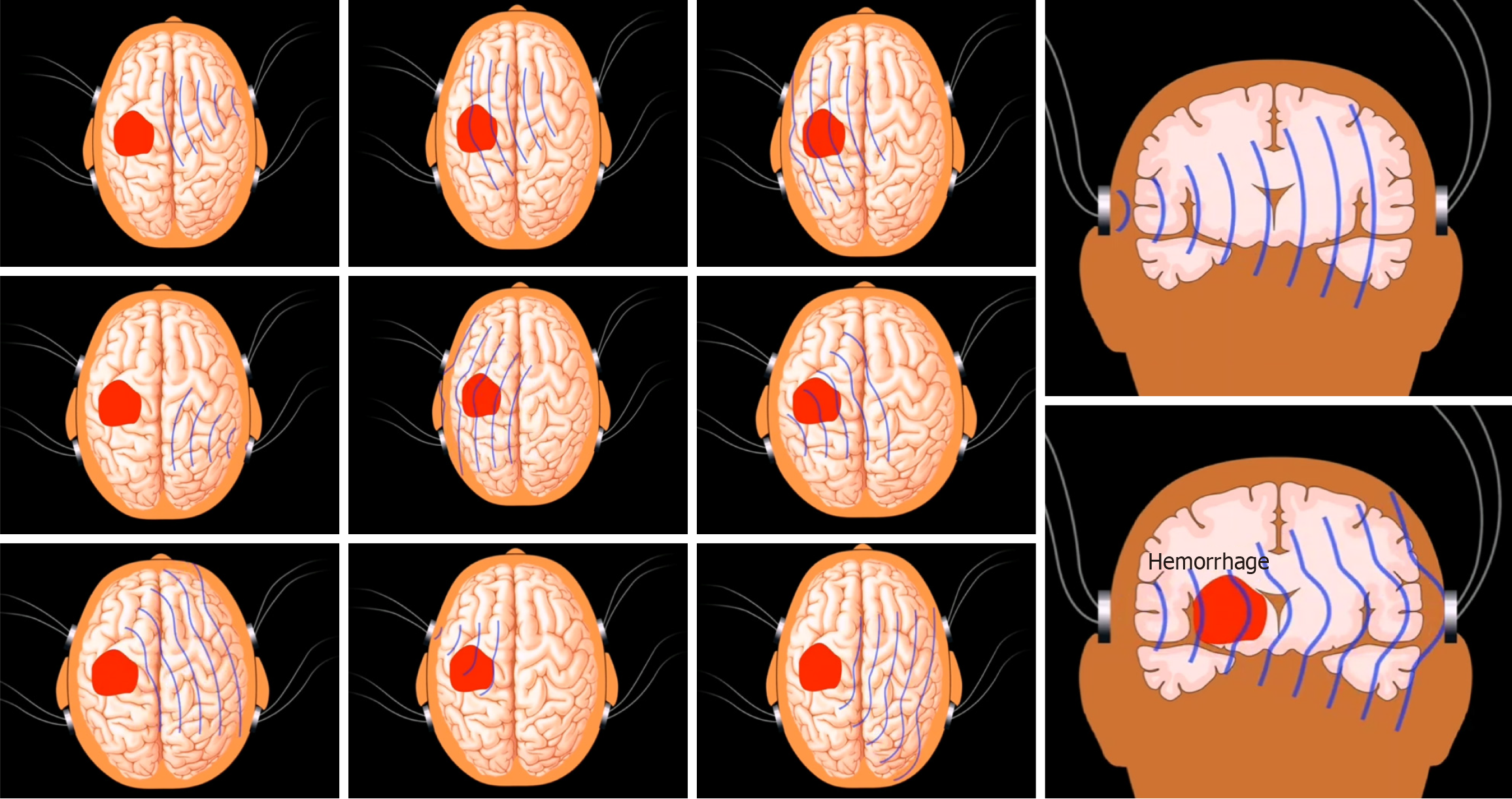
Preparation: We used two types of non-invasive brain edema dynamic monitoring system (Born-BE-III/IIIA and Born-BE-IV/IVA, Chongqing Bonenfuke Medical Equipment Co., Ltd). The systems were equipped with a portable non-interruptible power supply. As consumables, we used a disposable brain detection electrode and 75% alcohol. In addition, each patient underwent CT or MRI of the head.
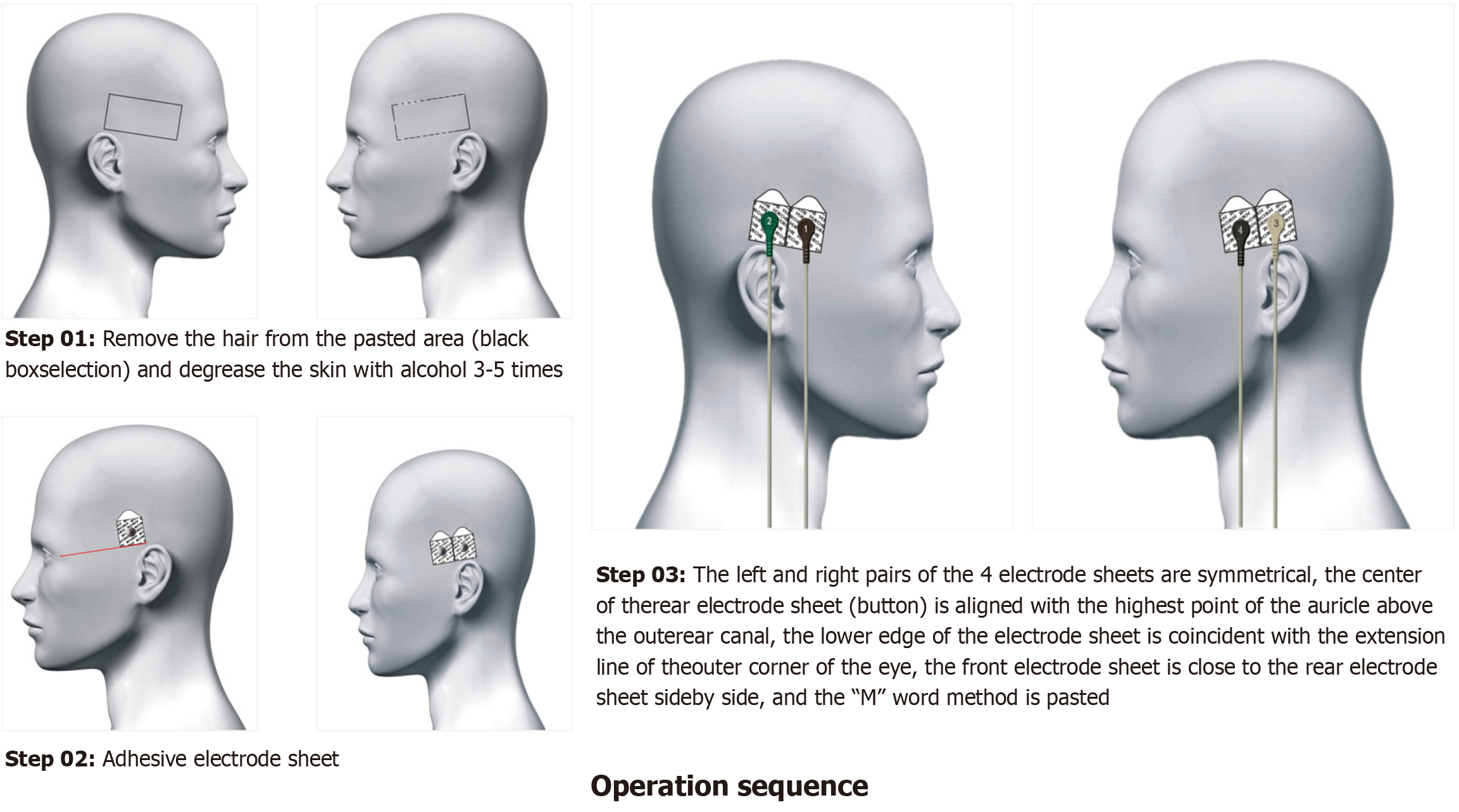
Operating sequence: First, it was necessary to ensure that power cables, lead cables, electrodes, clippers, razors, alcohol, and cotton swabs were ready. Then, we removed the hair from the pasted position and then degreased the skin with alcohol (3-5 times). Then, we connected the device with the lead wire, and fastened the electrode sheets in the following order: Brown, green, gray, and black. The left and right pairs of the four electrode sheets were symmetrical and the center of the rear electrode sheet (button) was aligned with the highest point of the auricle above the outer ear canal. The lower edge of the electrode sheet was coincident with the extension line of the outer corner of the eye. The front electrode sheet was positioned close to the rear electrode sheet side-by-side (Figure 2).
Patients in the control group received routine empirical treatment; those who met the surgical indications (China’s Guidelines for the Diagnosis and Treatment of Cerebral Hemorrhage in 2022) received surgical treatment. Cerebral CT scanning was executed according to the state of illness, and the amount of mannitol to cause dehydration was empirically and dynamically adjusted. Non-invasive dynamic monitoring of brain edema was performed in all groups, and a dynamic monitoring instrument for non-invasive dynamic brain edema monitoring (BORN-BE-III, Chongqing Bonenfuke Medical Equipment Co., Ltd) was used twice a day for 15 minutes each time. We observed the DC to dynamically adjust the amount of mannitol. Furthermore, cerebral CT scans were reviewed dynamically and surgical treatment was performed for those who reached the surgical indication, otherwise conservative treatment was performed. During the treatment of patients in both groups, we stopped mannitol treatment immediately if the levels of blood urea nitrogen and creatinine were elevated; in addition, we stopped furosemide and all nephrotoxic drugs. During the treatment of cerebral hemorrhage, we performed non-invasive dynamic monitoring for cerebral edema; this could accurately observe DC changes in a timely manner. This allowed us to evaluate the range of intracranial hematoma and edema, and more accurately administer mannitol during treatment.
When the DC ranged from 110 to 140, only conventional treatment was applied, such as oxygen inhalation and nu
When the DC ranged from 140 to 160, general treatment and a small dose of a dehydrating drug were used to dynamically observe changes. If the DC suddenly increased by 10 to 20 within more than 10 minutes, 125 mL of mannitol was rapidly administered to evaluate the patient’s vital signs, consciousness, and limb movement, and determine whether to review the cerebral CT scans. When the DC ranged from 160 to 180, patients with cerebral hemorrhage are in the peak period of brain tissue edema, intracranial pressure is very high, and large doses of dehydrating drugs are needed. At this time, the condition is critical, complex, and changeable, and there are risks of brain hernia leading to respiratory and circulation suspension at any time; clinical experience is essential to adapt appropriately. Even the administration of dehydrating agents may not be an effective control measure. It is important to review head CT scans and perform immediate surgery if there are indications for surgery.
When the DC ranges from 180 to 200, there is a considerable hematoma in the cerebral hemorrhage, and the hematoma will compress on the surrounding brain tissue, a condition which can readily form cerebral hernia. When CT examination shows indications for surgery, emergency surgical treatment should be performed to relieve the pressure created by the hematoma and reduce brain edema; at the same time, large doses of dehydrating drugs should be used to reduce intracranial pressure.
When the DC is > 200, there is a large amount of blood loss and the formation of cerebral hernia. Emergency surgical treatment should be performed in combination with large doses of dehydrating agents. Nevertheless, the effect of treatment is likely to be poor, and the prognosis is poor.
In the present study, all of the selected patients were diagnosed with cerebral hemorrhage, and DC measurements were predominantly high values, occasionally < 120, thus indicating the co-existence of hematoma and edema. Patients with surgical indications were referred for neurosurgery, while patients in the control group received conservative treatment.
Six months after discharge, patients were followed by telephone or by outpatient visit using the extended Glasgow outcome scale (GOS) (2010 version)[6]. According to the later survival status of patients, the prognosis of patients was divided into five levels. In this study, a GOS score of 5 was considered as good, while a 4 was fair, and a score of 1 to 3 was poor.
We recruited patients with hypertensive cerebral hemorrhage who were admitted to the Department of Neurosurgery of the Second Affiliated Hospital of Xi’an Medical University between September 2018 and September 2019. The age distribution ranged from 30 to 90 years and there was no gender limitation. A total of 160 patients were included in the study; these patients were divided into a control group and an experimental group using the random number table method. Of the 80 pateints in the control group, there were 51 males and 29 females, aged 30-87 years, with a mean age of 62 years. In the control group, 38 patients underwent surgical treatment, 42 underwent conservative treatment, 49 suffered a cerebral hemorrhage in the basal ganglia, 14 suffered from lobar hemorrhage, 10 suffered from ventricular hemorrhage, and 7 suffered from ICH in the external capsular area. Of the 80 patients in the experimental group, 47 were male and 33 were female, aged 35-90 years, with a mean age of 63 years. In the experimental group, 41 patients were treated by surgery, 39 underwent conservative treatment, 52 had cerebral hemorrhage in the basal ganglia region, 15 had lobar hemorrhage, 1 had cerebellar hemorrhage, 10 had ventricular hemorrhage, and 2 had external capsule hemorrhage.
There were no significant differences between the two groups in terms of mean age, sex ratio, admission GCS score, and hematoma volume (P > 0.05) (Table 1).
| Information | EG (n = 80) | CG (n = 80) | t/χ2 value | P value |
| Average age (years) | 63 ± 12.5 | 62 ± 12.1 | 0.032 | 0.974 |
| Sex ratio (male/female) | 47/33 | 51/29 | 0.421 | 0.516 |
| GCS score | 10.7 ± 3.4 | 10.5 ± 3.1 | 0.389 | 0.698 |
| Hematoma volume (mL) | 32.8 ± 25.8 | 33.5 ± 23.3 | −0.167 | 0.867 |
Our analysis showed that 95% of control subjects had DC values ranging from 115 to 155. Due to the presence of hematoma in patients with cerebral hemorrhage, most of the patients showed variable degrees of increase. Of the 80 patients in the experimental group, the mean DC on the first day of onset was 155.8 ± 24.3. The mean DC on the third day was 121.2 ± 24.2, while the mean DC on the seventh day after onset was 129.1 ± 23.6. The mean DC on the 14th day of onset was 139.0 ± 18.0. These results showed that three days after the onset of cerebral hemorrhage, the DC decreased due to the peak value of cerebral edema, and that the DC gradually returned to the normal range as the disease improved. Thus, DC can be used to evaluate the occurrence and development of HICH.
The mean daily consumption of mannitol in the control group was 362.7 ± 117.7 mL, compared to 283.1 ± 93.6 mL in the experimental group (t = 4.882; P = 0.000). The mean number of days for mannitol administration in the control group and experimental group was 14.8 ± 5.2 and 11.8 ± 4.2, respectively (t = 3.888; P = 0.000) (Table 2).
| Group | CG (n = 80) | EG (n = 80) | t value | P value |
| Average daily use of mannitol | 362.7 ± 117.7 | 283.1 ± 93.6 | 4.882 | 0.000 |
| Course | 14.8 ± 5.2 | 11.8 ± 4.2 | 3.888 | 0.000 |
Several complications were recorded in the experimental group, including pulmonary infection in 18 patients (22.5%), electrolyte disturbance in 6 (7.5%), renal impairment in 2 (2.5%), and stress ulcer in 15 (18.8%). The complications in the control group were as follows: Pulmonary infection in 20 patients (25%), electrolyte disturbance in 30 (37.5%), renal impairment in 20 (25%), and stress ulcers in 16 (20%). There was no significant difference in the incidence of pulmonary infection or stress ulcers between the two groups (P > 0.05), but there were significant differences in electrolyte disturbance and renal dysfunction between the two groups (P < 0.05). These data confirmed that in the treatment of hypertensive cerebral hemorrhage, the use of non-invasive dynamic monitoring of cerebral edema can more accurately guide the use of dehydrating drugs, reduce the occurrence of complications, shorten the length of hospital stay, and reduce the cost of hospitalization (Table 3).
| Group | Cases | Pulmonary infection | Electrolyte disturbance | Renal function damage | Stress ulcer |
| EG | 80 | 18 (22.5) | 6 (7.5) | 2 (2.5) | 15 (18.8) |
| CG | 80 | 20 (25) | 30 (37.5) | 20 (25) | 16 (20) |
| χ2 value | 0.138 | 20.645 | 17.075 | 0.040 | |
| P value | 0.710 | 0.000 | 0.000 | 0.841 |
The 80 patients in the experimental group were followed at 6 months after discharge. According to GOS scoring rules, 32 patients were classified as good (40%), 36 as fair (45%), and 12 as poor (15%), as shown in Table 4. The 80 patients in the control group were also followed at 6 months after discharge. According to GOS score rules, 20 patients were classified as good (25%), 26 as fair (32.5%), and 34 as poor (42.5%). Statistical analysis by the rank sum test (Z = -3.398; P = 0.001; P < 0.05) indicated that the prognosis of patients in the experimental group (the non-invasive cerebral edema monitoring group) was better than that of patients in the control group.
| Group | Cases | Good | Fair | Poor |
| EG | 80 | 32 (40) | 36 (45) | 12 (15) |
| CG | 80 | 20 (25) | 26 (32.5) | 34 (42.5) |
The mean number of hospitalization days in the control group and the experimental group was 29.4 ± 7.9 and 23.9 ± 8.3, respectively. After statistical analysis (t = 4.277; P = 0.000; P < 0.05), we identified a statistical difference in the number of hospitalization days between the two groups. Compared with the control group, the number of hospitalization days for the experimental group was significantly reduced.
Previous studies have shown that cerebral edema after cerebral hemorrhage is an important pathophysiological process leading to disease aggravation, and is one of the important reasons for the damage of brain tissue structure and nerve function[7,8]. In addition, the further development of cerebral edema will lead to a progressive increase in intracranial pressure, and the increase of intracranial pressure will lead to a decrease in blood flow to the brain, resulting in cerebral ischemia and hypoxia, further aggravating peripheral cerebral edema, forming a vicious cycle, and eventually leading to cerebral hernia or death[9]. Therefore, monitoring the development of cerebral edema after cerebral hemorrhage can indirectly reflect the changes in intracranial pressure and guide further treatment[10,11].
At the beginning of ICH, the DC is higher than normal due to the formation of hematoma. With disease development, the hematoma is gradually absorbed, and the cerebral edema around the hematoma area will gradually increase[12]. At this time, the hematoma and edema coexist, and the DC will be slightly lower than normal. With medical or surgical treatment, the hematoma can be further absorbed or removed by surgery, and the use of dehydrating drugs can reduce the degree of brain edema; therefore, the DC will fall back to normal range. Our current results show that DC can be used to assess the evolution of the disease in patients with cerebral hemorrhage in real time and guide treatment.
Mannitol is currently the most commonly used dehydrating agent in neurosurgery to reduce intracranial pressure and reduce the degree of brain edema; however, the long-term clinical use of this drug will lead to more adverse reactions[13]. Of all the adverse reactions, the most common are electrolyte disturbances. When treating serious patients, a large amount of mannitol needs to be injected, and the accumulation of a large amount of mannitol in the body will increase the osmotic pressure of the plasma, thus resulting in an increased blood volume in the body, which may lead to cardiac failure and hyperkalemia in severe cases, and even be life-threatening in severe conditions[14]. After a period of treatment, an increase in urine volume will lead to a rapid reduction in blood volume; this may exacerbate oliguria. At the same time, the rapid use of a large dose of mannitol will increase osmotic pressure in the renal tubules and then cause damage to the renal tubule epithelial cells. At this time, the pathological manifestations are excessive swelling of the renal tubular epithelial cells, thus forming vacuoles. The clinical manifestations of patients will be a significantly reduced urine volume, and even acute renal failure in severe conditions[15]. Our research demonstrated that the use of dehydrating drugs can be more accurately controlled when using non-invasive dynamic monitoring of cerebral edema, thus reducing the occurrence of adverse reactions and complications after cerebral hemorrhage, promoting a better recovery, and shortening the length of hospital stay. Therefore, during the course of HICH treatment, the use of non-invasive dynamic monitoring of cerebral edema can make the use of dehydrating drugs more reasonable and accurate.
In addition, our analyses indicated that in the experimental group, the use of non-invasive dynamic monitoring of brain edema could identify intracranial conditions in real time and in a quantitative manner, dynamically assess the severity of brain edema, and increase accuracy when administering dehydrating drug. In this study, in order to accurately use dehydrating drugs in clinical application, we only recorded the total amount of mannitol used and the duration of treatment as research indicators. From this study, we were able to conclude that the non-invasive dynamic monitoring of cerebral edema significantly reduced the mean dosage and duration of mannitol when compared with empirical medication. During the course of treatment, dehydrating drugs, such as glycerin fructose and furosemide, are also used for different patients, which may have a certain impact on the therapeutic effect. However, the main drug used in this study is mannitol, which may have caused a certain deviation in the results of our statistical analyses, although our findings suggest that this will not have a fundamental impact on our conclusions. In subsequent studies, we will more comprehensively analyze the clinical significance of non-invasive brain edema monitoring for brain edema. In this study, we also found that the damage caused to renal function was more obvious in the control group than in the experimental group, and there was no statistical significance in mean age between the two group. Moreover, the treatment plan applied for the two groups was similar, and drug treatment did not differ significantly. Non-invasive brain edema monitoring more accurately guided the clinical use of mannitol, significantly reducing its dosage, and therefore had a certain protective effect on the kidney function of patients. Finally, the GOS was used to evaluate the prognosis and recovery of patients[16]. Our results suggested that the prognosis of patients who received dynamic and non-invasive monitoring of cerebral edema was significantly better than that of the control group, thus confirming that the dynamic adjustment of the treatment plan and the use of dehydrating drugs according to changes in the DC during treatment could significantly improve the prognosis of patients and reduce the mortality and disability rate.
In summary, this study systematically investigated the application of non-invasive cerebral edema dynamic monitoring during the entire treatment process for patients with hypertensive cerebral hemorrhage. We conducted real-time monitoring of changes in patient condition, dynamically adjusted the use of dehydrating drugs according to the DC, and finally evaluated the therapeutic effect and prognosis. For HICH patients, continuous non-invasive dynamic monitoring of cerebral edema during hospitalization can evaluate the severity of brain injury after hemorrhage, guide the use of dehydration drugs, reduce complications, shorten the length of hospital stay, and reduce the disability rate and mortality rate, and it can serve as a new adjuvant tool for treatment.
The authors would like to thank all those who contributed to this study.
| 1. | Shao A, Zhu Z, Li L, Zhang S, Zhang J. Emerging therapeutic targets associated with the immune system in patients with intracerebral haemorrhage (ICH): From mechanisms to translation. EBioMedicine. 2019;45:615-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Jiang A, Wu W, Ma L, Yan M, Zhao Z, Chen Q. Effect of Electroacupuncture on the Treatment of Pneumonia in Patients with Hypertensive Intracerebral Hemorrhage. World Neurosurg. 2023;175:e1124-e1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Zhu ZY, Hao LF, Gao LC, Li XL, Zhao JY, Zhang T, Zhang GJ, You C, Wang XY. Determinants of acute and subacute case-fatality in elderly patients with hypertensive intracerebral hemorrhage. Heliyon. 2023;9:e20781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Wang H, Tian L, Yang H, Chen K. Use of Dyna-computed tomography-assisted neuroendoscopic hematoma evacuation in the treatment of hypertensive intracerebral hemorrhage. Neurosurg Rev. 2023;46:254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Zheng H, Chen C, Zhang J, Hu Z. Mechanism and Therapy of Brain Edema after Intracerebral Hemorrhage. Cerebrovasc Dis. 2016;42:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 6. | Lu J, Marmarou A, Lapane K, Turf E, Wilson L; IMPACT Group; American Brain Injury Consortium Study Participation Centers. A method for reducing misclassification in the extended Glasgow Outcome Score. J Neurotrauma. 2010;27:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Wan Y, Holste KG, Hua Y, Keep RF, Xi G. Brain edema formation and therapy after intracerebral hemorrhage. Neurobiol Dis. 2023;176:105948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 8. | Li Z, Li M, Shi SX, Yao N, Cheng X, Guo A, Zhu Z, Zhang X, Liu Q. Brain transforms natural killer cells that exacerbate brain edema after intracerebral hemorrhage. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 9. | Zhan Y, Zou X, Wu J, Fu L, Huang W, Lin J, Luo F, Wang W. Neuroendoscopy surgery for hypertensive intracerebral hemorrhage with concurrent brain herniation: a retrospective study of comparison with craniotomy. Front Neurol. 2023;14:1238283. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | de Oliveira Manoel AL. Surgery for spontaneous intracerebral hemorrhage. Crit Care. 2020;24:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 11. | Ziai WC, Thompson CB, Mayo S, McBee N, Freeman WD, Dlugash R, Ullman N, Hao Y, Lane K, Awad I, Hanley DF; Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III) Investigators. Intracranial Hypertension and Cerebral Perfusion Pressure Insults in Adult Hypertensive Intraventricular Hemorrhage: Occurrence and Associations With Outcome. Crit Care Med. 2019;47:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Giede-Jeppe A, Gerner ST, Sembill JA, Kuramatsu JB, Lang S, Luecking H, Staykov D, Huttner HB, Volbers B. Peak Edema Extension Distance: An Edema Measure Independent from Hematoma Volume Associated with Functional Outcome in Intracerebral Hemorrhage. Neurocrit Care. 2024;40:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Cook AM, Morgan Jones G, Hawryluk GWJ, Mailloux P, McLaughlin D, Papangelou A, Samuel S, Tokumaru S, Venkatasubramanian C, Zacko C, Zimmermann LL, Hirsch K, Shutter L. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocrit Care. 2020;32:647-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 14. | Li S, He X, Ruan L, Ye T, Wen Y, Song Z, Hu S, Chen Y, Peng B, Li S. Protective Effect of Mannitol on Cisplatin-Induced Nephrotoxicity: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:804685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Dai H, Ding F, Ma J, Zhao S. Risk factors for acute kidney injury after intracranial hemorrhage. Neuro Endocrinol Lett. 2022;43:257-264. [PubMed] |
| 16. | Holden DN, Mucksavage JJ, Cokley JA, Kim KS, Tucker NL, Esordi MS, Cook AM. Hypertonic saline use in neurocritical care for treating cerebral edema: A review of optimal formulation, dosing, safety, administration and storage. Am J Health Syst Pharm. 2023;80:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |