Published online May 6, 2025. doi: 10.12998/wjcc.v13.i13.100735
Revised: November 10, 2024
Accepted: December 9, 2024
Published online: May 6, 2025
Processing time: 139 Days and 21.8 Hours
The introduction of pegaspargase has greatly advanced the treatment of acute lymphoblastic leukemia (ALL). In the literature, only one case of pegaspargase-induced multiple organ failure has been reported, and the patient died due to multiple organ failure.
Herein, we present a rare case of a 40-year-old man with ALL who developed multiple organ failure after treatment with pegaspargase. The patient had two rare phenomena reflecting poor prognosis, including the discrepancy between clinical manifestations and liver function and persistently low alpha-fetoprotein (AFP) levels from subacute liver failure. However, the patient was successfully treated using a multidisciplinary team approach.
This is the first case report of successful treatment of pegaspargase-induced multiple organ failure. The findings emphasize the importance of a multidisciplinary team approach in treating pegaspargase-induced multiple organ failure.
Core Tip: We reported a rare patient with acute lymphoblastic leukemia, who suffered from multiple organ failure following the first application of pegaspargase. To date, only one patient has been reported and the patient died due to multiple organ failure in the reviewed word literature. In the article, although the patient had two rare phenomena reflecting poor prognosis, the patient was the first case of successful treatment. Two rare phenomena including inconformity between clinical manifestation and liver function, and persistent low AFP concentrations from subacute liver failure patients will be discussed in this patient.
- Citation: Bao SX, Yuan XL, Yan L, Xu J. Pegaspargase induced multiple organ failure with acute lymphoblastic leukemia: A case report. World J Clin Cases 2025; 13(13): 100735
- URL: https://www.wjgnet.com/2307-8960/full/v13/i13/100735.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i13.100735
With the introduction of pegaspargase, acute lymphoblastic leukemia (ALL) treatment has improved. However, pegaspargase may have adverse effects, including liver dysfunction, hepatic steatosis, hypersensitivity, pancreatitis, and thrombosis[1]. Unfortunately, effective treatments for pegaspargase-induced multiple organ failure are limited. To date, only one case of pegaspargase-induced multiple organ failure has been reported in the literature, and the patient died due to multiple organ failure[2]. Herein, we reported the first case of the successful treatment of multiple organ failure initially induced by pegaspargase, highlighting the importance of a multidisciplinary team approach.
A 40-year-old man with newly diagnosed ALL-L2 was admitted to our hospital. The patient received induction chemotherapy according to the ALL-consensus protocol. On November 6, phase I induction chemotherapy was completed with VDCLP (vindesine 4 mg d1, d8, d15, d22 + cyclophosphamide 1.5 d1, d15 + liposomal doxorubicin 40 mg d1, 20 mg d2 + mileson 60 mg d1-14, 40 mg d15-21, 20 mg d22-28 + pegaspargase 3750 IU d14). On December 4, cytarabine (50 mg) and methotrexate (10 mg) were administered.
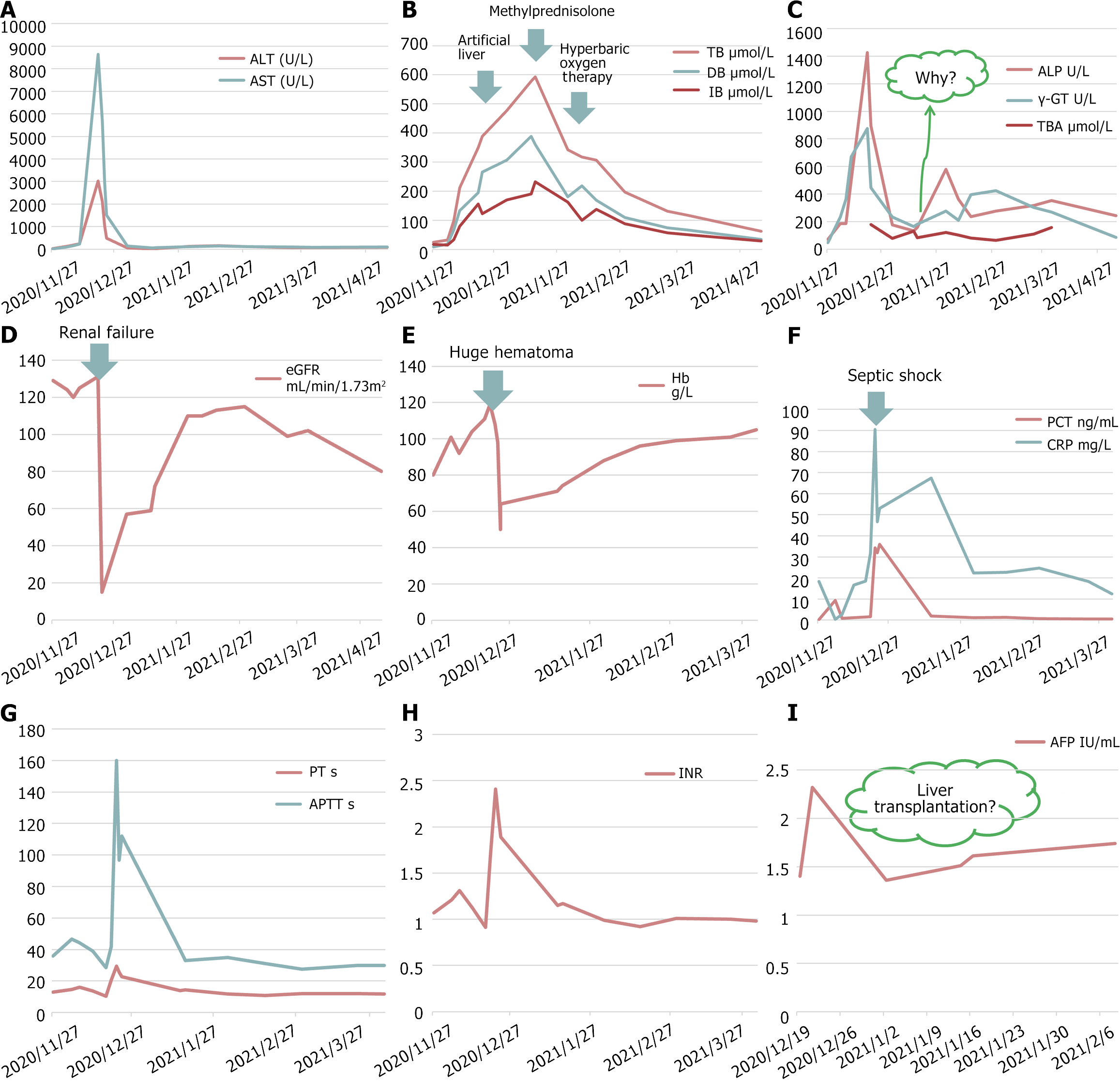
Twenty-seven days after receiving pegaspargase, the patient developed abnormal liver function, which progressively worsened. The patient was treated with hepatoprotective drugs, including compound glycyrrhizin and transmetil. On December 6, the patient experienced acute cholecystitis and was treated with ceftriaxone and levofloxacin. On December 18, a small, red, maculopapular rash appeared on the front chest, indicating chickenpox. On December 19, the patient was diagnosed with subacute liver failure characterized by critical hyperbilirubinemia and prolonged prothrombin time (Table 1 and Supplementary Table 1) and was treated with an artificial liver support system.
| Date | TB, (μmol/L) | DB (μmol/L) | TP (g/L) | Alb (g/L) | ALT (U/L) | AST (U/L) | ALP (U/L) | γ-GT (U/L) | TBA (μmol/L) | eGFR (mL/min/1.73m2) |
| 2020/10/31 | 8.4 | 1.8 | 71 | 42 | 117 | 73 | 83 | 56 | / | / |
| 2020/11/27 | 24.6 | 8.2 | 44 | 27 | 27 | 16 | 73 | 49 | / | 129 |
| 2020/12/4 | 31.7 | 17.4 | 52 | 29 | 121 | 89 | 187 | 235 | / | 124 |
| 2020/12/7 | 99.1 | 66.7 | 44 | 24 | 177 | 148 | 187 | 363 | / | 120 |
| 2020/12/10 | 212.2 | 133 | 44 | 25 | 235 | 225 | 499 | 669 | / | 125 |
| 2020/12/19 | 349.4 | 194.2 | 55 | 30 | 3018 | 8637 | 1425 | 876 | / | 131 |
| 2020/12/21 | 387.8 | 265.3 | 47 | 23 | 2115 | 5666 | 895 | 445 | 179.6 | 15 |
| 2021/1/2 | 476.5 | 306.7 | 41 | 23 | 51 | 144 | 177 | 235 | 79 | 57 |
| 2021/1/14 | 577.9 | 387.8 | 32 | 17 | 14 | 60 | 135 | 166 | 131.8 | 59 |
| 2021/1/16 | 593 | 360.7 | 54 | 34 | 23 | 65 | 158 | 181 | 83.7 | 72 |
| 2021/2/1 | 342.4 | 180.6 | 45 | 32 | 121 | 112 | 580 | 276 | 121.6 | 110 |
| 2021/2/8 | 317.7 | 218.3 | 53 | 38 | 162 | 150 | 363 | 210 | 102.3 | 110 |
| 2021/2/15 | 306 | 169 | 55 | 37 | 155 | 141 | 237 | 395 | 81.3 | 113 |
| 2021/3/1 | 196.9 | 109.1 | 47 | 32 | 111 | 108 | 276 | 424 | 64.9 | 115 |
| 2021/3/22 | 130.9 | 74.1 | 45 | 28 | 62 | 91 | 317 | 303 | 110.6 | 99 |
| 2021/4/1 | 116.4 | 66.2 | 47 | 29 | 61 | 86 | 354 | 271 | 157.5 | 102 |
| 2021/5/7 | 62.5 | 33.5 | 55 | 34 | 82 | 92 | 243 | 86 | / | 80 |
| 2021/6/24 | 45.3 | 21.8 | 61 | 34 | 50 | 30 | 194 | 73 | / | / |
On December 20, the patient experienced septic shock characterized by high fever (axillary temperature 39.2 °C), hypotension (blood pressure 80/40 mmHg), increased heart rate (124 beats/min), and elevated inflammation indicators (Supplementary Table 2). The patient was treated with imipenem/cilastatin and levofloxacin for bacterial infections, and caspofungin for fungal infection, noradrenaline for maintaining blood pressure, and immunoglobulin for anti-inflammatory treatment. On December 21, the patient experienced anuria, and renal function tests revealed renal failure (Table 1). Thus, the patient was managed with hemodialysis. On December 23, the patient developed a large hematoma at the root of the left thigh after arterial blood was drawn, and routine blood tests showed a significant decrease in hemoglobin (50 g/L). Hemostasis was achieved by compression and red blood cell transfusion.
A multidisciplinary team comprising infectious disease, nephrology, vascular surgery, blood transfusion, and hematology specialists were involved in patient management. The patient continued to have a high fever, and vancomycin was administered for anti-infection on December 27. Despite using the artificial liver support system, the total bilirubin level gradually increased, γ-glutamyl transpeptidase (γ-GT) and alkaline phosphatase (ALP) levels decreased, and coagulation function improved. Hyperbaric oxygen therapy and methylprednisolone (40 mg, qd, ivgtt) were administered, and the artificial liver support system was continued. Methylprednisolone was gradually reduced and was changed to oral methyldehydrocortisone. Subsequently, γ-GT and ALP levels increased, and cholestyramine was administered. Figure 1 shows the time course of laboratory parameters associated with multiple organ failure.
On January 15, 2021, the patient experienced symptoms such as cough and expectoration. Chest CT showed scattered oozing in both lungs (Supplementary Figure 1), sputum culture revealed Stenotrophomonas maltophilia infection, and levofloxacin was administered. The patient’s condition was stable, and he was discharged on April 1, 2021.
Oral hepatoprotective drugs, including transmetil and ursodeoxycholic acid, were continued after discharge. The patient fully recovered from the severe complications of pegaspargase. Bone marrow biopsy showed complete remission on May 7, 2021. On May 11, 2021, lumbar puncture and intrathecal chemotherapy (cytarabine 50 mg + methotrexate 10 mg + dexamethasone 5 mg) were administered. On June 9, 2021, cytarabine 50 mg, methamine pterin 10 mg, dexamethasone 5 mg, vindesine 4 mg d1, and milesone 60 mg d1-d7 were administered. Later, the patient’s condition was stable.
A 40-year-old man was diagnosed as ALL-L2 on Oct 2020. The patient received induction chemotherapy according to the ALL-consensus protocol. On November 6,2020, phase I induction chemotherapy was completed with VDCLP (vindesine 4 mg d1, d8, d15, d22 + cyclophosphamide 1.5 d1, d15 + liposomal doxorubicin 40 mg d1, 20 mg d2 + mileson 60 mg d1-14, 40 mg d15-21, 20 mg d22-28 + pegaspargase 3750 IU d14). On December 4, 2020, cytarabine (50 mg) and methotrexate (10 mg) were administered.
The patient had a history of hypertension for 6 years, with a maximum of 180/110 mmHg. The patient had been taking valsartan amlodipine once a day and carvedilol once a day orally for a long time, and the blood pressure can be well controlled.
There is no history of genetic diseases or tumors in the family
The patient was admitted with ALL without any obvious positive signs. Subsequently, with the use of chemotherapy drugs, he developed symptoms such as papules, chickenpox, yellow staining of the skin and sclera, wet rales on lung auscultation, edema, etc.
The laboratory examinations are shown in Table 1
The imaging examinations are shown in Supplementary Figure 1.
Multidisciplinary active treatment. Infection control from the infection department, hemodialysis from the nephrology department, hemostasis from vascular surgery department, ALL control from the hematology department, and adequate provision of red blood cells and plasma from the transfusion department.
Multiple organ failure; septic shock; severe anemia; ALL; primary hypertension (grade 3, very high risk).
Anti infection, liver protection, hemodialysis, artificial liver support system, blood transfusion, nutritional support and other treatments.
Later, the patient’s condition was stable.
Pegaspargase enhances the therapeutic effect of ALL treatment. Liver dysfunction, pancreatitis, hypersensitivity, or thrombosis were the important adverse effects of pegaspargase[1,2]. Unfortunately, few successful treatments for pegaspargase-induced multiple organ failure have been reported.
A previous study showed that pegaspargase induces rapid development of cholestasis and hepatic steatosis[3]. The vitamin B complex and levocarnitine regimen have been reported to be an effective and safe therapy for pegaspargase-induced hepatotoxicity[4]. Pegaspargase-induced multiple organ failure is highly rare, and its mortality rate is high. Plasmapheresis improved the outcome of severe complications after pegaspargase treatment in three patients with ALL[5]. In the literature, only one case of pegaspargase-induced multiple organ failure has been reported, and the patient died due to multiple organ failure. The possible mechanisms explaining the findings observed in this patient are impaired liver mitochondrial function and alterations in very-low-density lipoprotein metabolism and secretion[2].
In our case, pegaspargase-induced subacute liver failure, varicella, and acute cholecystitis caused septic shock, which induced multiple organ failure, including liver and kidney failure, coagulation dysfunction, and immune system failure. The survival rate of patients with more than four organ failures is very low. However, our patient was successfully treated using a multidisciplinary team approach, and recovery of multiple organs was achieved.
Two interesting phenomena that reflect the poor prognosis should be discussed. First, the patient had kaolin-like stools, the total bilirubin gradually increased, and the levels of γ-GT, ALP, and total bile acid (TBA) declined. This may be due to vanishing bile duct syndrome (VBDS), which is associated with adverse drug reactions, infections, neoplasms, ischemia, allograft rejection, and autoimmune disease. The prognosis of VBDS depends on the capacity for biliary regeneration and the etiological trigger of biliary epithelial apoptosis[6]. The diagnosis of VBDS is confirmed when less than 50% of the bile ducts are seen on liver biopsy[7]. In our case, the most likely causes of VBDS were adverse drug reactions (vinblastine) and infections (varicella and acute cholecystitis). Capillary cholangitis is another possible cause. The patient had acute cholecystitis and was most likely to develop a secondary bacterial infection based on cholestasis, causing capillary cholangitis. Pegaspargase-induced liver damage may also cause capillary cholangitis. Unfortunately, liver puncture was not performed due to the patient’s poor condition and high jaundice level. The patient was then treated with methylprednisolone and ursodeoxycholic acid, which is a choleretic agent that has antioxidant activity, enhances glutathione levels, and inhibits liver cell apoptosis. The jaundice level decreased, the γ-GT, ALP, and TBA levels increased, and cholestyramine was administered. Liver function gradually recovered after comprehensive treatment.
Second, alpha-fetoprotein (AFP) is considered a marker of hepatocyte regeneration, and the capacity for hepatocyte regeneration is vital for the reversal of liver injury. However, the patient’s AFP level remained normal during the progression or recovery period of liver failure. A previous study showed that stratified AFP can accurately predict mortality in patients with liver failure[8]. Rising AFP levels are associated with transplant-free survival in non-acetaminophen acute liver failure[9]. In patients with acute-on-chronic liver failure, a value of log10AFP ≥ 2.04 indicated a better prognosis with 62.5% sensitivity and 76.9% specificity, which could be a useful marker for predicting the outcomes of acute-on-chronic liver failure[10]. Usually, a persistently low AFP level is considered a regenerative failure in protracted cases, ultimately leading to death. Our patient who had persistently low AFP levels ultimately survived. One possible reason was that liver tissue repair occurred, followed by subsequent intensive care.
Liver failure is associated with persistently low AFP levels, VBDS, and capillary cholangitis. To the best of our knowledge, this is the first case report of successful treatment of multiple organ failure initially induced by pegaspargase. Unfortunately, liver puncture was not performed due to the patient’s poor condition.
This is the first case report of successful treatment of pegaspargase-induced multiple organ failure. The findings emphasize the importance of a multidisciplinary team approach in treating pegaspargase-induced multiple organ failure.
We would like to thank the patient that was involved in this study.
| 1. | Cooper SL, Young DJ, Bowen CJ, Arwood NM, Poggi SG, Brown PA. Universal premedication and therapeutic drug monitoring for asparaginase-based therapy prevents infusion-associated acute adverse events and drug substitutions. Pediatr Blood Cancer. 2019;66:e27797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Bodmer M, Sulz M, Stadlmann S, Droll A, Terracciano L, Krähenbühl S. Fatal liver failure in an adult patient with acute lymphoblastic leukemia following treatment with L-asparaginase. Digestion. 2006;74:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Kamal N, Koh C, Samala N, Fontana RJ, Stolz A, Durazo F, Hayashi PH, Phillips E, Wang T, Hoofnagle JH; Drug-Induced Liver Injury Network. Asparaginase-induced hepatotoxicity: rapid development of cholestasis and hepatic steatosis. Hepatol Int. 2019;13:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Lu G, Karur V, Herrington JD, Walker MG. Successful treatment of pegaspargase-induced acute hepatotoxicity with vitamin B complex and L-carnitine. Proc (Bayl Univ Med Cent). 2016;29:46-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Göpel W, Schnetzke U, Hochhaus A, Scholl S. Functional acute liver failure after treatment with pegylated asparaginase in a patient with acute lymphoblastic leukemia: potential impact of plasmapheresis. Ann Hematol. 2016;95:1899-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Lv T, Yu H, Han X, Wee A, Liu J, Li M, Xu J, Hu X, Li J, Duan W, Wang T, Jia J, Zhao X. Histopathological Features Predicting Long-term Clinical Outcomes in Patients with Vanishing Bile Duct Syndrome. J Clin Transl Hepatol. 2023;11:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Huang GQ, Xie YY, Zhu PW, Wang XD, Lin Z, Wang Y, Ye JP, Wang YM, Chen YX, Jin XZ, Van Poucke S, Chen YP, Zheng MH. Stratified alpha-fetoprotein pattern accurately predicts mortality in patients with acute-on-chronic hepatitis B liver failure. Expert Rev Gastroenterol Hepatol. 2018;12:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Singh S, Hynan LS, Rule JA, Lee WM. Changes in alpha-foetoprotein and Gc-globulin in relation to outcomes in non-acetaminophen acute liver failure. Liver Int. 2019;39:2368-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Wang X, Shen C, Yang J, Yang X, Qin S, Zeng H, Wu X, Tang S, Zeng W. Alpha-Fetoprotein as a Predictive Marker for Patients with Hepatitis B-Related Acute-on-Chronic Liver Failure. Can J Gastroenterol Hepatol. 2018;2018:1232785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Bilgir O, Calan M, Bilgir F, Cagliyan G, Arslan O. An experience with plasma exchange treatment of acute lymphoblastic leukemia in a case with fulminant hepatitis related to L-asparaginase. Transfus Apher Sci. 2013;49:328-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |