Published online Jan 6, 2025. doi: 10.12998/wjcc.v13.i1.99746
Revised: October 3, 2024
Accepted: October 24, 2024
Published online: January 6, 2025
Processing time: 100 Days and 18.6 Hours
Ependymoma with lipomatous differentiation is a rare type of ependymoma. The ZFTA fusion-positive supratentorial ependymoma is a novel tumor type in the 2021 World Health Organization classification of central nervous system tumors. ZFTA fusion-positive lipomatous ependymoma has not been reported to date.
We reported a case of a 15-year-old Chinese male who had a sudden convulsion lasting approximately six minutes. Magnetic resonance imaging showed a round cystic shadow of approximately 1.9 cm × 1.5 cm × 1.9 cm under the right parieto-occipital cortex. Microscopic examination showed characteristic perivascular pseudorosettes and adipose differentiation in the cytoplasm. Immunohistochemical staining showed that the tumor cells were negative for cytokeratin, NeuN, Syn and p53, but positive for GFAP, vimentin and S-100 protein. Signi
Based on these findings, the patient was diagnosed as a ependymoma with ZFTA fusion and lipomatous differentiation. This case report provides information on the microscopic morphological features of ependymoma with ZFTA fusion and lipomatous differentiation, which can help pathologists to make a definitive diagnosis of this tumor.
Core Tip: Ependymoma with lipomatous differentiation is a rare type of ependymoma. The ZFTA fusion-positive supratentorial ependymoma is a novel tumor type in the 2021 World Health Organization classification of central nervous system tumors. ZFTA fusion-positive lipomatous ependymoma has not been reported to date. This case report provides information on the microscopic morphological features of ependymoma with ZFTA fusion and lipomatous differentiation, and highlights the possibility that ZFTA fusion and lipomatous differentiation may co-occur, adding a new layer to the molecular classification of ependymomas.
- Citation: Zhao XY, Yu JH, Wang YH, Liu YX, Xu L, Fu L, Yi N. Lipomatous ependymoma with ZFTA: RELA fusion-positive: A case report. World J Clin Cases 2025; 13(1): 99746
- URL: https://www.wjgnet.com/2307-8960/full/v13/i1/99746.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i1.99746
Ependymoma with lipomatous differentiation is a rare type of ependymoma, characterized by well-defined lipomatous differentiation, whose biological behavior is generally considered to be World Health Organization (WHO) grade 2[1-5]. ZFTA (formerly known as C11orf95) fusion-positive supratentorial ependymoma is a new tumor type listed in the 2021 WHO classification of central nervous system (CNS) tumors[6-8]. To the best of our knowledge, there are no reports of lipomatous ependymoma with ZFTA fusion-positive. After obtaining informed consent from the patient and the legal guardian, we discussed one such rare case and reviewed the existing literature to provide further insights into the clinicopathological nature of this rare tumor.
The patient is a 15-year-old Chinese male who presented with a sudden convulsion lasting approximately six minutes without an apparent cause.
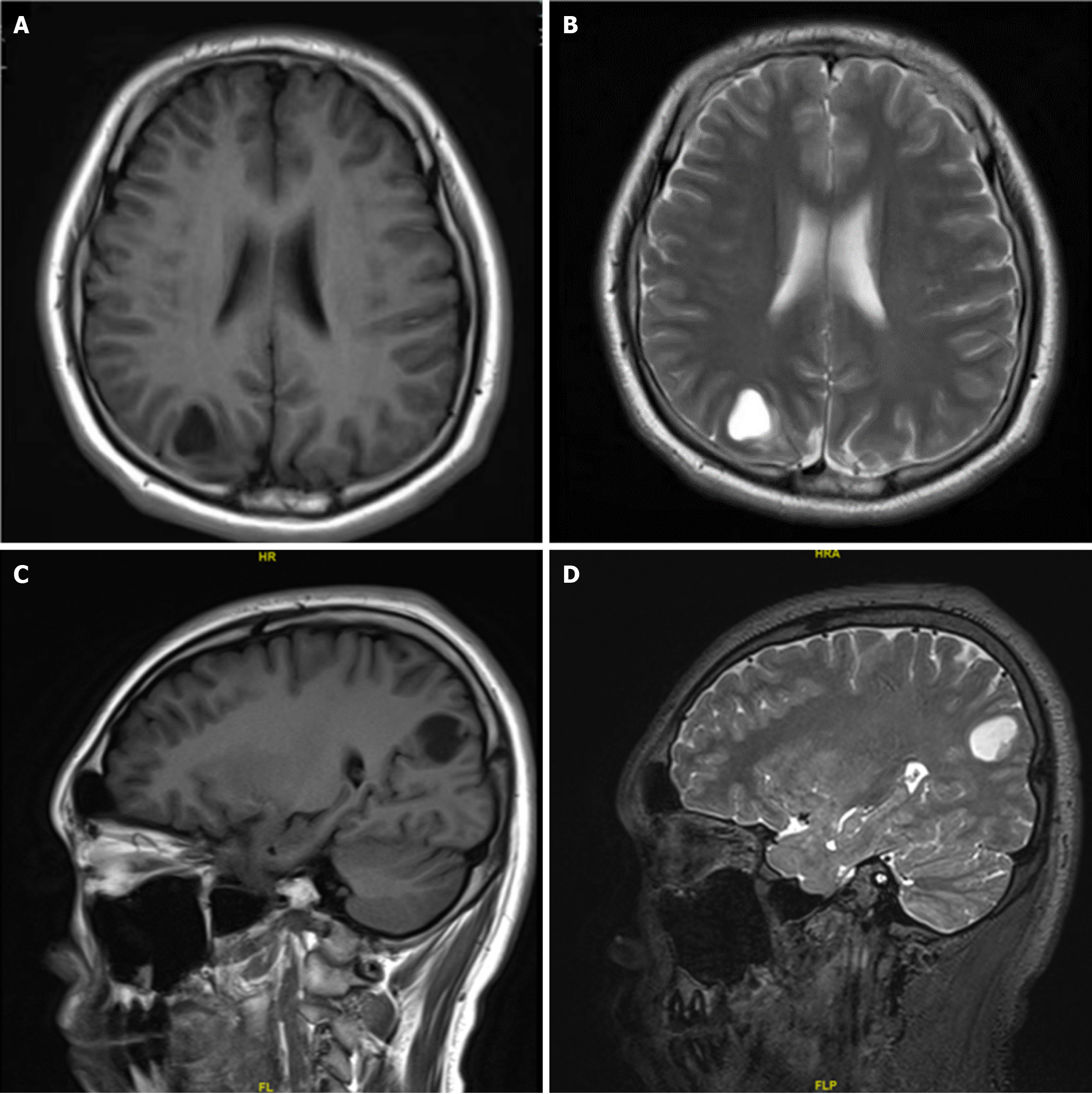
A 15-year-old boy was admitted to the Department of Neurosurgery at our hospital who presented with a sudden convulsion lasting approximately six minutes without an apparent cause. Magnetic resonance imaging (MRI) showed a round cystic shadow of approximately 1.9 cm × 1.5 cm × 1.9 cm under the right parieto-occipital cortex, with a regular envelope, clear and smooth margins, and slightly compressed surrounding brain tissue.
The patient had been previously healthy.
No obvious abnormalities were found in the personal and family history.
The patient’s physical examination reveals no abnormalities.
The resected specimens were fixed with 10% neutral-buffered formalin and embedded in paraffin blocks. Then tissue blocks were cut into 4 μm sections, deparaffinized in xylene, rehydrated with graded alcohols, and immunostained with the following antibodies: Cytokeratin, GFAP, S-100 protein, vimentin, synaptophysin (Syn), oligodendrocyte trans
MRI showed a round cystic shadow of approximately 1.9 cm × 1.5 cm × 1.9 cm under the right parieto-occipital cortex, with a regular envelope, clear and smooth margins, and slightly compressed surrounding brain tissue (Figure 1).
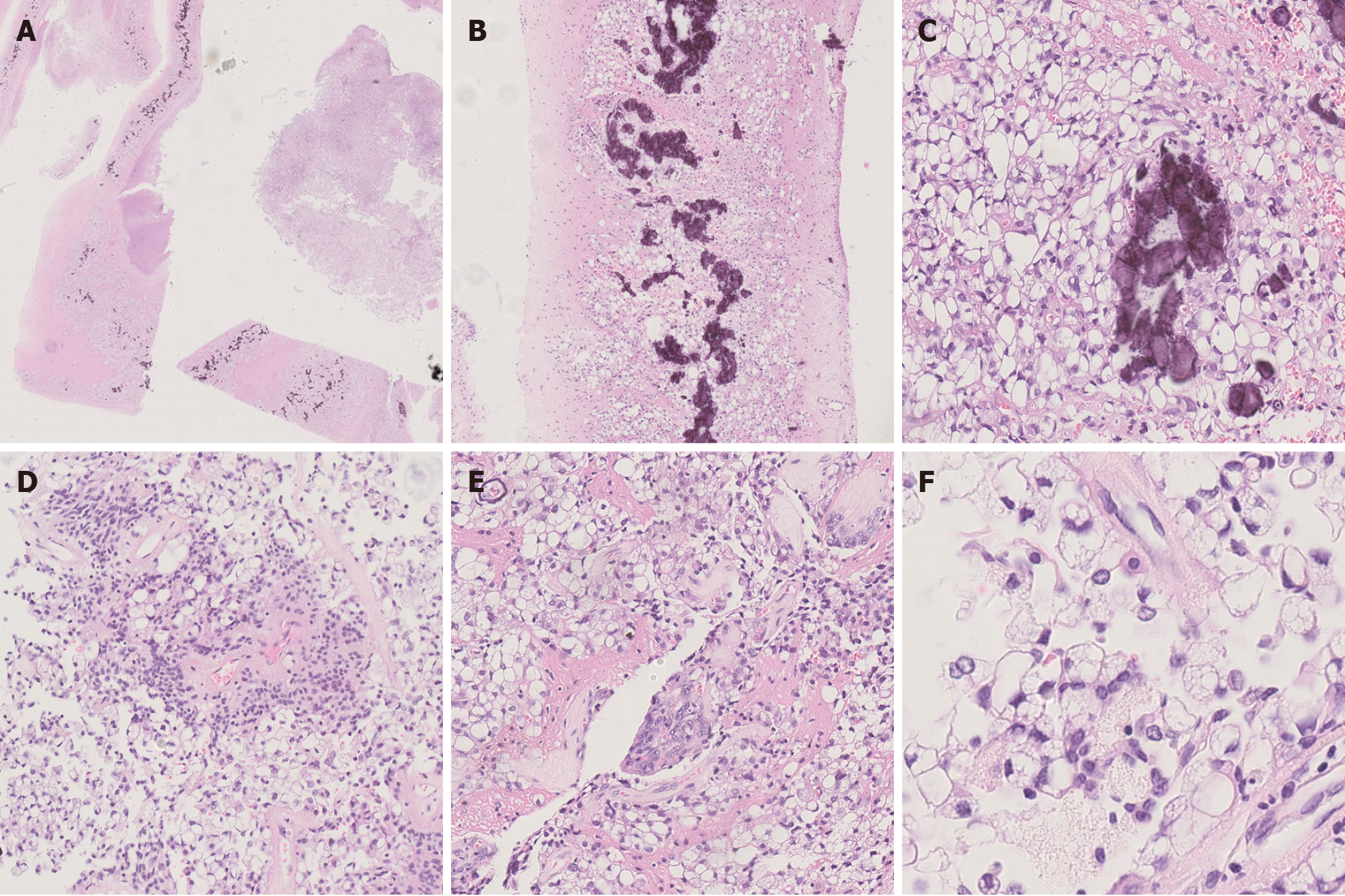
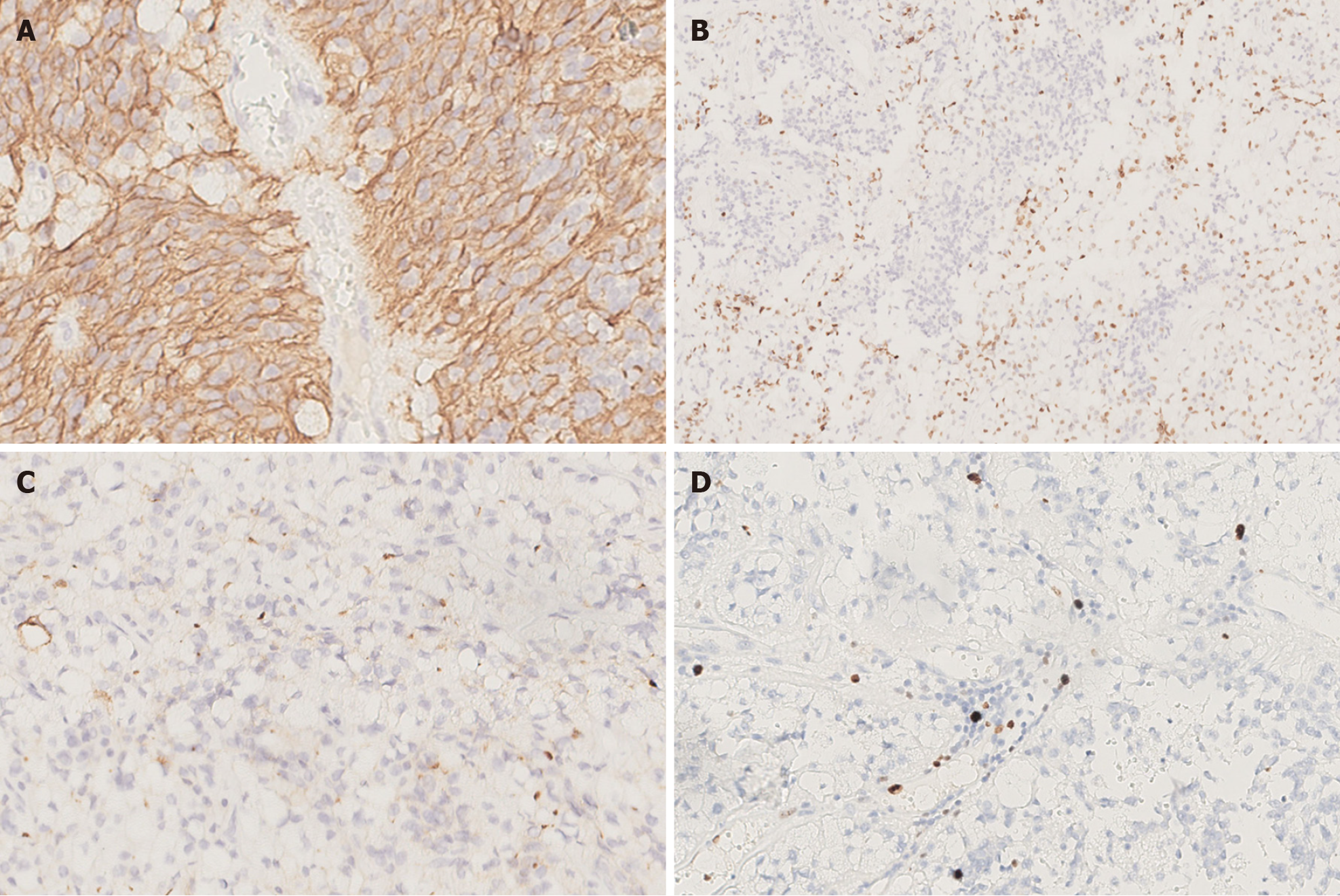
Microscopic examination showed that the tissue boundary in the cystic wall-like structure was clear, and density of tumor cells in the cyst cavity was moderate, with no obvious necrosis. Calcifications were seen on the cyst wall, and large vacuoles were seen in the cytoplasm of tumor cells on the cyst wall. Characteristic perivascular pseudorosettes were seen. We also observed localized vascular endothelial cell proliferation, resembling a glomerular structure. The tumors were irregularly arranged and primarily sheet-like, and the cytoplasm of most tumor cells contained large vacuoles of different sizes, pushing the crescentic nucleus along the cell membrane to the periphery, similar to signet ring cells. The mitosis was rare (Figure 2). Immunohistochemical staining showed that the tumor cells were negative for cytokeratin, NeuN, Syn and p53, but positive for GFAP, vimentin and S-100 protein. The tumor cells were negative and individual cells were positive for Olig-2. Moreover, significant punctate intracytoplasmic EMA immunoreactivity was observed. The level of Ki-67 immunoreactivity was about 5% (Figure 3).
Total DNA was extracted from formalin-fixed, paraffin-embedded sections. The targets of examined genes or chromo
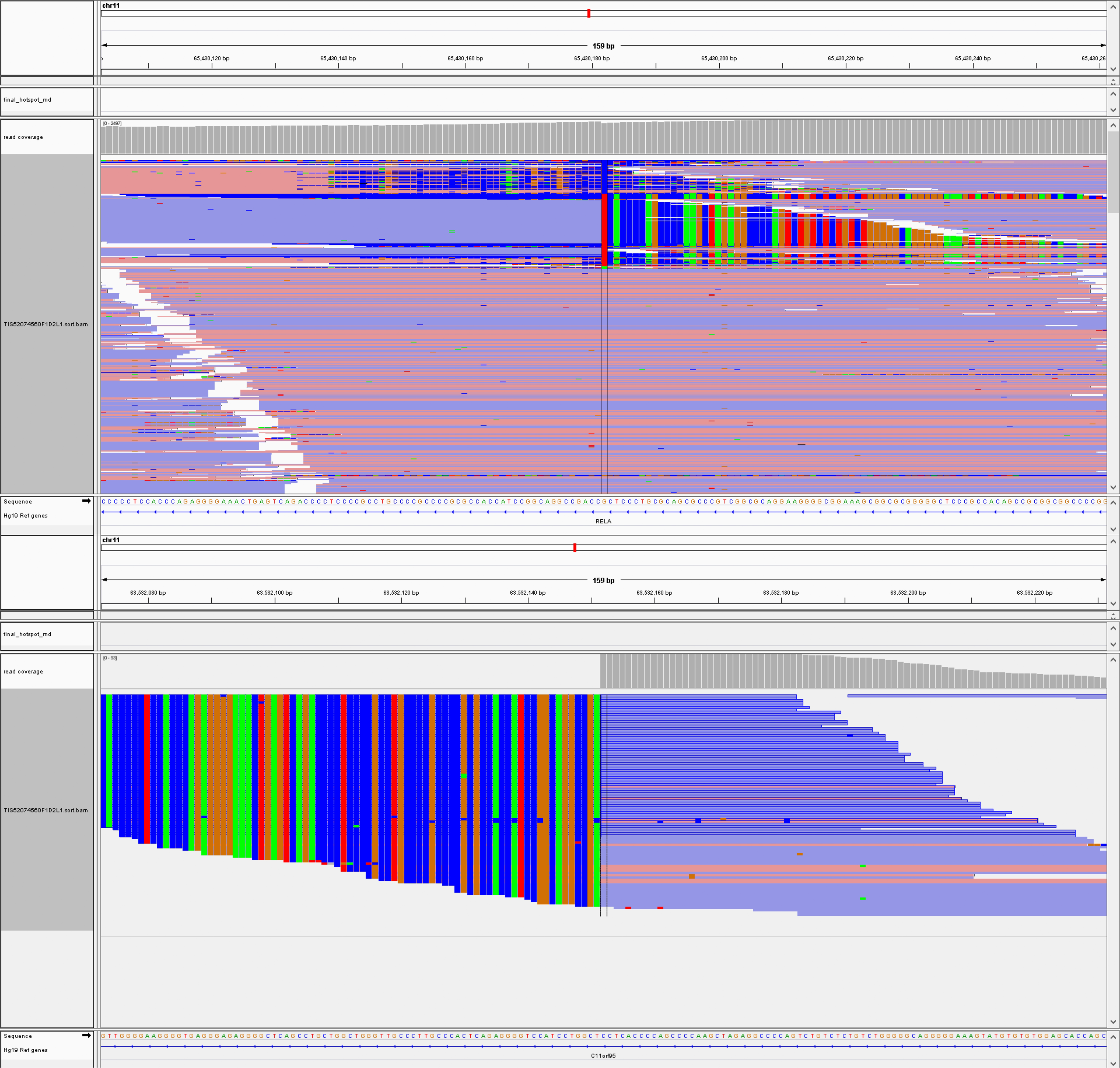
Genetic analysis revealed ZFTA: RELA fusion (Figure 4), while no homozygous deletion of CDKN2A and/or CDKN2B, mutations in IDH1 or IDH2, TERT promoter mutations, KIAA1549-BRAF fusion or deletion of 1p/19q were found.
Based on these findings, the patient was diagnosed as WHO grade 2 ependymoma with ZFTA fusion and lipomatous differentiation.
A craniotomy with total excision of the tumor was performed.
The follow-up time was 36 months, no evidence of disease recurrence was found in MRI.
Ependymoma is a relatively rare CNS tumor that originates in the spina ventricles or central canal as a glioma composed of tumorigenic ventricular meningeal cells that can occur at any age, but is more common in children. Ependymomas account for 2% of all CNS tumors and 3%-5% of gliomas in adults[9]. Among them, ependymoma with lipomatous differentiation is a more rare histological type seen in young patients, mainly in the supratentorial (ST) location. Ruchoux et al[5] in 1998 first described three cases of classical ependymoma with lipomatous differentiation. Histologically, scattered single or patchy mature adipose-like cells were seen. The vacuoles within the tumor cells varied in size but were all single. The nucleus was pushed to the periphery by a large single cytoplasmic vesicle, which formed a ring-like pattern.
The accompanying lipomatous changes may be due to differentiation or disturbance of cellular metabolism rather than as a result of metaplasia[2]. The vacuoles in ependymoma were thought to be microrosettes and degenerated and swollen cytoplasmic processes of tumor cells[3]. The diagnosis of ependymoma with lipomatous differentiation requires differentiation from other intracranial tumors with lipomatous differentiation. In primary CNS tumors, tumor cell adipocyte differentiation morphology is mainly seen in meningiomas, medulloblastomas and neurocytomas[10-12]. Lipomatous meningioma microscopic presentation typically shows a meningioma-like region and adipocyte-like cell mixture. It can be identified by immunomarkers such as GFAP and somatostatin receptor 2 (SSTR2). The latter two types of tumors generally have neuronal differentiation and are therefore easier to distinguish by histopathological observation and immunomarkers.
According to research statistics, half of the lipomatous ependymomas reported to date have recurred, the follow-up duration is 8 months to 4 years, and most cases were histological grade 3[1]. The current case showed no necrosis and the Ki-67 index was about 3%, so this case was classified as WHO grade 2. To date, only 30 cases of lipomatous ependy
Before 2015, the classification of ependymal tumor was mainly based on histopathological features, which was classified as WHO grade 1-3. However, the correlation between this grade and prognosis is unclear, and it cannot effectively judge and predict the malignancy of tumors and the survival of patients[13]. Moreover, ependymal tumors show significant heterogeneity in their histopathological and molecular characteristics, so it is difficult to obtain a definitive diagnosis primarily based on morphology. Histopathological variants described separately in the 2016 WHO classification of CNS tumors are no longer classified as subtypes of ependymoma because it has been recognized that tanycytic, papillary, or clear cell morphology has no clinical value by itself[14].
Recent large-scale genome sequencing studies of ependymal tumor identified nine molecular subsets associated with different genomic alterations, clinical behavior, age distribution, and anatomical locations[15]. The 2021 WHO classification of the CNS is a new classification system for ependymal tumors based on the anatomical location and molecular characteristics of CNS tumors[6-8]. Based on the results obtained by genetic and epigenetic profiling studies, there are at least nine molecular groups of ependymal tumors in three CNS locations[16]. ST ependymoma has two high-frequency fusion genes, ZFTA: RELA fusion and YAP1: MAMLD1 fusion. Parker et al[17] found that ZFTA: RELA fusion of the 11q chromosome was the most recurrent genetic alteration in ependymoma, affecting more than 70% of ST tumors and predominantly occurring in older patients, which may be caused by chromothripsis. ZFTA: RELA fusion is capable of cellular transformation and tumor initiation when expressed in the developing mouse brain or when neural stem cells are expressed after transplantation[17]. RELA is a well-known major transcription factor in the NF-κB pathway, which is intimately associated with various pathophysiological processes such as inflammation and cancer[18]. While the normal molecular function of the ZFTA protein is currently unknown, studies have shown that the zinc finger domain of this gene plays an important role in the pathogenesis of tumors that may lead to new DNA binding and transcriptional regulation[17]. These findings suggest that the ZFTA: RELA fusion protein and its direct target gene may be candidates for therapy and may provide clues for future treatments.
Studies have found that ST ependymoma with ZFTA: RELA fusion-positive has a poor prognosis, and the survival rate of patients is remarkably lower than that of ST ependymoma with YAP1 fusion-positive[8,15]. Recent retrospective studies suggested that progression-free survival in ependymal tumor with non-RELA: ZFTA fusion-positive might be worse than in ependymal tumor with ZFTA: RELA fusion-positive[19]. Additionally, homozygous deletion of CDKN2A on 9p21.3 is a powerful independent indicator of unfavorable prognosis[20]. In our patient, no homozygous deletion of CDKN2A was detected and no recurrence or metastasis was found for 36 months after surgery. The prognosis of ependymoma with ZFTA: RELA fusion-positive remains unknown.
In this paper, we reported the first case of lipomatous ependymoma with ZFTA fusion-positive. Since both lipomatous ependymoma and ependymoma with ZFTA fusion-positive are rare, more case reports and long-term follow-up studies are needed on the prognostic impact of adipose differentiation and ZFTA fusion-positive and whether they are linked.
| 1. | Gaur K, Batra VV, Gupta R, Sharma MC, Narang P, Pandey PN. Lipomatous ependymoma: report of a rare differentiation pattern with a comprehensive review of literature. Brain Tumor Pathol. 2016;33:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Sharma MC, Arora R, Lakhtakia R, Mahapatra AK, Sarkar C. Ependymoma with extensive lipidization mimicking adipose tissue: a report of five cases. Pathol Oncol Res. 2000;6:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Hirato J, Nakazato Y, Iijima M, Yokoo H, Sasaki A, Yokota M, Ono N, Hirato M, Inoue H. An unusual variant of ependymoma with extensive tumor cell vacuolization. Acta Neuropathol. 1997;93:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Salazar MF, Tena-Suck ML, Ortiz-Plata A, Salinas-Lara C, Rembao-Bojórquez D. Lipomatous/Extensively Vacuolated Ependymoma with Signet-Ring Cell-Like Appearance: Analysis of a Case with Extensive Literature Review. Case Rep Pathol. 2017;2017:8617050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Ruchoux MM, Kepes JJ, Dhellemmes P, Hamon M, Maurage CA, Lecomte M, Gall CM, Chilton J. Lipomatous differentiation in ependymomas: a report of three cases and comparison with similar changes reported in other central nervous system neoplasms of neuroectodermal origin. Am J Surg Pathol. 1998;22:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4523] [Cited by in RCA: 6445] [Article Influence: 1611.3] [Reference Citation Analysis (1)] |
| 7. | Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. 2022;128:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 184] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 8. | Kresbach C, Neyazi S, Schüller U. Updates in the classification of ependymal neoplasms: The 2021 WHO Classification and beyond. Brain Pathol. 2022;32:e13068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Lavrador JP, Oliveira E, Teixeira JC, Lopes JP, Pimentel J, Carvalho MH. Adult Supratentorial Extraventricular Anaplastic Ependymoma: Therapeutic Approach and Clinical Review. Asian J Neurosurg. 2018;13:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Radwan W, Lucke-Wold B, Cheyuo C, Ahn J, Gyure K, Bhatia S. Lipomatous meningioma: Case report and review of the literature. Case Stud Surg. 2016;2:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Soylemezoglu F, Soffer D, Onol B, Schwechheimer K, Kleihues P. Lipomatous medulloblastoma in adults. A distinct clinicopathological entity. Am J Surg Pathol. 1996;20:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Gupta K, Salunke P, Kalra I, Vasishta RK. Central liponeurocytoma: case report and review of literature. Clin Neuropathol. 2011;30:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Ellison DW, Kocak M, Figarella-Branger D, Felice G, Catherine G, Pietsch T, Frappaz D, Massimino M, Grill J, Boyett JM, Grundy RG. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 10839] [Article Influence: 1204.3] [Reference Citation Analysis (0)] |
| 15. | Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, Wani K, Tatevossian R, Punchihewa C, Johann P, Reimand J, Warnatz HJ, Ryzhova M, Mack S, Ramaswamy V, Capper D, Schweizer L, Sieber L, Wittmann A, Huang Z, van Sluis P, Volckmann R, Koster J, Versteeg R, Fults D, Toledano H, Avigad S, Hoffman LM, Donson AM, Foreman N, Hewer E, Zitterbart K, Gilbert M, Armstrong TS, Gupta N, Allen JC, Karajannis MA, Zagzag D, Hasselblatt M, Kulozik AE, Witt O, Collins VP, von Hoff K, Rutkowski S, Pietsch T, Bader G, Yaspo ML, von Deimling A, Lichter P, Taylor MD, Gilbertson R, Ellison DW, Aldape K, Korshunov A, Kool M, Pfister SM. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell. 2015;27:728-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 841] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 16. | Ellison DW, Aldape KD, Capper D, Fouladi M, Gilbert MR, Gilbertson RJ, Hawkins C, Merchant TE, Pajtler K, Venneti S, Louis DN. cIMPACT-NOW update 7: advancing the molecular classification of ependymal tumors. Brain Pathol. 2020;30:863-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 17. | Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Y, Lee R, Tatevossian RG, Phoenix TN, Thiruvenkatam R, White E, Tang B, Orisme W, Gupta K, Rusch M, Chen X, Li Y, Nagahawhatte P, Hedlund E, Finkelstein D, Wu G, Shurtleff S, Easton J, Boggs K, Yergeau D, Vadodaria B, Mulder HL, Becksfort J, Gupta P, Huether R, Ma J, Song G, Gajjar A, Merchant T, Boop F, Smith AA, Ding L, Lu C, Ochoa K, Zhao D, Fulton RS, Fulton LL, Mardis ER, Wilson RK, Downing JR, Green DR, Zhang J, Ellison DW, Gilbertson RJ. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature. 2014;506:451-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 494] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 18. | DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1228] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 19. | Tauziède-Espariat A, Siegfried A, Nicaise Y, Kergrohen T, Sievers P, Vasiljevic A, Roux A, Dezamis E, Benevello C, Machet MC, Michalak S, Puiseux C, Llamas-Gutierrez F, Leblond P, Bourdeaut F, Grill J, Dufour C, Guerrini-Rousseau L, Abbou S, Dangouloff-Ros V, Boddaert N, Saffroy R, Hasty L, Wahler E, Pagès M, Andreiuolo F, Lechapt E, Chrétien F, Blauwblomme T, Beccaria K, Pallud J, Puget S, Uro-Coste E, Varlet P; RENOCLIP-LOC, the BIOMECA (Biomarkers for Ependymomas in Children, Adolescents) consortium. Supratentorial non-RELA, ZFTA-fused ependymomas: a comprehensive phenotype genotype correlation highlighting the number of zinc fingers in ZFTA-NCOA1/2 fusions. Acta Neuropathol Commun. 2021;9:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Korshunov A, Witt H, Hielscher T, Benner A, Remke M, Ryzhova M, Milde T, Bender S, Wittmann A, Schöttler A, Kulozik AE, Witt O, von Deimling A, Lichter P, Pfister S. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol. 2010;28:3182-3190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |