Published online Mar 26, 2024. doi: 10.12998/wjcc.v12.i9.1578
Peer-review started: November 2, 2023
First decision: January 9, 2024
Revised: January 22, 2024
Accepted: February 25, 2024
Article in press: February 25, 2024
Published online: March 26, 2024
Processing time: 144 Days and 0.3 Hours
Frey syndrome, also known as ototemporal nerve syndrome or gustatory sw
To investigate the preventive effect of acellular dermal matrix (ADM) on Frey syndrome after parotid tumor resection and analyzed the effects of Frey syn
Retrospective data from 82 patients were analyzed to assess the correlation bet
Among the 82 patients, the incidence of Frey syndrome was 56.1%. There were no significant differences in sex, age, or operation time between the two groups (P > 0.05). However, there was a significant difference between ADM implantation and occurrence of Frey syndrome (P < 0.05). ADM application could reduce the va
ADM can effectively prevent Frey syndrome and delay its onset.
Core Tip: The use of acellular dermal matrix in parotid tumor surgery can reduce the incidence of Frey syndrome, especially when the diameter of the surgically removed parotid tissue is greater than 4 cm.
- Citation: Chai XD, Jiang H, Tang LL, Zhang J, Yue LF. Factors influencing Frey syndrome after parotidectomy with acellular dermal matrix. World J Clin Cases 2024; 12(9): 1578-1584
- URL: https://www.wjgnet.com/2307-8960/full/v12/i9/1578.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i9.1578
Frey syndrome, also known as ototemporal nerve syndrome or gustatory sweating syndrome, is one of the most common complications of parotid gland surgery. This condition is characterized by abnormal sensations in the facial skin accom
In this retrospective analysis of clinical data from 126 patients who underwent parotid gland surgery in the Department of Oral and Maxillofacial Surgery at Anshun People's Hospital between January 2018 and December 2020, a total of 82 patients were deemed eligible for the study. Patients with parotid gland inflammation, patients who had undergone lymph node dissection, and patients who had undergone periparotid gland surgery during the same period were ex
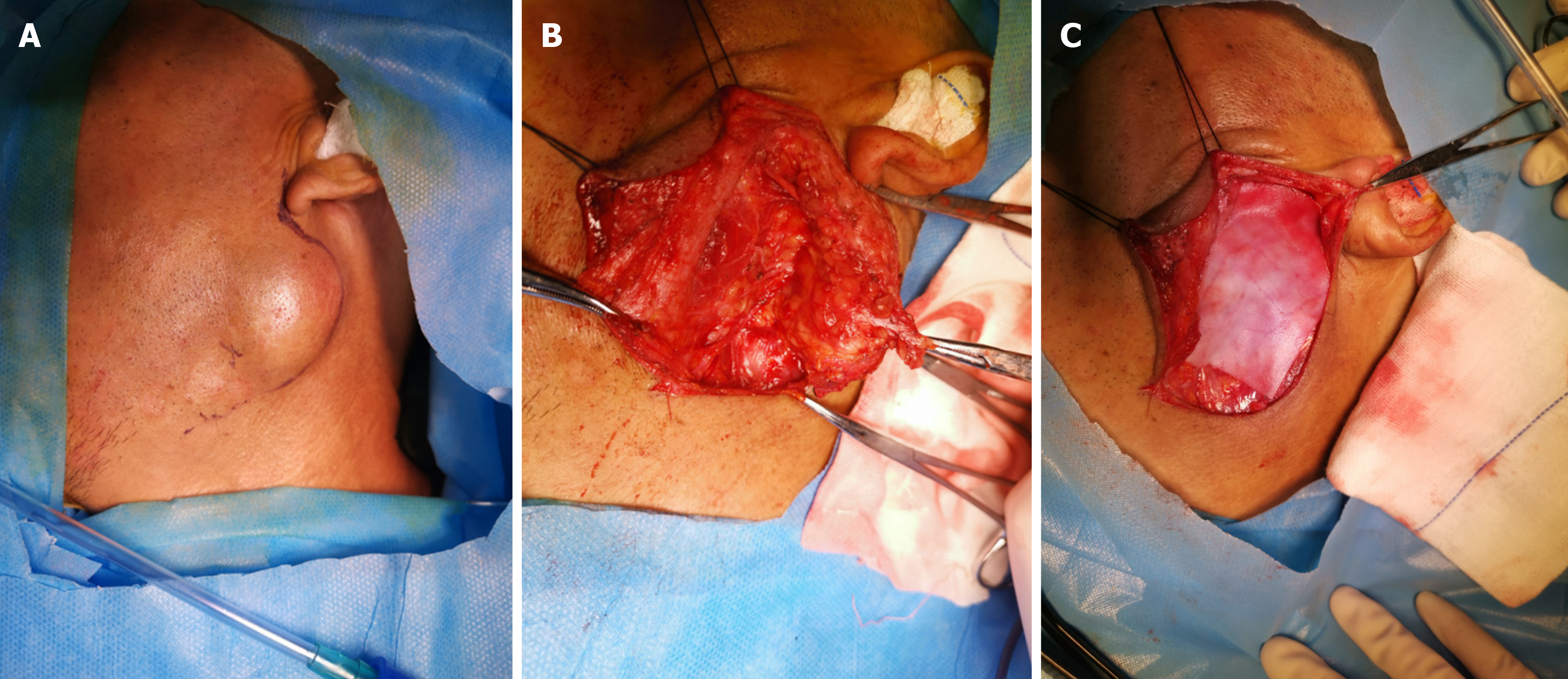
A modified "S" incision[9] was made in the conventional parotid area. The platysma muscle under the skin was incised, the parotid gland envelope was opened, and the envelope was preserved for tumors that did not invade it. The facial nerve was dissected retrogradely, and any tumor-invading segments of the facial nerve were resected. Partial parotid resection or total parotid lobectomy was performed, depending on the size and location of the tumor. The surgical method randomly categorized the patients into either the partial parotid resection group or the total parotid lobectomy group. The patients were randomly divided into a tissue patch implantation group and a control group according to their preoperative informed consent and willingness to undergo tissue patch implantation. After surgery, all patients un
Patients were contacted by phone each month after surgery, during which they were questioned regarding symptoms, such as facial flushing, facial paresthesia, and facial sweating during eating. These responses were used to assess Frey syndrome using a subjective questionnaire. Positive Frey syndrome was defined as the presence of any of the four indi
Data analysis was performed using SPSS (version 25.0) to assess the correlations between age, sex, surgical method, size of surgically removed samples, time of occurrence of postoperative Frey syndrome, and intraoperative application of ADM for the prevention of Frey syndrome. A logistic regression model was established to analyze the risk factors associated with Frey syndrome. Additionally, a receiver operating characteristic (ROC) curve was constructed to predict the diagnostic value of certain risk factors for Frey syndrome. The significance level for the tests was set at α = 0.05.
A total of 82 patients were included in this study, 46 of whom developed Frey syndrome, an incidence rate of 56.1%. Among them, 43 (52.4%) experienced facial paresthesia after eating, 29 (35.4%) experienced facial flushing after eating, 13 (15.9%) exhibited facial sweating after eating, and 4 (4.9%) reported that these symptoms seriously affected their daily lives (Table 1).
| Symptoms | Number and proportion of cases, n = 82 |
| Facial paresthesia after eating | 43 (52.4) |
| Flushed cheeks after eating | 29 (35.4) |
| Facial sweating after eating | 13 (15.9) |
| Feeling that life is severely affected after surgery | 4 (4.9) |
The 82 patients were categorized into the Frey and non-Frey groups (Table 2). No significant differences were observed in terms of sex, age, or operation time between the two groups. However, a significant difference was noted in the occu
| Clinical features | Frey group (n = 46) | Non-Frey group (n = 36) | P value |
| Sex (male) | 26 (56.52%) | 20 (55.56%) | 0.93 |
| Age (yr) | 48.09 ± 14.95 | 47.08 ± 16.31 | 0.97 |
| Surgically removed sample size (maximum diameter, cm) | 3.40 ± 0.97 | 2.878 ± 0.79 | 0.0291 |
| Method of surgery | |||
| ADM implants | |||
| Partial removal of parotid gland | 8 | 9 | 0.295 |
| Complete removal of parotid gland | 3 | 8 | |
| No-implant ADM | |||
| Partial removal of parotid gland | 21 | 18 | 0.0061 |
| Complete removal of parotid gland | 14 | 1 | |
| Procedure time (min) | 198.54 ± 43.87 | 185.97 ± 57.75 | 0.92 |
| Implanted ADM | |||
| Yes | 11 | 17 | 0.0271 |
| No | 35 | 19 | |
| Time of Frey sign occurrence (months) | |||
| Implant ADM | 7.54 ± 3.2 | 0 | 0.0011 |
| Non-implant ADM | 3.43 ± 2.33 | 0 |
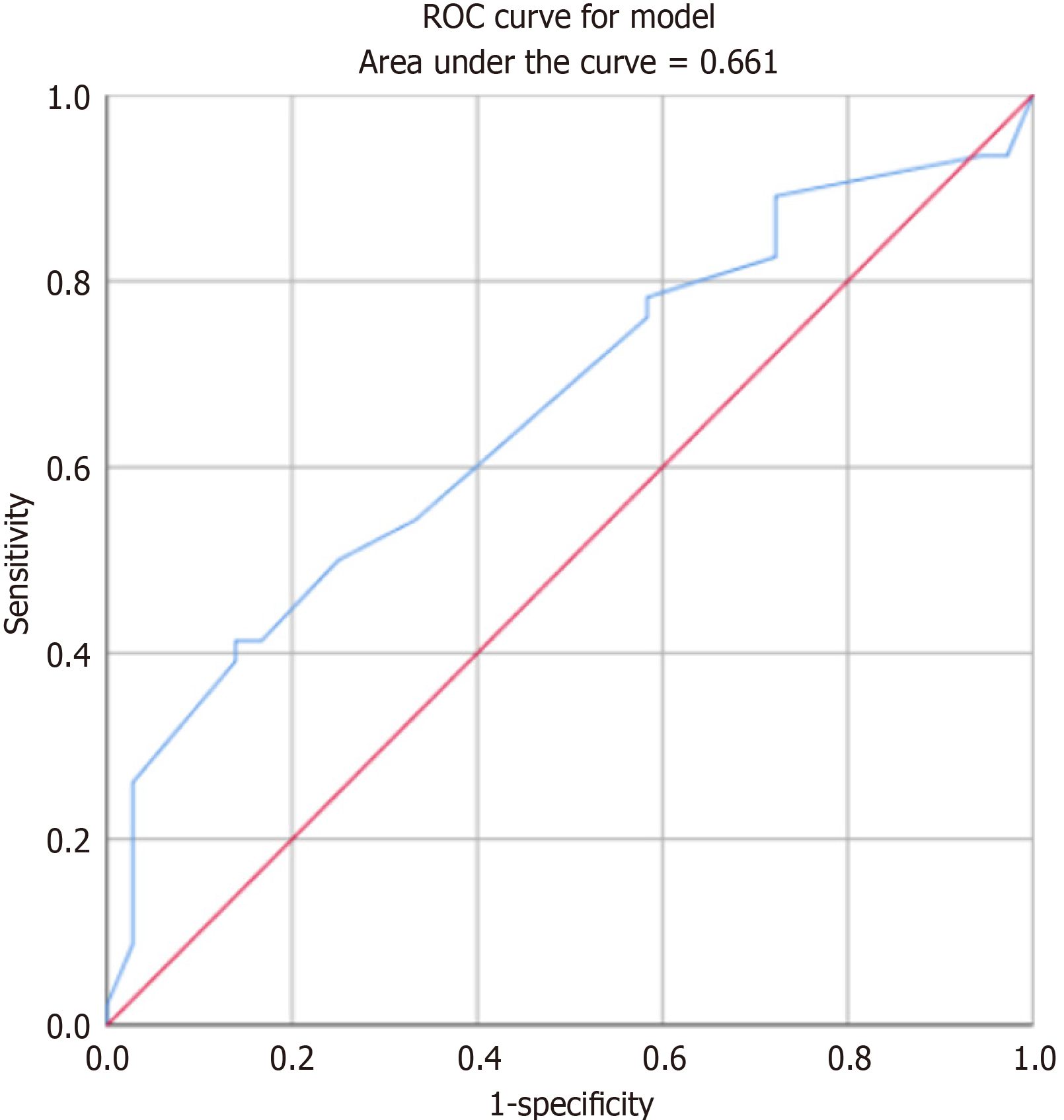
ROC curve analysis was performed to assess the relationship between the maximum diameter of the surgically re
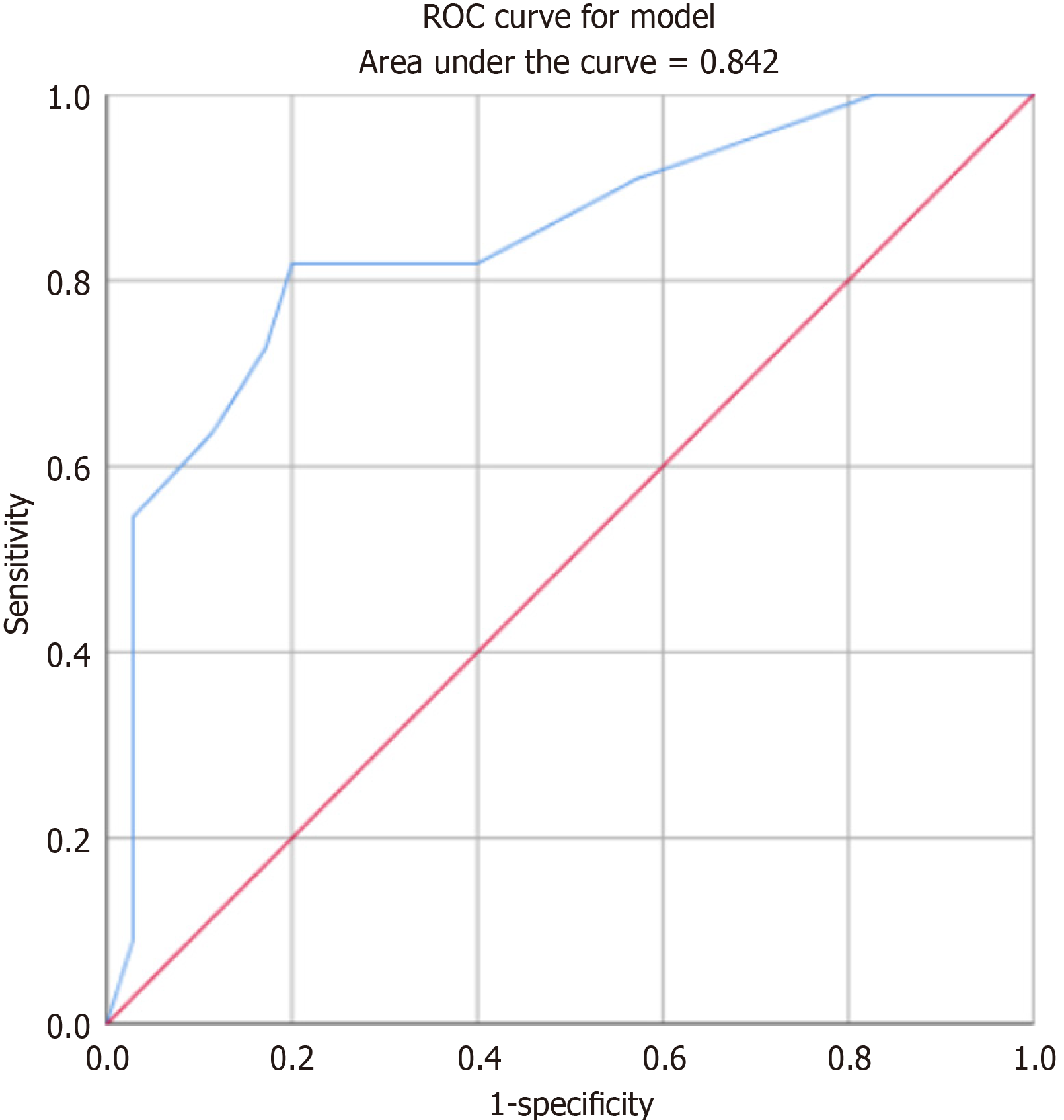
ROC curve analysis comparing the timing of ADM implantation with the timing of the occurrence of symptoms of Frey syndrome revealed that ADM could significantly delay the occurrence of Frey syndrome, with an AUC of 0.842 (Figure 3).
Frey syndrome is now commonly believed to be most likely caused by parotid gland surgery or injury. Destruction of parotid gland cyst integrity exposes the parasympathetic branch, which controls the parotid gland acinar secretion in the auriculotemporal nerve issued by the trigeminal nerve within the parotid gland and leads to its misplacement with the sympathetic nerve, which controls the skin sweat glands. Consequently, upon seeing or eating food, an individual’s parasympathetic branch is stimulated, resulting in secretion from skin sweat glands, leading to facial paresthesia, flushing, or sweating[10]. Frey syndrome occurred in 56.1% of the patients in this study, a rate similar to that reported in most studies[11]. ROC analysis of the tumor sample diameter demonstrated that a resected sample with a larger area was correlated with a higher probability of Frey syndrome occurrence (AUC = 0.661). This finding was consistent with the results presented by Lin et al[12]. Therefore, it was concluded that the probability of Frey syndrome increased when the resected tumor diameter exceeded 4 cm[12,13]. ADM can effectively prevent the occurrence of Frey syndrome after resection. Favorable outcomes have been achieved using sternocleidomastoid flap[14] and superficial muscle aponeurotic system flap[15]. For the prevention of Frey syndrome. However, it is important to note that the flap preparation process inevitably prolongs the operation time. In this study, there was no significant difference in operation time between the ADM and non-ADM groups, indicating that this method did not extend the operation time in the context of Frey syndrome prevention. Significant differences in the incidence of Frey syndrome were evident among different surgical methods without ADM. Total parotid excision was more likely to result in Frey syndrome, likely because of the removal of excessive parotid tissue that could potentially damage more ototemporal nerve endings[16]. This can contribute to more dislocation-related complications during nerve injury reconstruction. The use of ADM reduced the variability in the occurrence of Frey syndrome between surgical methods. According to the currently available parotid surgery guidelines[17] for benign tumors, preference is given to extracapsular resection or endoscopic minimally invasive surgery[18,19] to reduce the risk of Frey syndrome. However, in cases of tumors > 4 cm in diameter or located deeper within the parotid gland, total parotid excision combined with ADM[20] to decrease postoperative recurrence and prevent Frey syndrome is recommended.
Regarding the timing of Frey syndrome occurrence, non-implantation of ADM resulted in Frey syndrome occurring approximately 3 months after the operation, consistent with the neural reconstruction theory that the occurrence time for the middle auriculotemporal nerve is abnormal[16]. After ADM implantation, the onset of Frey syndrome was delayed by approximately three months. This delay might be attributed to the severity of the auriculotemporal nerve injury and the absorption timeframe of the ADM.
In conclusion, the application of ADM affects Frey syndrome prevention. However, it is important to note that ADM degrades within approximately six months, and Frey syndrome may still occur after this degradation. Nonetheless, because of the limited number of Frey syndrome cases following ADM implantation in this study, the results may be biased. Additionally, controlling the diameter of the excised samples can help prevent the occurrence of Frey syndrome.
Frey syndrome, also known as ototemporal nerve syndrome or guest-sweating syndrome, is one of the most common complications of parotid gland surgery. It is characterized by abnormal facial skin sensations, flushing, or sweating when the patient thinks, sees, or eats.
This inclusion has inadvertently increased the recorded incidence of Frey syndrome. This retrospective study investigated the factors influencing the acellular dermal matrix (ADM) in the prevention of Frey syndrome in patients who have undergone parotid surgery.
Because of the effects of frey syndrome, there was a need to find a way to reduce its incidence
The data of 82 patients were retrospectively analyzed using SPSS 25.0, and the correlations between sex, age, resection sample size, operation time, operation mode, ADM use, and postoperative Frey syndrome were analyzed.
The incidence of Frey syndrome was 56.1% among the 82 patients. There were no significant differences in sex, age, or operation time between the two groups (P > 0.05). There was a significant difference between ADM implantation and the onset of symptoms of Frey syndrome (P < 0.05). ADM can reduce the variation in Frey syndrome onset. ADM can delay the onset of Frey signs.
the application of ADM affects Frey syndrome prevention. However, it is important to note that ADM degrades within approximately six months, and Frey syndrome may still occur after this degradation. Additionally, controlling the diameter of the excised samples can help prevent the occurrence of Frey syndrome.
The incidence of Frey syndrome was reduced by surgery and the implantation of ADM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kochurova EV, Russia S-Editor: Zhang H L-Editor: A P-Editor: Xu ZH
| 1. | Mantelakis A, Lafford G, Lee CW, Spencer H, Deval JL, Joshi A. Frey's Syndrome: A Review of Aetiology and Treatment. Cureus. 2021;13:e20107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Guntinas-Lichius O, Gabriel B, Klussmann JP. Risk of facial palsy and severe Frey's syndrome after conservative parotidectomy for benign disease: analysis of 610 operations. Acta Otolaryngol. 2006;126:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Linder TE, Huber A, Schmid S. Frey's syndrome after parotidectomy: a retrospective and prospective analysis. Laryngoscope. 1997;107:1496-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Choi HG, Kwon SY, Won JY, Yoo SW, Lee MG, Kim SW, Park B. Comparisons of Three Indicators for Frey's Syndrome: Subjective Symptoms, Minor's Starch Iodine Test, and Infrared Thermography. Clin Exp Otorhinolaryngol. 2013;6:249-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Lee YC, Liao WC, Yang SW, Luo CM, Tsai YT, Tsai MS, Lee YH, Hsin LJ. Systematic review and meta-analysis of modified facelift incision versus modified Blair incision in parotidectomy. Sci Rep. 2021;11:24106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tuncel A, Karaman M, Sheidaei S, Tatlıpınar A, Esen E. A comparison of incidence of Frey's syndrome diagnosed based on clinical signs and Minor's test after parotis surgery. Kulak Burun Bogaz Ihtis Derg. 2012;22:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Neumann A, Rosenberger D, Vorsprach O, Dazert S. [The incidence of Frey syndrome following parotidectomy: results of a survey and follow-up]. HNO. 2011;59:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Durgut O, Basut O, Demir UL, Ozmen OA, Kasapoglu F, Coskun H. Association between skin flap thickness and Frey's syndrome in parotid surgery. Head Neck. 2013;35:1781-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Khafif A, Niddal A, Azoulay O, Holostenco V, Masalha M. Parotidectomy via Individualized Mini-Blair Incision. ORL J Otorhinolaryngol Relat Spec. 2020;82:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Drummond PD. Mechanism of gustatory flushing in Frey's syndrome. Clin Auton Res. 2002;12:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Motz KM, Kim YJ. Auriculotemporal Syndrome (Frey Syndrome). Otolaryngol Clin North Am. 2016;49:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Lin HJ, Hsiao JR, Chang JS, Hu CL, Chen TR, Lee WT, Huang CC, Ou CY, Tsai SW, Lu YC, Tsai ST, Chao WY, Chang CC. Resected specimen size: A reliable predictor of severe Frey syndrome after parotidectomy. Head Neck. 2019;41:2285-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Lee CC, Chan RC, Chan JY. Predictors for Frey Syndrome Development After Parotidectomy. Ann Plast Surg. 2017;79:39-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Filho WQ, Dedivitis RA, Rapoport A, Guimarães AV. Sternocleidomastoid muscle flap preventing Frey syndrome following parotidectomy. World J Surg. 2004;28:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Cristofaro MG, Cordaro R, Barca I, Giudice A. Efficacy of SMAS flap technique to prevent Frey's syndrome and aesthetic outcomes. A retrospective cohort analysis. Ann Ital Chir. 2021;92:683-690. [PubMed] |
| 16. | de Bree R, van der Waal I, Leemans CR. Management of Frey syndrome. Head Neck. 2007;29:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Yu GY, Ma DQ, Peng X, Wang SL, Li LJ, Yu CQ, Cheng Y. Guidelines On the diagnosis and treatment of salivary gland tumors. Zhonghua Kongqiang Yixue Zazhi. 2010;45:131-134. [DOI] [Full Text] |
| 18. | Gao L, Liang QL, Ren WH, Li SM, Xue LF, Zhi Y, Song JZ, Wang QB, Dou ZC, Yue J, Zhi KQ. Comparison of endoscope-assisted versus conventional resection of parotid tumors. Br J Oral Maxillofac Surg. 2019;57:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Zou HW, Gao J, Liu JX, Qu ZL, Du ZS, Zhao H, Zhao M, Chen HY. Feasibility and advantages of endoscope-assisted parotidectomy: a systematic review and meta-analysis. Br J Oral Maxillofac Surg. 2021;59:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Li C, Xu Y, Zhang C, Sun C, Chen Y, Zhao H, Li G, Fan J, Lei D. Modified partial superficial parotidectomy versus conventional superficial parotidectomy improves treatment of pleomorphic adenoma of the parotid gland. Am J Surg. 2014;208:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |