Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1510
Peer-review started: December 12, 2023
First decision: January 9, 2024
Revised: January 18, 2024
Accepted: February 22, 2024
Article in press: February 22, 2024
Published online: March 16, 2024
Processing time: 90 Days and 15.4 Hours
The prognosis for patients with advanced metastatic cervix cancer (MCC) is poor, and this disease continues to pose a considerable therapeutic challenge. Despite the administration of first-line regimens consisting of cisplatin, paclitaxel, and bevacizumab, survival rates for patients with metastasis remain poor. The emergence of bispecific antibodies (BsAbs) offers a novel treatment option for patients diagnosed with MCC.
In this report, we present a patient with MCC who was treated with cadonilimab monotherapy at a dose of 6 mg/kg every two weeks after chemotherapy was proven to be intolerable. The patient exhibited a sustained complete response for a duration of 6 months, demonstrating an optimistic outlook.
This case illustrates the considerable efficacy of cadonilimab for treating advanced MCC. Therefore, BsAb therapy is a promising strategy for effectively treating patients with advanced MCC and should be considered as an option when patients are intolerant to standard chemotherapy.
Core Tip: Advanced metastatic cervical cancer (MCC) has a poor prognosis and a low survival rate. The emergence of bispecific antibodies offers a novel treatment option for patients with MCC. Cadonilimab is a tetravalent bispecific antibody designed to simultaneously block the programmed death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) pathways while substantially reducing toxicities compared to combination therapy with PD-1 and CTLA-4 antibodies. We report the case of a patient with advanced MCC who achieved a complete response with cadonilimab monotherapy despite chemotherapy-induced grade IV myelosuppression.
- Citation: Zhu R, Chen TZ, Sun MT, Zhu CR. Advanced cervix cancer patient with chemotherapy-induced grade IV myelosuppression achieved complete remission with cadonilimab: A case report. World J Clin Cases 2024; 12(8): 1510-1516
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1510.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1510
Among all cancers, cervix cancer (CC) is the fourth most common cancer among women worldwide, following breast, colorectal, and lung cancer. The average age at diagnosis of CC globally is 53 years, while the average age at death is 59 years[1]. Additionally, CC ranks as the fourth leading cause of cancer-related deaths in women, following breast, lung, and colorectal cancers. Surgery remains the primary treatment option for early-stage CC, while concurrent radiotherapy is commonly employed for intermediate-stage and advanced-stage CC. Even when patients are treated with the first-line regimen of cisplatin and paclitaxel combined with bevacizumab, the median overall survival (mOS) for patients with recurrent or metastatic cervical cancer (R/MCC) is only 17.0 months[2]. R/MCC is widely considered an incurable condition. The advent of immunotherapy in recent years has provided a promising avenue for treating R/MCC.
In the past two decades, tumor immunotherapy has emerged as a prominent modality in combination with surgery, radiation therapy, or chemotherapy for cancer treatment. The emergence of immune checkpoint inhibitors (ICIs), such as programmed death protein 1 (PD-1)/programmed death protein 1 ligand (PD-L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), has revolutionized cancer immunotherapy on a global scale[3]. However, the advent of PD-1/CTLA-4 bispecific antibodies (BsAbs) offers a novel and highly specific treatment option that demonstrates enhanced efficacy for patients with R/MCC who have experienced failure with previous platinum-containing chemotherapy[4]. In this context, we present a comprehensive account of the process involved in achieving complete response (CR) following treatment with PD-1/CTLA-4 BsAb in a patient diagnosed with advanced metastatic cervix cancer (MCC) and grade IV myelosuppression after chemotherapy.
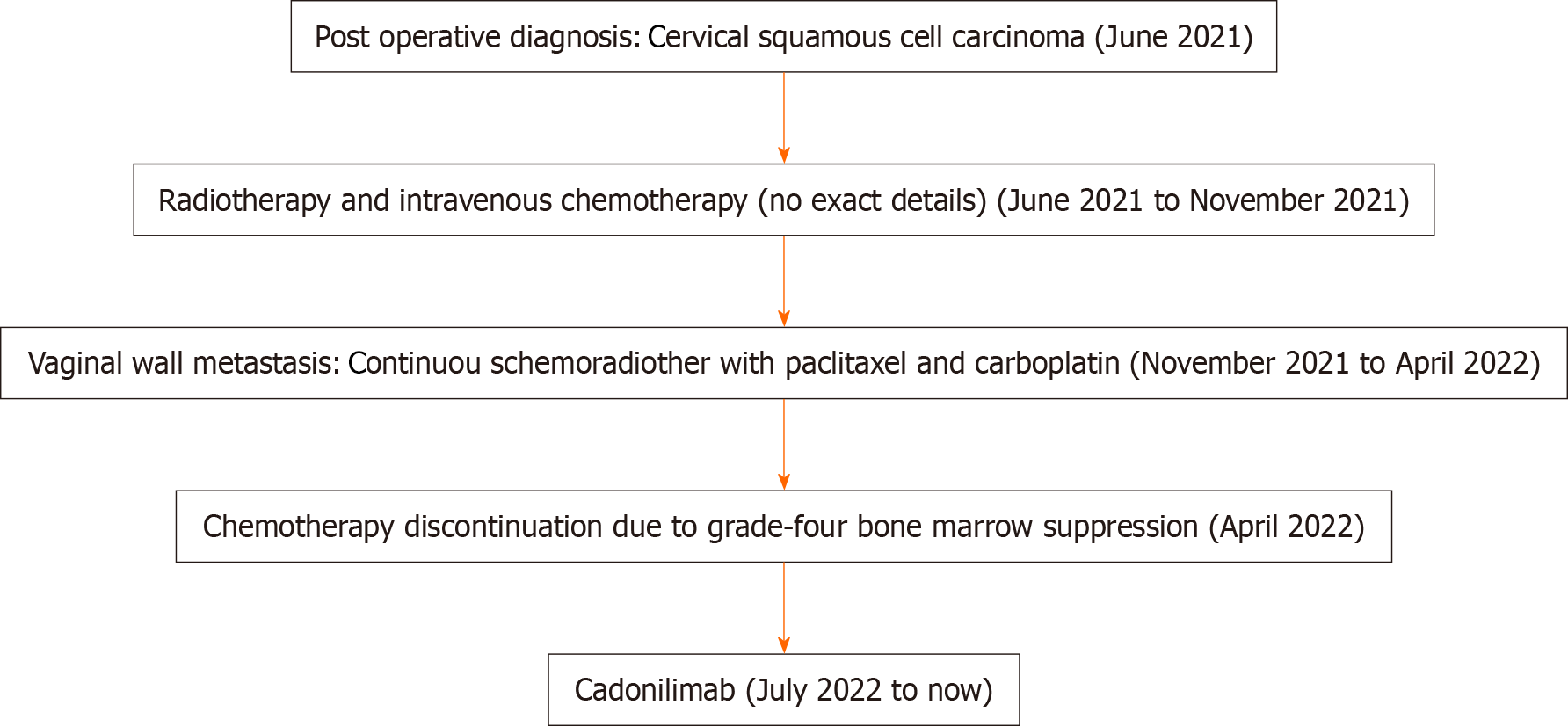
A 59-year-old woman was admitted to the oncology department of our hospital (the First Affiliated Hospital of Soochow University) due to vaginal metastasis of CC, seeking further treatment.
Chemotherapy could not be continued due to grade IV myelosuppression induced by chemotherapy.
The patient presented to the Second Affiliated Hospital of Soochow University in June 2021 with a 2-month history of abnormal vaginal bleeding. Specialized examination revealed the presence of a cauliflower-like mass in the cervix. After laparoscopic extensive total uterus removal, bilateral adnexectomy, bilateral pelvic infundibular ligament high ligation, and pelvic lymphadenectomy, postoperative pathology confirmed the diagnosis of extensive squamous cell carcinoma involving the entire uterus and both adnexa. Adjuvant chemoradiotherapy was administered following the surgical intervention.
In November 2021, a mass was identified in the vaginal wall and confirmed to be squamous cell carcinoma through biopsy and related pathological and immunohistochemical findings. A total of 28 radiotherapy sessions were performed at the Second Affiliated Hospital of Soochow University, starting in December 2021. Additionally, the patient received three cycles of intravenous chemotherapy with paclitaxel combined with carboplatin from January to April 2022. Chemotherapy was discontinued due to the occurrence of grade four bone marrow suppression.
The patient had a documented history of hypothyroidism and consistently adhered to the prescribed medication. She had a family history of genetic disease, history of other chronic diseases, history of cancer, etc.
A detailed general examination of the patient was performed and no obvious positive signs were found.
On July 8, 2022, the female tumor marker levels were as follows: CEA, 146 ng/mL; CA125, 94 IU/mL; and CA199, 83.1 IU/mL (Table 1). The following three indicators of thyroid function were measured: T4, 54.9 nmol/L; TSH, 31.5 µIU/mL; and TPO-Ab, 941 IU/mL (Table 1). No abnormalities were found in routine blood, biochemical, or cardiac activity.
| Measurement | 07/08/2022 | 08/27/2020 | 10/28/2022 | 01/28/2023 | 02/04/2023 | 04/27/2023 | 07/24/2023 |
| CEA (ng/mL) | 146 | 22.5 | 3.5 | 2.68 | 8.23 | 5.95 | 7.69 |
| CA125 (IU/mL) | 94 | 17.3 | 16.3 | 10.2 | 14.3 | 14 | 13.6 |
| CA199 (IU/mL) | 83.1 | 18.4 | 17.5 | 38.9 | 36.4 | 28.4 | 30.6 |
| SCCA (ng/mL) | 0.754 | 0.776 | 0.989 | 1.23 | 0.96 | 1.07 | 0.985 |
| T3 (nmol/mL) | 1.69 | 1.01 | 1.37 | 0.71 | 0.97 | 0.95 | 0.93 |
| T4 (nmol/mL) | 89.8 | 62.9 | 74.2 | 22.2 | 45.2 | 58.1 | 46.5 |
| TSH (µIU/mL) | 33.8 | 90.1 | 92.1 | > 100 | > 100 | > 100 | > 100 |
| TPO-Ab (IU/mL) | 996 | > 1000 | 993 | > 1000 | 900 | 785 | 320 |
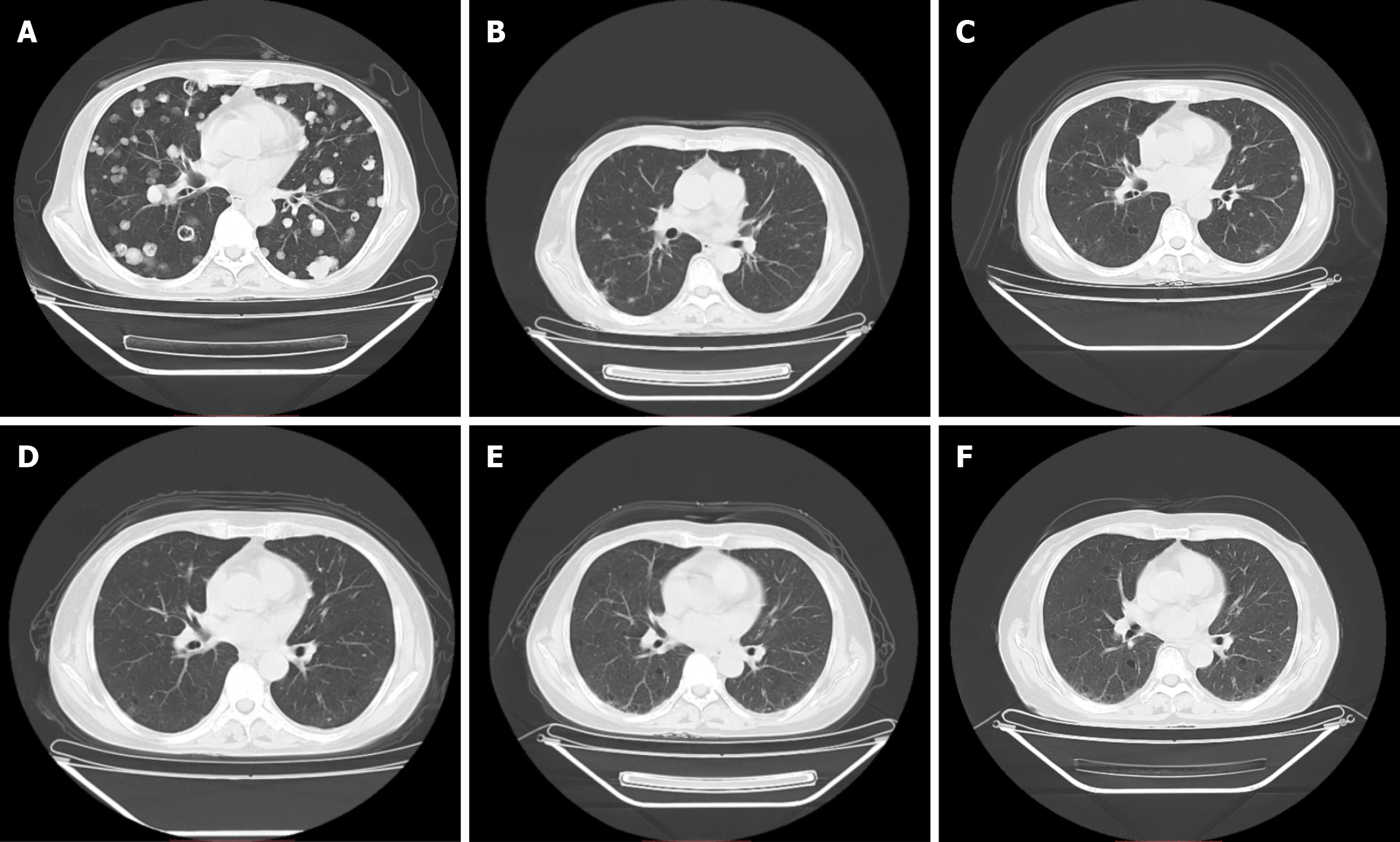
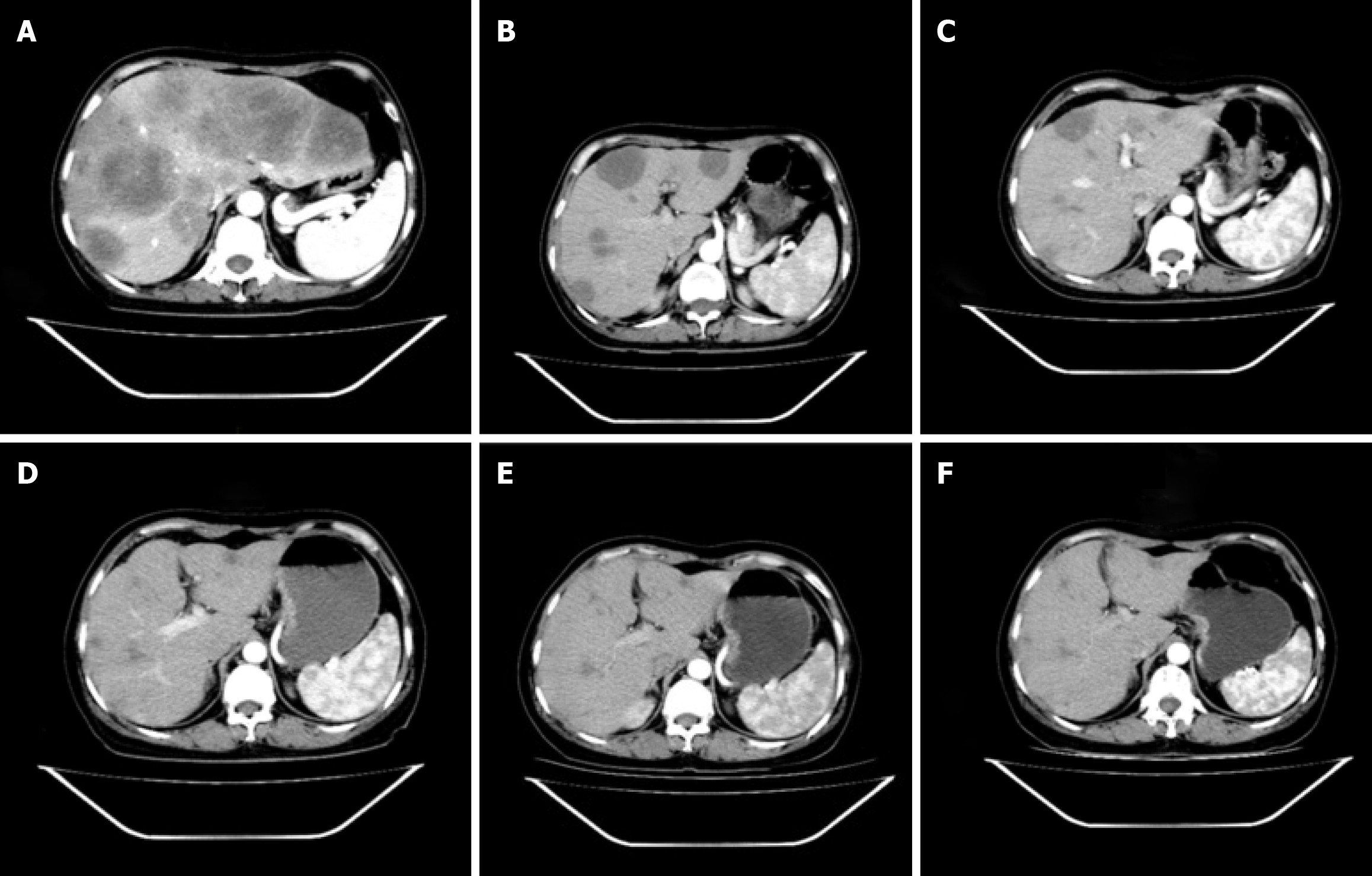
Computed tomography (CT) revealed notable metastases in both the lungs and liver (Figures 1A and 2A).
The patient was diagnosed with cervical squamous cell cancer accompanied by vaginal metastasis, and the disease stage was determined to be T1N0M1 (IVB).
After excluding contraindications, cadonilimab 6 mg/kg q2w was administered for immunotherapy. Figure 3 illustrates the diagnosis and treatment of the patient.
On August 27, 2023, we reexamined the patient's chest and abdomen CT and observed considerable improvement in multiple lung and liver metastases (Figures 1B and 2B). The levels of CEA (22.50 ng/mL), CA125 (17.30 IU/mL), and CA199 (18.40 IU/mL) were lower than their previous values (Table 1). Thyroid function was assessed on August 27, 2022, with a T3 level of 1.01 nmol/L, a T4 level of 62.90 nmol/L, a TSH level of 90.100 µIU/mL, and a TPO-Ab concentration exceeding 1000 IU/mL (Table 1); the Euthyrox dosage was increased from 25 µg qd to 75 µg qd orally due to considerations related to immune medications. No other immune-related side effects were observed during the comprehensive evaluation of partial response (PR). Assessments of the patient's condition were scheduled for October 28, 2022; January 18, 2023; April 27, 2023; and July 24, 2023 (Table 1, Figures 1C-F and 2C-F). We were pleased to find that on April 27, 2023, the patient achieved CR according to the Revised Response Tumors (RECIST) guidelines (version 1.1) and maintained CR until July 24, 2023 (the time of most recent assessment). We will continue to follow up with the patient and strictly review the changes in her conditions.
Here, we report the efficacy, feasibility, and tolerability of cadonilimab monotherapy in patients diagnosed with MCC who previously underwent platinum-based chemotherapy but experienced treatment failure. The patient did not experience any adverse effects during treatment with cadonilimab; however, the Euthyrox dosage was increased from 25 µg qd to 75 µg qd due to considerations related to immune medications.
The first-line treatment option for R/MCC consists of carboplatin, paclitaxel, and bevacizumab. However, this disease is often curable and is typically associated with a limited survival rate when treated using conventional methods[5]. The landscape of cancer treatment has substantially transformed in recent decades with immunotherapies, particularly ICIs for which PD-1, PD-L1, and CTLA-4 are targeted. These treatments are increasingly used for CC.
The combination of CTLA-4 and PD-1 inhibitors has received approval for three indications, namely, metastatic melanoma (MM), advanced renal cell carcinoma, and colorectal cancer with MMR and MSI-H aberrations[6]. Extensive investigations have been conducted on the combination of CTLA-4 and PD-1 inhibitors in patients with MM, demonstrating the efficacy of this combination across multiple clinical trials. The combination of nivolumab and ipilimumab in previously untreated patients with advanced melanoma, as demonstrated in a phase 1 trial (NCT01927419) conducted by Postow et al[7], resulted in a significantly improved objective response rate and progression-free survival compared to those from treatment with ipilimumab alone. Notably, 22% of the participants achieved CR. The subsequent findings from a phase 2 trial (NCT01927419) conducted by Hodi et al[8] revealed a significant improvement in the two-year overall survival rate among patients who received nivolumab and ipilimumab compared with those who received only ipilimumab as treatment (63.8% vs 53.6%). The Landmark Check Mate 067 clinical trial (NCT01844505) assessed the efficacy of combination therapy with nivolumab plus ipilimumab compared to that of monotherapy with either nivo
Cadonilimab is a tetravalent BsAb designed to simultaneously block the PD-1 and CTLA-4 pathways while signifi
Four completed single-agent studies of cadonilimab (AK104-101, AK104-201, AK104-202 and AK104-204) were conducted to investigate the efficacy of this drug in various tumor types, including cervical cancer, nonsmall cell lung cancer, nasopharyngeal cancer, hepatocellular carcinoma, esophageal cancer, mesothelioma, melanoma, colorectal cancer, stomach cancer, endometrial cancer, ovarian cancer, breast cancer, renal cell carcinoma, small cell lung cancer, neuroendocrine tumors, prostate adenocarcinoma, head and neck squamous cell carcinoma, pancreatic ductal adenocarcinoma, and cholangiocarcinoma. In a case report by Peng et al[14], a patient diagnosed with stage IV HER-2-positive gastric adenocarcinoma who presented with advanced liver and lung metastases was documented. The patient was enrolled in a clinical trial (NCT03852251) and achieved CR following treatment with cadonilimab combined with chemotherapy. However, immune-associated pulmonary toxicity and hepatitis related to treatment were observed.
Cadonilimab is one of the many BsAbs. To date, a total of seven BsAbs have received marketing approval worldwide, namely, catumaxomab (which was withdrawn from the market in 2017), blinatumomab, emicizumab, amivantamab, faricimab, cadonilimab, and mosunetuzumab. Additionally, more than 200 BsAbs are currently undergoing clinical and preclinical research stages[15].
The patient in this case had to receive single-agent cadonilimab due to grade 4 bone marrow suppression resulting from chemotherapy. We next investigated the potential synergistic effects of combining cadonilimab with radiotherapy and chemotherapy in future clinical treatments. Moreover, we must be attentive to the potential occurrence of complications resulting from the combination of radiotherapy, chemotherapy, and immunotherapy.
This case demonstrated the marked effectiveness of single-agent cadonilimab treatment in patients with advanced MCC in which chemotherapy was not well tolerated. The therapy was well tolerated; however, vigilant monitoring for immune-related adverse events is recommended for patients with a prior medical history. BsAbs offer a promising therapeutic approach for patients with advanced MCC and should be considered an option after standard chemotherapy fails.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bernardes A, Portugal; Das Mohapatra SS, India S-Editor: Gong ZM L-Editor: A P-Editor: Zheng XM
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, DiSaia PJ, Copeland LJ, Creasman WT, Stehman FB, Brady MF, Burger RA, Thigpen JT, Birrer MJ, Waggoner SE, Moore DH, Look KY, Koh WJ, Monk BJ. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 449] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 3. | Gennigens C, Jerusalem G, Lapaille L, De Cuypere M, Streel S, Kridelka F, Ray-Coquard I. Recurrent or primary metastatic cervical cancer: current and future treatments. ESMO Open. 2022;7:100579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Keam SJ. Cadonilimab: First Approval. Drugs. 2022;82:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 5. | Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 1518] [Article Influence: 253.0] [Reference Citation Analysis (0)] |
| 6. | Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 672] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 7. | Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2210] [Cited by in RCA: 2221] [Article Influence: 222.1] [Reference Citation Analysis (0)] |
| 8. | Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor DR, Salama AK, Taylor MH, Ott PA, Horak C, Gagnier P, Jiang J, Wolchok JD, Postow MA. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 754] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 9. | Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 1051] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 10. | Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2362] [Cited by in RCA: 2780] [Article Influence: 347.5] [Reference Citation Analysis (0)] |
| 11. | Pang X, Huang Z, Zhong T, Zhang P, Wang ZM, Xia M, Li B. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. MAbs. 2023;15:2180794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 12. | Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D, Allison JP. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell. 2017;170:1120-1133.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 980] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 13. | Wu XH, Ji JF, Lou HM, Li YX, Feng M, Xu N, Li YZ, Wang J, Huang Y, Lou G, An RF, Li CZ, Zhou Q, Huang X, Zhao EF, Liu TS, Fan QX, Li GL, Li BY, Xia Y. Efficacy and safety of cadonilimab, an anti-PD-1/CTLA4 bi-specific antibody, in previously treated recurrent or metastatic (R/M) cervical cancer. Gynecol Oncol. 2022;166:S47-S48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Peng J, Zhu Q, Peng Z, Chen Z, Liu Y, Liu B. Patients with positive HER-2 amplification advanced gastroesophageal junction cancer achieved complete response with combined chemotherapy of AK104/cadonilimab (PD-1/CTLA-4 bispecific): A case report. Front Immunol. 2022;13:1049518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 15. | Kang J, Sun T, Zhang Y. Immunotherapeutic progress and application of bispecific antibody in cancer. Front Immunol. 2022;13:1020003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |