Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1481
Peer-review started: December 9, 2023
First decision: January 5, 2024
Revised: January 20, 2024
Accepted: February 25, 2024
Article in press: February 25, 2024
Published online: March 16, 2024
Processing time: 94 Days and 2.6 Hours
In recent years, confocal laser endomicroscopy (CLE) has become a new endoscopic imaging technology at the microscopic level, which is extensively performed for real-time in vivo histological examination. CLE can be performed to distinguish benign from malignant lesions. In this study, we diagnosed using CLE an asymptomatic patient with poorly differentiated gastric adenocarcinoma.
A 63-year-old woman was diagnosed with gastric mucosal lesions, which may be gastric cancer, in the small curvature of the stomach by gastroscopy. She consented to undergo CLE for morphological observation of the gastric mucosa. Through the combination of CLE diagnosis and postoperative pathology, the intraoperative CLE diagnosis was considered to be reliable. According to our experience, CLE can be performed as the first choice for the diagnosis of gastric cancer.
CLE has several advantages over pathological diagnosis. We believe that CLE has great potential in the diagnosis of benign and malignant gastric lesions.
Core Tip: Confocal laser microscopic endoscope (CLE) is a relatively new endoscopic imaging technology. It provides a real-time microscopic vison of mucosal epithelium physiology and pathology in natural state. Gastric cancer is a common disease in clinic. Early symptoms are hidden, and patients do not pay enough attention to them. In this case, a woman was diagnosed gastric poorly differentiated adenocarcinoma by CLE in the small curvature by gastroscope. Through the combination of CLE and pathology, the intraoperative CLE diagnosis was considered to be reliable. According to our experience, CLE can be used as the first choice for the diagnosis of gastric cancer.
- Citation: Lou JX, Wu Y, Huhe M, Zhang JJ, Jia DW, Jiang ZY. Diagnosis of poorly differentiated adenocarcinoma of the stomach by confocal laser endomicroscopy: A case report. World J Clin Cases 2024; 12(8): 1481-1486
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1481.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1481
Confocal laser endomicroscopy (CLE) is a relatively new endoscopic imaging technology. It is a supplement to histopathology and also provides real-time microscopic visualization of mucosal epithelium physiology and pathology in the natural state. The magnification can reach 500 times or more. Because of its real-time detection and virtual tissue imaging, it can be performed for the endoscopic study of living cells. For these reasons, CLE is usually called “optical biopsy.”
Gastric cancer is a common disease worldwide. Early symptoms are hidden, and patients do not pay enough attention to them. Many cases are in the middle and late stages when found by gastroscopy. To improve the diagnosis rate of early gastric cancer, pathological biopsy is often performed for gastric mucosal lesions > 1 cm. Although the gold standard for the diagnosis of gastric cancer is still pathological biopsy, obtaining an effective pathological diagnosis depends on the accuracy of the sampling site and the experience of pathologists. Human factors have a great influence, so there are still opportunities for enhancement in the diagnosis of early gastric cancer.
CLE based on a probe can diagnose gastric cancer through the working channel of the gastroscope. Therefore, we performed CLE combined with pathological biopsy to obtain the diagnosis of gastric cancer, with the risk of gastric malignant tumor, which provides a clinical basis for subsequent treatment.
On November 6, 2023, a 63-year-old woman presented to The Second Affiliated Hospital of Baotou Medical College (Baotou, China). The patient had intermittent epigastric discomfort without obvious inducement six months ago, had taken omeprazole and other drugs, and the effect was not good.
The patient has not lost weight since the onset of the disease. She did not complain of abdominal pain.
The patient has denied the history of heart disease, hypertension and diabetes.
The patient's father has cardiac cancer.
During gastroscopy, the patient temperature was 36.6 °C, heart rate 78 bpm, respiratory rate 18 breaths/min, blood pressure 134/75 mmHg, and oxygen saturation in room air 99%.
The Gastric function examination: Pepsinogen Ⅰ 296.19 g/L, Pepsinogen Ⅱ 17.41 g/L, Gastrin 17 49.10 pg/mL; There were no abnormalities in electrocardiogram, blood routine examination and infection test.
No relevant imaging examination was done.
The patient was diagnosed with poorly differentiated adenocarcinoma of the stomach.
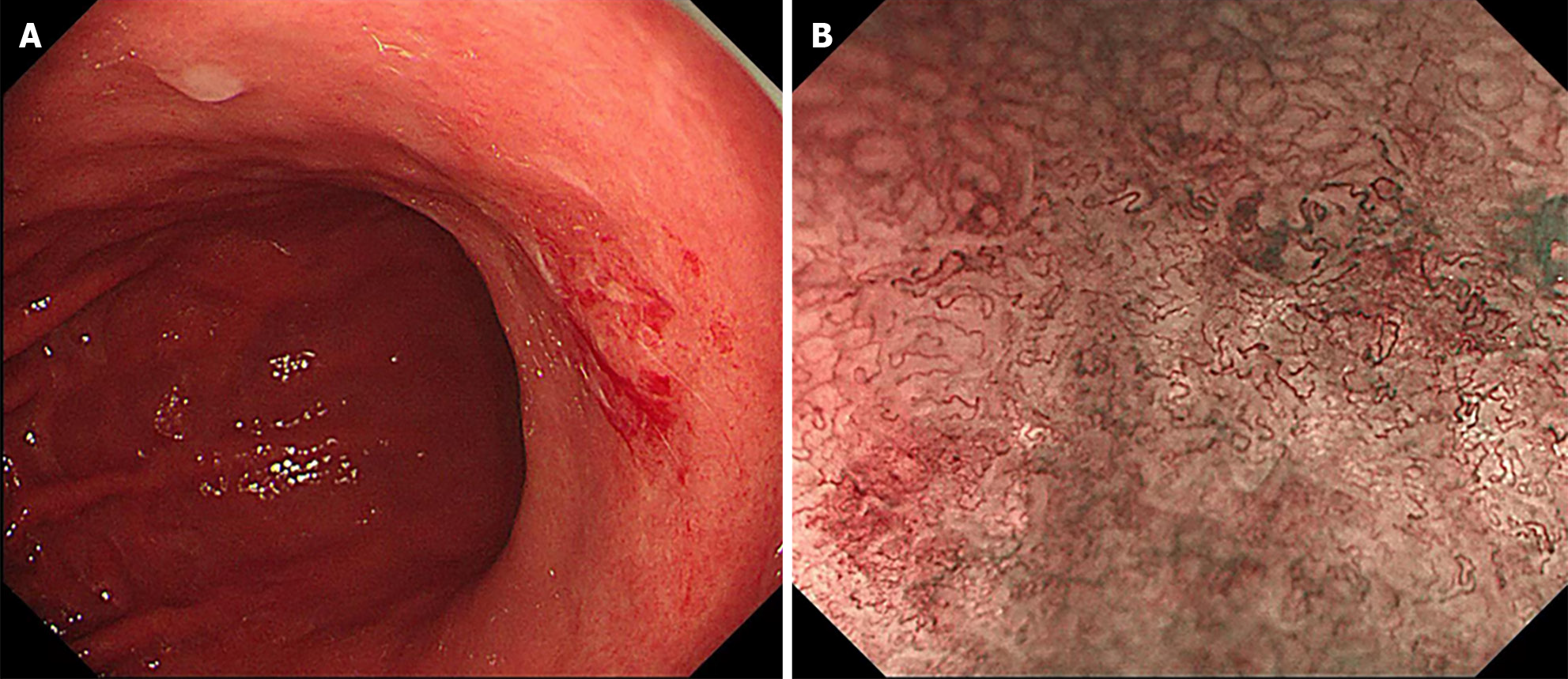
The patient, a 63-year-old woman, had been experiencing intermittent abdominal distension with hiccups for more than 0.5 years, which was aggravated for more than 1 month. The patient underwent a gastroscopy in the Digestive Endoscopy Center of the Second Affiliated Hospital of Baotou Medical College on November 6, 2023. White-light gastroscopy revealed that in the gastric antrum, the mucosa was red and white, mainly red, with peristalsis, and the mucosa was rough on the front wall of small curvature. In the stomach body, the shape and peristalsis of the folds were normal, the flaky mucosa was red, the center was depressed, and a small ulcer had formed on the small curvature (Figure 1A). Narrow band imaging magnification indicated that the flaky mucosa was brown with clear boundaries between surrounding tissues, glands were missing from the center, blood vessels were twisted and climbing, and the mucosa was brittle (Figure 1B). It was highly suggested that the small curvature of the stomach had a high probability of becoming cancerous.
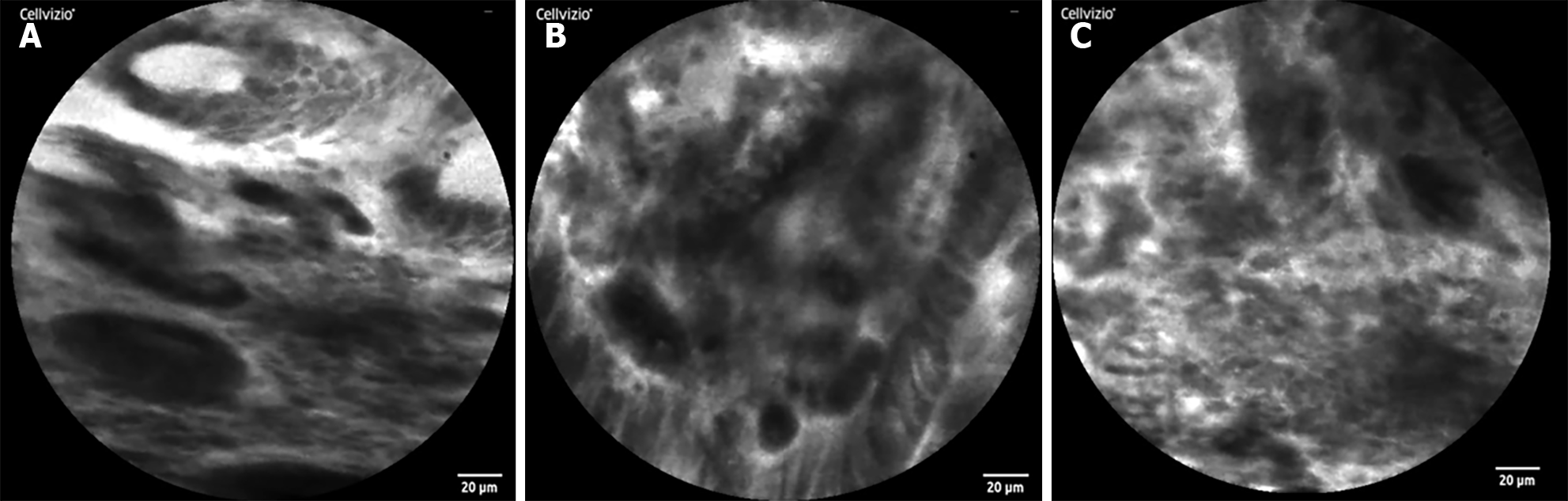
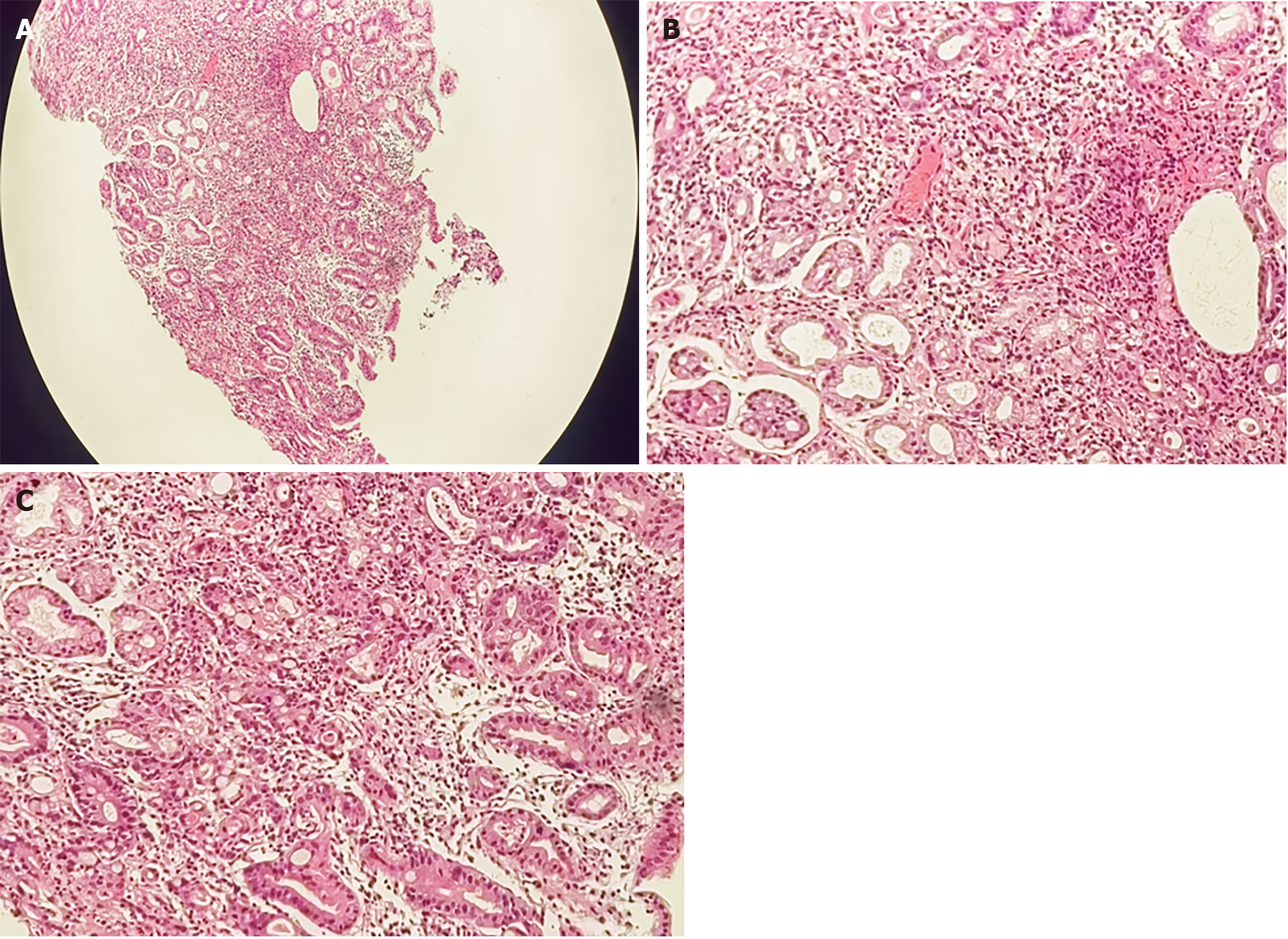
To further determine the characteristics of the lesion, after acquiring the consent of the family members, a CLE was performed. Because intravenous fluorescein sodium stain in CLE examination is required, to avoid serious allergic reactions in the patient, a sodium fluorescein allergy test was performed. After a negative result was obtained, a CLE examination was performed at the mucosal depression on the small curvature of the stomach, suggesting that the normal gastric pit structure disappeared, the glandular duct structure disappeared, and the cell polarity was abnormal (Figure 2). Biopsy with forceps in the positive part of the CLE examination revealed poor tissue quality, poor elasticity, and easy bleeding. Pathology suggested adenocarcinoma (moderately poorly differentiated, intramucosal, hand-held type) (Figure 3). CLE and pathological results indicated that the patient had a poorly differentiated adenocarcinoma of the small curvature of the stomach.
Upon active communication with the patient and her family about the severity of the disease and the follow-up treatment plan, the patient agreed to undergo elective surgical treatment.
The gold standard for diagnosis of most gastrointestinal disorders is endoscopic tissue sampling with histopathology. The differentiation between malignant and benign lesions is crucial for further management. However, random biopsies involve flaws that may be incurred, such as sampling errors[1,2]. CLE is a new type of microscope that integrates optical microscopy technology, laser scanning technology, and computer image processing software, and it is the only endoscopic imaging technology that can pathologically diagnose living tissues in real time. CLE is known as optical biopsy, magnifying the cross-sectional gray-scale image 500 times to observe the digestive tract mucosa at the subcellular level. CLE has only been put into clinical application for more than 10 years, which has extraordinary application value in many gastrointestinal diseases, especially in early tumor screening and precancerous lesion monitoring, and has ushered in a new era for endoscopic diagnosis of gastrointestinal diseases. Studies have revealed that the sensitivity and specificity of CLE in distinguishing gastric mucosal tumors from nontumor lesions can reach 90.2% and 98.5%, respectively[3]. The sensitivity, specificity, and accuracy of CLE in the diagnosis of early gastric cancer reached 88.9%, 99.3%, and 98.9%, respectively[4]. The prospective study of Bok et al[5] found that the accuracy of CLE in diagnosing gastric cancer was higher than that of general endoscopy (90.7% vs 85.2%), and the accuracy of combined diagnosis of CLE and histopathology could reach 98.1%. It is difficult to distinguish superficial gastric microcarcinomas from noncancerous lesions under conventional white-light endoscopy, which further increases the number of unnecessary biopsies. CLE has changed this situation because it can directly provide histological observation in vivo, and it may be extensively performed in the diagnosis and treatment of digestive tract tumors as a new technology to replace histopathological biopsy in the future. Furthermore, CLE is easy to be controlled by doctors. With only a short learning curve, doctors can achieve a reliable CLE diagnostic level through training. In this regard, pathological biopsy diagnosis is not easily comparable[6].
Gastric cancer has a high incidence in China and is one of the most common malignant digestive tract tumors worldwide. The mortality of gastric cancer accounts for more than 20% of the total tumor mortality[7]. Because the early symptoms are hidden or patients do not pay enough attention to them, many patients are in the middle and late stages when they are diagnosed by gastroscopy, and most patients still have recurrence and metastasis after comprehensive treatment, with high mortality. In gastric cancer, poorly differentiated adenocarcinoma is a type with a relatively high degree of malignancy. The clinical manifestations are dull pain in the upper abdomen, fullness, and discomfort, and some patients may experience no discomfort. When cases are found, they are usually in the middle and late stages, with rapid onset and poor prognosis[8]. Therefore, the treatment of poorly differentiated gastric adenocarcinoma is relatively difficult, and the treatment of early poorly differentiated gastric adenocarcinoma is still mainly surgical resection[9]. Early detection and intervention are the most effective and economical therapeutic strategies.
Through CLE, our patient was diagnosed early, which provided good conditions for subsequent surgical treatment. According to a literature review, many genes play a regulatory role in the occurrence and development of gastric cancer, such as increased expression of notch homolog 1, recombinant hairy and enhancer of split 1, and calcium activated neutral proteinase 1, which regulate the invasion ability of gastric cancer cells by participating in cell adhesion and epithelial–mesenchymal transition[10]. To date, the pathogenesis of poorly differentiated gastric adenocarcinoma remains to be elucidated, and the prognosis of surgical treatment and postoperative comprehensive treatment is poor. Therapeutic large scale clinical trials showed that public health drive to reduce the prevalence of Helicobacter pylori as a strategy to reduce the incidence of gastric cancer in the population[7]. At present, the relationship between poorly differentiated gastric adenocarcinoma and Helicobacter pylori infection has not been supported by clear research evidence. Therefore, the primary goal is to improve the diagnosis rate of early gastric cancer through endoscopy to improve the prognosis of early gastric cancer. We believe that CLE can be performed as an examination tool for the diagnosis of early gastric cancer and can be broadly popularized in this field.
Based on our experience, CLE technology should be performed as the first choice for the diagnosis of early gastric cancer. We believe that CLE has great potential in this field.
We are grateful for the understanding and cooperation of the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Limaiem F, Tunisia S-Editor: Liu H L-Editor: A P-Editor: Yu HG
| 1. | Huang B, Tian S, Zhan N, Ma J, Huang Z, Zhang C, Zhang H, Ming F, Liao F, Ji M, Zhang J, Liu Y, He P, Deng B, Hu J, Dong W. Accurate diagnosis and prognosis prediction of gastric cancer using deep learning on digital pathological images: A retrospective multicentre study. EBioMedicine. 2021;73:103631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Iwamuro M, Kondo E, Takata K, Yoshino T, Okada H. Diagnosis of follicular lymphoma of the gastrointestinal tract: A better initial diagnostic workup. World J Gastroenterol. 2016;22:1674-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Yao K. How is the VS (vessel plus surface) classification system applicable to magnifying narrow-band imaging examinations of gastric neoplasias initially diagnosed as low-grade adenomas? Gastric Cancer. 2012;15:118-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Li WB, Zuo XL, Li CQ, Zuo F, Gu XM, Yu T, Chu CL, Zhang TG, Li YQ. Diagnostic value of confocal laser endomicroscopy for gastric superficial cancerous lesions. Gut. 2011;60:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Bok GH, Jeon SR, Cho JY, Cho JH, Lee WC, Jin SY, Choi IH, Kim HG, Lee TH, Park EJ. The accuracy of probe-based confocal endomicroscopy versus conventional endoscopic biopsies for the diagnosis of superficial gastric neoplasia (with videos). Gastrointest Endosc. 2013;77:899-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | East JE, Vleugels JL, Roelandt P, Bhandari P, Bisschops R, Dekker E, Hassan C, Horgan G, Kiesslich R, Longcroft-Wheaton G, Wilson A, Dumonceau JM. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy. 2016;48:1029-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 7. | Chen YC, Malfertheiner P, Yu HT, Kuo CL, Chang YY, Meng FT, Wu YX, Hsiao JL, Chen MJ, Lin KP, Wu CY, Lin JT, O'Morain C, Megraud F, Lee WC, El-Omar EM, Wu MS, Liou JM. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 165] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 8. | Hibdon ES, Razumilava N, Keeley TM, Wong G, Solanki S, Shah YM, Samuelson LC. Notch and mTOR Signaling Pathways Promote Human Gastric Cancer Cell Proliferation. Neoplasia. 2019;21:702-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | ASGE standards of practice committee; Forbes N, Elhanafi SE, Al-Haddad MA, Thosani NC, Draganov PV, Othman MO, Ceppa EP, Kaul V, Feely MM, Sahin I, Buxbaum JL, Calderwood AH, Chalhoub JM, Coelho-Prabhu N, Desai M, Fujii-Lau LL, Kohli DR, Kwon RS, Machicado JD, Marya NB, Pawa S, Ruan W, Sheth SG, Storm AC, Thiruvengadam NR, Qumseya BJ; (ASGE Standards of Practice Committee Chair). American Society for Gastrointestinal Endoscopy guideline on endoscopic submucosal dissection for the management of early esophageal and gastric cancers: summary and recommendations. Gastrointest Endosc. 2023;98:271-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 10. | Du X, Cheng Z, Wang YH, Guo ZH, Zhang SQ, Hu JK, Zhou ZG. Role of Notch signaling pathway in gastric cancer: a meta-analysis of the literature. World J Gastroenterol. 2014;20:9191-9199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |