Published online Feb 26, 2024. doi: 10.12998/wjcc.v12.i6.1144
Peer-review started: October 11, 2023
First decision: December 5, 2023
Revised: December 13, 2023
Accepted: January 12, 2024
Article in press: January 12, 2024
Published online: February 26, 2024
Processing time: 131 Days and 19.9 Hours
This study presents a case of rapidly developing respiratory failure due to anti
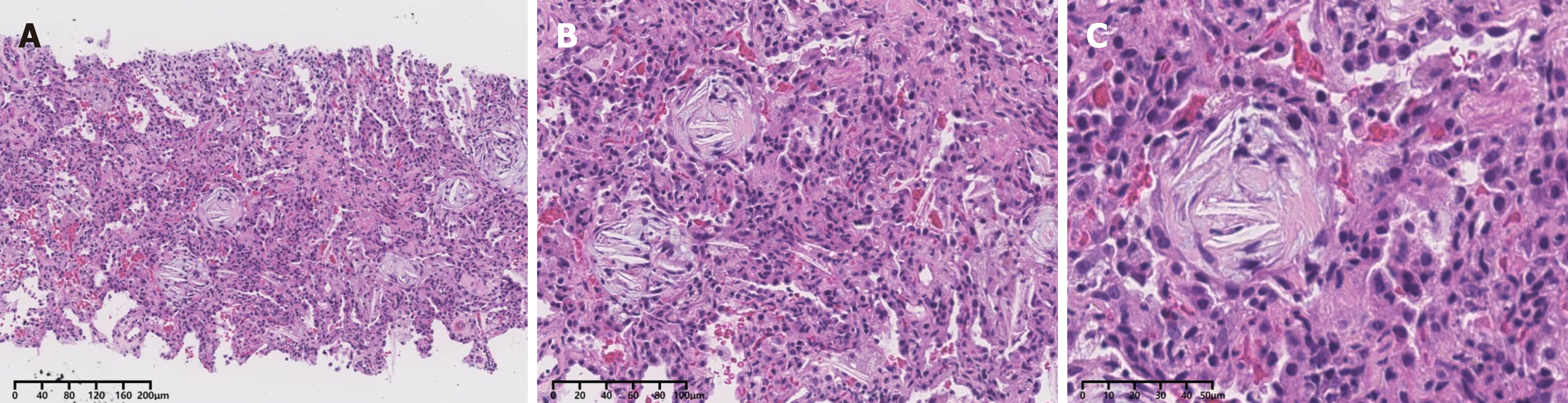
A 33-year-old man with a diagnosis of KS was admitted to the Department of Pulmonary and Critical Care Medicine of a tertiary hospital in China for fever and shortness of breath 2 wk after the onset of COVID-19. Computed tomography of both lungs revealed diffuse multiple patchy heightened shadows in both lungs, accompanied by signs of partial bronchial inflation. Metagenomic next-generation sequencing of the bronchoalveolar lavage fluid suggested absence of pathogen. A biopsy specimen revealed organizing pneumonia with alveolar septal thickening. Additionally, extensive auto-antibody tests showed strong positivity for anti-SSA, anti-SSB, anti-Jo-1, and anti-Ro-52. Following multidisciplinary discussions, the patient received a final diagnosis of AS, leading to rapidly progressing respiratory failure.
This study underscores the clinical progression of AS-associated interstitial lung disease subsequent to viral infections such as COVID-19 in patients diagnosed with KS.
Core Tip: Antisynthetase syndrome (AS) presents as an idiopathic inflammatory muscle disease typified by the presence of anti-Jo1 antibodies. Mainstream treatments encompass corticosteroids and immunosuppressants. Occasionally, certain rheumatic immune diseases can be precipitated by infectious diseases. Herein, we present a case detailing the rapid onset of respiratory failure due to AS subsequent to coronavirus disease 2019 (COVID-19) in an individual diagnosed with Klinefelter syndrome (KS). Following a multidisciplinary discussion, the conclusive diagnosis for the patient’s rapid respiratory decline was AS. This investigation accentuates the clinical progression of AS-associated interstitial lung disease following viral infections such as COVID-19 in individuals with KS.
- Citation: Wu XX, Cui J, Wang SY, Zhao TT, Yuan YF, Yang L, Zuo W, Liao WJ. Clinical evolution of antisynthetase syndrome-associated interstitial lung disease after COVID-19 in a man with Klinefelter syndrome: A case report. World J Clin Cases 2024; 12(6): 1144-1149
- URL: https://www.wjgnet.com/2307-8960/full/v12/i6/1144.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i6.1144
Since January 2023, reported cases of coronavirus disease 2019 (COVID-19) have notably surged in China. A large study revealed that more than 30% of patients with severe COVID-19 concurrently exhibit a second active clinical condition[1]. Although the impact of COVID-19 on individuals with rheumatic disorders has been partially evaluated, its influence on the progression of these diseases remains incompletely understood[2-4]. Antisynthetase syndrome (AS) is an idiopathic inflammatory muscle disease characterized by anti-Jo1 antibodies, showcasing diverse clinical presentations and a fe
A 33-year-old man was admitted to the Department of Pulmonary and Critical Care Medicine at a tertiary hospital lo
His symptoms commenced 2 d before admission, marked by fever and difficulty breathing. Upon arrival, the patient’s condition required high-flow nasal cannula oxygen (HFNC) to maintain normal transcutaneous oxygen saturation.
His past medical history included KS and novel coronavirus pneumonia diagnosis. At the age of approximately 10-years-old, the patient had a confirmed diagnosis of KS in a tertiary children’s hospital because of small external genitalia. Two weeks before the current hospitalization, the patient had fever and tested positive for COVID-19 by nucleic acid assay at a local county hospital. A chest computed tomography (CT) scan revealed a few patchy ground glass shadows outside the upper lungs. Following a 5-d oral paxlovid treatment and subsequent chest CT confirming lesion absorption, he was dis
The patient denied any family history related to malignant tumors, rheumatoid diseases, or immune conditions.
Upon admission, his initial vital signs were as follows: blood pressure, 100/80 mmHg; pulse rate, 130 beats per minute; respiratory rate, 40 breaths per minute; and body temperature, 38°C. He exhibited flushed cheeks, agitated nostrils, and shallow, rapid breathing, along with significant wet rales heard in both lungs. No other abnormalities were observed du
His laboratory findings indicated a red blood cell count of 3.94 × 1012/L, white blood cell count of 2.95 × 109/L, lympho
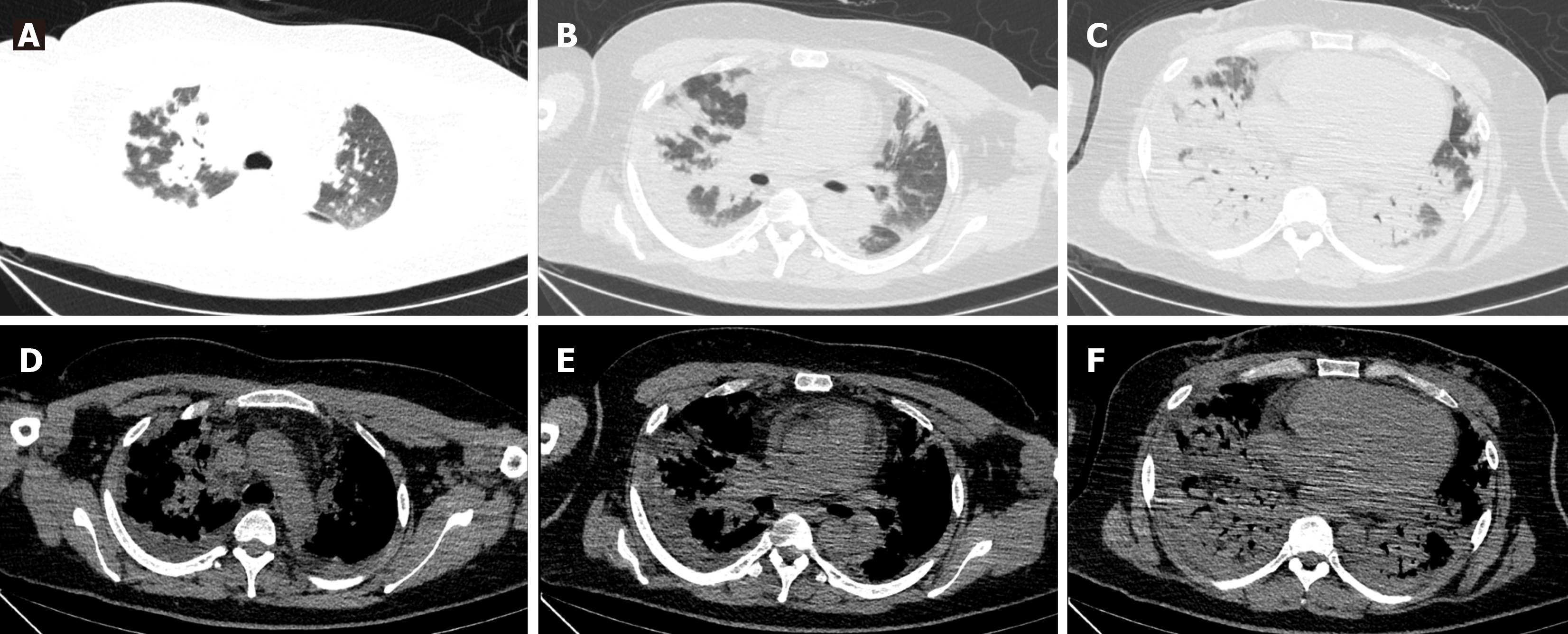
Lung CT examination revealed diffuse multiple patchy areas of increased shadows in both lungs, displaying a partial bronchial inflation sign (Figure 1).
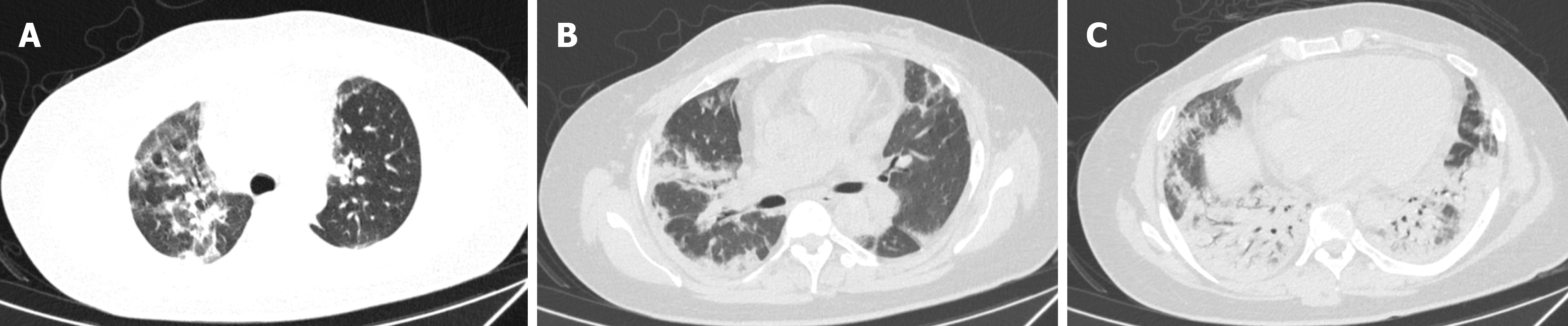
On the 2nd day, a bronchoscopy was conducted on suspicion of infectious pneumonia. The cells in the bronchoalveolar lavage fluid (BALF) were counted, showing a notably elevated ratio of neutrophils at 53%, lymphocytes at 15%, and eosinophils at 9%. Subsequently, microbial next-generation sequencing of BALF showed no detectable pathogens. On the 5th day, additional testing for autoimmune disease-related antibodies indicated strong positive results for anti-SSA, anti-SSB, anti-Jo-1, and anti-Ro-52. To prevent infection, the patient received cefoperazone and sulbactam. Moreover, a dosage of methylprednisolone (40 mg once daily) was initiated 6 d after hospitalization. Remarkably, the diffuse multiple patches of increased density in both upper lungs significantly diminished (Figure 2). To ascertain the nature of the diffuse mul
Following a comprehensive multidisciplinary team meeting (MDT) discussion, the patient had a final diagnosis of AS-associated interstitial lung disease (ILD).
Subsequently, the patient underwent treatment with methylprednisolone at 500 mg paxlovid for 3 d, followed by a regimen of methylprednisolone at 80 mg paxlovid in conjunction with tacrolimus at 1 mg bid.
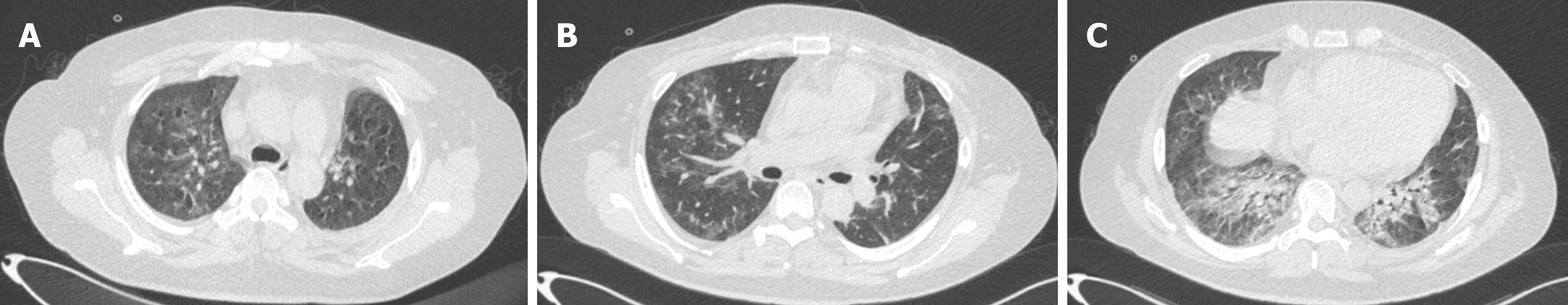
By day 30, the patient regained the ability to walk without requiring oxygen inhalation. A follow-up chest CT examina
In this case study, we report a case of rapidly developing respiratory failure 2 wk after COVID-19 onset in a man with KS. A previous study revealed that all KS patients with COVID-19 experienced mild symptoms, suggesting that the presence of an extra X chromosome might contribute to a more favorable clinical outcome[7]. However, the patient with KS in this study encountered respiratory failure shortly after contracting COVID-19. To address this, we performed a color ultra
AS is a rare autoimmune disease characterized by the presence of aminoacyl-transfer RNA synthetase antibodies, often accompanied by clinical manifestations such as ILD, myopathy, and nonerosive arthritis. AS occurs distinct from other in
Currently, there is no standardized treatment for AS-ILD because of the absence of randomized controlled trials. The selection of immunosuppression is usually consistent with treatment strategies adopted for ILD secondary to inflammatory myopathies. Treatment options encompass corticosteroids and immunosuppressants, with rituximab often reser
While the prevalence of autoimmune diseases in KS remains unknown, it is believed that the estimated frequency is higher in men, nearly reaching the levels observed in women. This could be elucidated by several genes on the X chro
In summary, we have described a rare clinical case of AS-associated ILD following COVID-19 in a male patient with KS in China. Clinicians should be cognizant of this rare clinical entity, and timely MDT discussions can significantly contribute to the diagnosis of AS in patients with KS, especially those facing potentially severe respiratory failure.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Freund O, Israel S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Xu ZH
| 1. | Freund O, Azolai L, Sror N, Zeeman I, Kozlovsky T, Greenberg SA, Epstein Weiss T, Bornstein G, Tchebiner JZ, Frydman S. Diagnostic delays among COVID-19 patients with a second concurrent diagnosis. J Hosp Med. 2023;18:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 2. | Bozzalla Cassione E, Zanframundo G, Biglia A, Codullo V, Montecucco C, Cavagna L. COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann Rheum Dis. 2020;79:1382-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79:667-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 361] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 4. | Ferri C, Giuggioli D, Raimondo V, Dagna L, Riccieri V, Zanatta E, Guiducci S, Tavoni A, Foti R, Cuomo G, De Angelis R, Cozzi F, Murdaca G, Cavazzana I, Romeo N, Codullo V, Ingegnoli F, Pellegrini R, Varcasia G, Rossa AD, De Santis M, Abignano G, Colaci M, Caminiti M, L'Andolina M, Lubrano E, Spinella A, Lumetti F, De Luca G, Bellando-Randone S, Visalli E, Bilia S, Giannini D, Masini F, Pellegrino G, Pigatto E, Generali E, Dall'Ara F, Mariano GP, Barsotti S, Pettiti G, Zanframundo G, Brittelli R, Aiello V, Scorpiniti D, Ferrari T, Caminiti R, Campochiaro C, D'Angelo S, Iannone F, Matucci-Cerinic M, Doria A, Miccoli M, Fallahi P, Antonelli A; COVID-19 & Autoimmune Systemic Diseases Italian Study Group. COVID-19 and systemic sclerosis: clinicopathological implications from Italian nationwide survey study. Lancet Rheumatol. 2021;3:e166-e168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Rovenský J, Kovalancík M, Payer J, Kohler K. Klinefelter syndrome with antisynthetase syndrome: why might they be associated? J Clin Rheumatol. 2003;9:62-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Rovenský J, Imrich R, Lazúrová I, Payer J. Rheumatic diseases and Klinefelter's syndrome. Ann N Y Acad Sci. 2010;1193:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Aliberti L, Gagliardi I, Lupo S, Verrienti M, Bondanelli M, Zatelli MC, Ambrosio MR. Investigation of COVID-19 infection in subjects with Klinefelter syndrome. J Endocrinol Invest. 2022;45:1065-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Vertui V, Zanframundo G, Castañeda S, Biglia A, Palermo BL, Cavazzana I, Meloni F, Cavagna L. Clinical evolution of antisynthetase syndrome after SARS-CoV2 infection: a 6-month follow-up analysis. Clin Rheumatol. 2022;41:2601-2604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Fu H, Zheng Z, Zhang Z, Yang Y, Cui J, Wang Z, Xue J, Chi S, Cao M, Chen J. Prediction of progressive pulmonary fibrosis in patients with anti-synthetase syndrome-associated interstitial lung disease. Clin Rheumatol. 2023;42:1917-1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Sawal N, Mukhopadhyay S, Rayancha S, Moore A, Garcha P, Kumar A, Kaul V. A narrative review of interstitial lung disease in anti-synthetase syndrome: a clinical approach. J Thorac Dis. 2021;13:5556-5571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Zanframundo G, Faghihi-Kashani S, Scirè CA, Bonella F, Corte TJ, Doyle TJ, Fiorentino D, Gonzalez-Gay MA, Hudson M, Kuwana M, Lundberg IE, Mammen A, McHugh N, Miller FW, Monteccucco C, Oddis CV, Rojas-Serrano J, Schmidt J, Selva-O'Callaghan A, Werth VP, Sakellariou G, Aggarwal R, Cavagna L. Defining anti-synthetase syndrome: a systematic literature review. Clin Exp Rheumatol. 2022;40:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 12. | Witt LJ, Curran JJ, Strek ME. The Diagnosis and Treatment of Antisynthetase Syndrome. Clin Pulm Med. 2016;23:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Kalayci Yigin A, Alay MT, Uğurlu S, Seven M. The First Case Report of 47,XXY/46,XX/46,XY Mosaic Klinefelter Syndrome Patient With Mixed Connective Tissue Disorder. Am J Mens Health. 2023;17:15579883231165173. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |