Published online Feb 16, 2024. doi: 10.12998/wjcc.v12.i5.966
Peer-review started: November 16, 2023
First decision: December 26, 2023
Revised: December 30, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: February 16, 2024
Processing time: 75 Days and 17.6 Hours
The diagnosis of sepsis combined with acute respiratory distress syndrome (ARDS) has increased owing to the enhanced awareness among medical professionals and the continuous development of modern medical technologies, while early diagnosis of ARDS still lacks specific biomarkers. One of the main patho
To investigate the expression of PMAs in the serum of patients with sepsis complicated by ARDS and its clinical significance.
We selected 72 hospitalized patients diagnosed with sepsis as the study population between March 2019 and March 2022. Among them, 30 patients with sepsis and ARDS formed the study group, while 42 sepsis patients without ARDS comprised the control group. After diagnosis, venous blood samples were imme
The study found that the levels of PNAs and PLyAs in the serum of the study group were higher than those in the control group, but the difference was not statistically significant (P > 0.05). However, the expression of PMAs in the serum of the study group was significantly upregulated (P < 0.05) and positively correlated with the APACHE II score (r = 0.671, P < 0.05). When using PMAs as a diagnostic indicator, the area under the curve value was 0.957, indicating a high diagnostic value (P < 0.05). Furthermore, the optimal cutoff value was 8.418%, with a diagnostic sensitivity of 0.819 and specificity of 0.947.
In summary, the serum levels of PMAs significantly increase in patients with sepsis and ARDS. Therefore, serum PMAs have the potential to become a new biomarker for clinically diagnosing sepsis complicated by ARDS.
Core Tip: Our research aimed to investigate the expression of platelet mononuclear cell aggregates (PMAs) in the serum of patients with sepsis complicated by acute respiratory distress syndrome (ARDS) and its clinical significance. The results indicate that the serum levels of PMAs significantly increase in patients with sepsis and ARDS. Therefore, serum PMAs have the potential to become a new biomarker for clinically diagnosing sepsis complicated by ARDS.
- Citation: Huang CM, Li JJ, Wei WK. Clinical significance of platelet mononuclear cell aggregates in patients with sepsis and acute respiratory distress syndrome. World J Clin Cases 2024; 12(5): 966-972
- URL: https://www.wjgnet.com/2307-8960/full/v12/i5/966.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i5.966
Sepsis is a common and critical clinical condition, and in recent years, the number of cases diagnosed as sepsis with concomitant acute respiratory distress syndrome (ARDS) has increased, owing to the enhanced awareness among medical professionals and the continuous development of modern medical diagnostic technologies[1]. Due to the high mortality rate associated with sepsis, it has attracted widespread attention in the clinical community. Despite various diagnostic tools available, early diagnosis of ARDS still lacks specific biomarkers. Recent studies have confirmed that one of the main pathogenic mechanisms of sepsis-associated ARDS involves the actions of various pathological injuries and inflammatory factors[2,3].
In this study, platelets and white blood cells in the patient's body are activated, leading to an increase in surface adhesion molecules. These adhesion molecules further form platelet-white blood cell aggregates, including platelet-neutrophil aggregates (PNAs), platelet aggregates (PLyAs), and platelet-mononuclear cell aggregates (PMAs)[4]. Activated platelets bind to monocytes and neutrophils, with the noteworthy observation that the binding of platelets to monocytes precedes that to neutrophils. PMAs are considered one of the markers of platelet activation[5]. The objective of this study is to investigate whether PMAs in the serum of sepsis patients with ARDS can serve as effective biomarkers for the early diagnosis of this complication.
This study included 72 adult sepsis patients admitted to our hospital between March 2019 and March 2022. The diagnosis was in accordance with the " International Guidelines for Management of Sepsis and Septic Shock: 2016[6]." Following the 2012 Berlin definition[7], patients were categorized into the study group (sepsis with ARDS, n = 30) and the control group (sepsis alone, n = 42). Exclusion criteria comprised pregnancy with blood system diseases, pure blood system diseases, HIV infection, ongoing chemotherapy, use of immunosuppressive agents or antiplatelet drugs, pulmonary interstitial fibrosis, and acute exacerbation of chronic obstructive pulmonary disease. The study was approved by our hospital's medical ethics committee, and written informed consent was obtained from all patients or their authorized representatives.
Three milliliters of peripheral venous blood were collected from all confirmed sepsis patients immediately upon admission, placed in anticoagulant tubes, and preserved and transported to the Shanghai Lanwei Medical Laboratory for further testing via ice pack refrigeration. To ensure accuracy, the entire blood collection process strictly adhered to standardized procedures to prevent platelet activation-induced errors.
Patients underwent blood gas analysis, and the oxygenation index PaO2/FiO2 was calculated. Flow cytometry was employed to classify platelet-mononuclear cell aggregates in the peripheral blood of both sepsis patient groups, including PLyAs, PMAs, and PNAs. The site of infection was recorded, and the nature of the pathogenic bacteria was determined through blood culture.
Within 24 h of admission, all confirmed sepsis patients had their physiological indicators meticulously recorded by the attending physician, who then calculated the Acute Physiology and Chronic Health Evaluation (APACHE) II score, noting the worst values.
Statistical analysis was conducted using SPSS 20.0 software. Descriptive data are presented as mean ± SD, and t-tests were used for comparisons. Receiver operating characteristic (ROC) curves were generated to determine the optimal cutoff value, sensitivity, and specificity of serum PMAs in diagnosing sepsis with ARDS. The significance level was set at P < 0.05.
According to the data in Table 1, there were no significant differences (P > 0.05) observed in general information, such as age, gender, infection site, pathogen, and oxygenation index PaO2/FiO2, between sepsis patients with ARDS and those with sepsis alone.
| Study group (n = 30) | Control group (n = 42) | χ2/t | P value | |
| Male/female | 16/14 | 25/17 | 0.475 | 0.523 |
| Age | 51.6 ± 11.4 | 48.6 ± 14.7 | 1.234 | 0.224 |
| Infection | 1.587 | 0.904 | ||
| Urinary tract infection | 4 (13.3) | 5 (11.9) | ||
| Hematogenous infection | 3 (10) | 4 (9.5) | ||
| Abdominal infection | 6 (20) | 8 (19.1) | ||
| Pulmonary infection | 15 (50) | 22 (52.4) | ||
| Others | 2 (6.7) | 3 (7.1) | ||
| Microbiology | 2.88 | 0.518 | ||
| Fungus | 3 (10) | 5 (11.9) | ||
| G- | 12 (40) | 17 (40.5) | ||
| G+ | 5 (16.7) | 8 (19) | ||
| Mixed infection | 6 (20) | 7 (16.7) | ||
| Unknown cause | 4 (13.3) | 5 (11.9) | ||
| PaO2/FiO2 | 141.85 ± 29.44 | 145.35 ± 30.28 | 11.27 | 0.912 |
As shown in Table 2, the serum levels of PNAs and PLyAs in sepsis patients with ARDS were slightly higher than those in the sepsis-alone group; however, these differences were not statistically significant (P > 0.05). Nevertheless, the serum levels of PMAs in sepsis patients with ARDS were significantly higher than those in the sepsis-alone group, and this difference was statistically significant (P < 0.05).
According to the data in Table 3, the APACHE II scores of sepsis patients with ARDS were significantly higher than those of sepsis patients without ARDS, and this difference was statistically significant (P < 0.05).
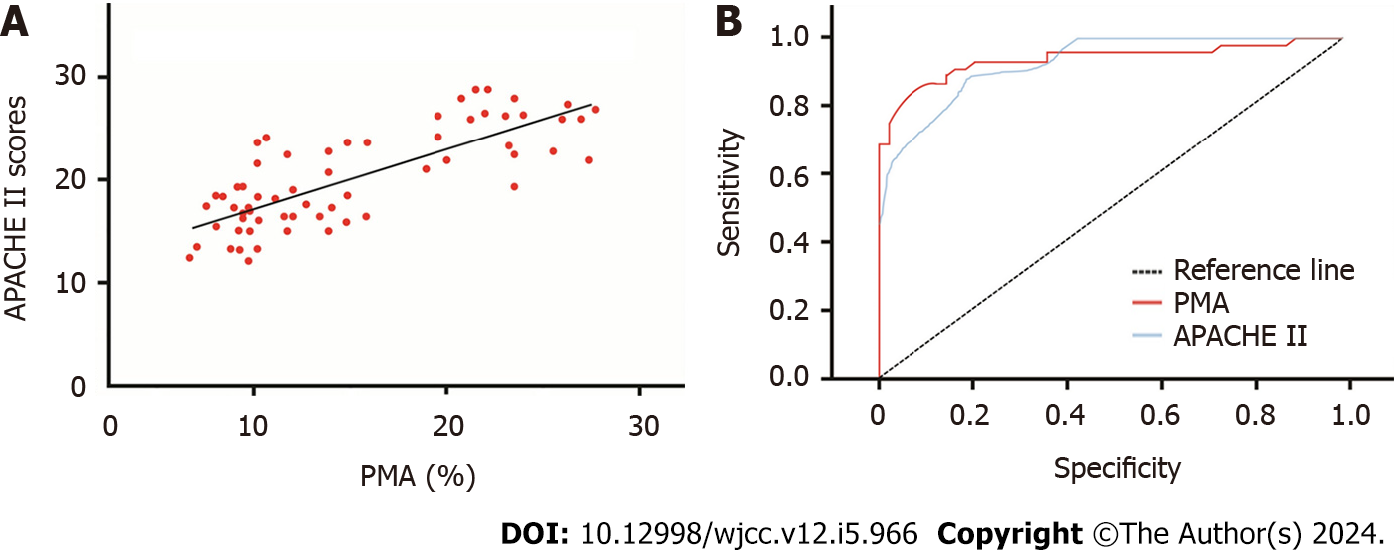
As illustrated in Figure 1A, the PMAs levels in sepsis patients with ARDS were significantly higher than those in sepsis patients without ARDS. Further linear correlation analysis revealed a positive correlation between PMAs and APACHE II scores in patients (r = 0.671, P < 0.05).
Using PMAs and APACHE II scores as test variables and ARDS as the state variable, ROC curves were fitted. When using PMAs as the test variable, the area under the curve (AUC) was 0.957, indicating a significant diagnostic value (P < 0.05). The optimal cutoff value for PMAs was 8.418%, with a diagnostic sensitivity of 0.819 and specificity of 0.947. When using APACHE II scores as the test variable, the AUC was 0.940, indicating a significant diagnostic value (P < 0.05). The optimal cutoff value for APACHE II scores was 17.115, with a diagnostic sensitivity of 0.837 and specificity of 0.844. Refer to Tables 4 and 5, and Figure 1B for detailed results.
| Parameter | AUC | SE | P value | 95%CI | |
| Upper limit | Lower limit | ||||
| PMAs | 0.957 | 0.022 | < 0.05 | 0.914 | 0.974 |
| APACHE Ⅱ | 0.93 | 0.021 | < 0.05 | 0.872 | 0.981 |
| Parameter | Cutoff value | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
| PMAs | 8.418 | 0.819 | 0.947 | 0.956 | 0.819 |
| APACHE Ⅱ | 17.115 | 0.837 | 0.844 | 0.829 | 0.877 |
Sepsis, as a common complication of severe infections, trauma, acute abdomen, and major surgeries in clinical practice, spans multiple disciplines such as internal medicine, surgery, and gynecology. It leads to multi-organ dysfunction, poor prognosis, and a high mortality rate[8]. The lungs are particularly susceptible to the effects of sepsis, causing pathological damage that is closely related to patient prognosis. Sepsis-induced multi-organ pathology includes the aggregation of white blood cells and platelets at the site of infection, disseminated intravascular coagulation, endothelial damage, resulting in the loss of surfactant on the alveolar surface, and activation of oxidative stress responses. These mechanisms collectively contribute to the development of severe lung injury[9]. Due to the activation of inflammatory reactions and coagulation mechanisms in sepsis patients, they are prone to developing ARDS, with pathological manifestations in the lungs characterized by increased permeability of the alveolar-capillary barrier, pulmonary tissue edema, and severe hypoxemia. After the onset of typical injury symptoms, some sepsis patients may rapidly deteriorate within a short period, progressing to ARDS, thus affecting their prognosis[10]. Venous blood samples are the most readily available and suitable for laboratory testing. Among various specimens, patient serum is primarily used as a biological specimen for accurately and rapidly assessing the severity of sepsis.
In sepsis, damage to endothelial cells leads to activating inflammatory cells and platelets. Activated inflammatory cells release a large number of inflammatory and cellular factors through a cascade reaction, promoting endothelial cell apoptosis and monocyte release of chemokines. Platelets and white blood cells interact in the microcirculation of damaged tissues, forming platelet-white blood cell aggregates. This process further accelerates the release of inflammatory factors such as interleukin and tumor necrosis factor-alpha[11]. The worsening of the inflammatory response leads to endothelial cell swelling, necrosis and shedding, further worsening the patient's condition. Therefore, platelet-white blood cell aggregates in the serum play a crucial intermediary role between platelet activation and inflammatory response.
The results of this study indicate that the serum levels of PMAs in sepsis patients with ARDS were significantly higher than those in sepsis patients without ARDS (P < 0.05), confirming the utility of PMAs as a beneficial indicator for diagnosing sepsis with ARDS. The APACHE II scoring system is commonly used to assess the severity and prognosis of critically ill patients[12], have confirmed the application of the APACHE II score in predicting mortality in sepsis patients. The current study demonstrates that the APACHE II scores of sepsis patients with ARDS were significantly higher than those of sepsis patients without ARDS and were positively correlated with serum PMAs levels (P < 0.05). This further confirms the clinical importance of serum PMAs in the early diagnosis of sepsis with ARDS.
One limitation of this study is the relatively small sample size, with a total of 72 hospitalized patients included in the analysis. The limited sample size may affect the generalizability of the findings to a broader population. Additionally, the study focused on patients from a single hospital, which may introduce institutional biases and limit the external validity of the results. Including a more diverse and larger sample from multiple medical centers could enhance the robustness and applicability of the study findings. Furthermore, the retrospective nature of the study poses inherent limitations. The reliance on historical data collected from medical records may lead to incomplete or missing information. The retrospective design also prevents the researchers from controlling the data collection process, potentially introducing biases in the selection of patients or in the measurement of variables. A prospective study with a carefully designed protocol and standardized data collection procedures would provide stronger evidence and allow for better control of confounding variables. The study primarily focused on the expression of PMAs in the serum as a potential biomarker for sepsis complicated by ARDS. While the findings suggest a significant association, the study does not explore the underlying mechanisms or causality between elevated PMAs levels and the development of ARDS in sepsis patients. Further mechanistic studies are needed to elucidate the pathways through which PMAs may contribute to the pathogenesis of ARDS in sepsis. Finally, the study does not address the specificity of PMAs as a biomarker, and its utility in distinguishing sepsis with ARDS from other conditions that may present with similar clinical manifestations. Future research should explore the specificity and sensitivity of PMAs in differentiating various respiratory and systemic disorders to better understand its diagnostic value in a broader clinical context.
In conclusion, the elevation of serum PMAs levels is closely associated with the release of inflammatory factors. Although the exact mechanism of PMAs still requires further research, current studies suggest that its changes have important clinical significance in early diagnosing sepsis with ARDS. Therefore, PMAs may serve as one of the biomarkers for early diagnosing sepsis with ARDS.
The diagnosis of sepsis combined with acute respiratory distress syndrome (ARDS) has increased owing to the enhanced awareness among medical professionals and the continuous development of modern medical technologies, while early diagnosis of ARDS still lacks specific biomarkers. One of the main pathogenic mechanisms of sepsis-associated ARDS involves the actions of various pathological injuries and inflammatory factors, such as platelet and white blood cells activation, leading to an increase of surface adhesion molecules. These adhesion molecules further form platelet-white blood cell aggregates, including platelet-mononuclear cell aggregates (PMAs). PMAs has been identified as one of the markers of platelet activation, here we hypothesize that PMAs might play a potential biomarker for the early diagnosis of this complication.
To investigate whether PMAs could be a potential biomarker for the early diagnosis of sepsis combined with ARDS.
To investigate the clinical significance of PMAs in patients with sepsis complicated by ARDS.
72 patients diagnosed with sepsis were enrolled in the study between March 2019 and March 2022. Among them, 30 patients with sepsis and ARDS formed the study group, while 42 sepsis patients without ARDS comprised the control group. After diagnosis, venous blood samples were immediately collected from all patients. Flow cytometry was employed to analyze the expression of PMAs, platelet neutrophil aggregates (PNAs), and Platelet Aggregates (PLyAs) in the serum. Additionally, the Acute Physiology and Chronic Health Evaluation (APACHE) II score was calculated for each patient, and Receiver operating characteristic curves were generated to assess diagnostic value.
The levels of PNAs and PLyAs in the serum of the study group were higher than those in the control group, but the difference was not statistically significant (P > 0.05). However, the expression of PMAs in the serum of the study group was significantly upregulated (P < 0.05) and positively correlated with the APACHE II score (r=0.671, P < 0.05). When using PMAs as a diagnostic indicator, the area under the curve value was 0.957, indicating a high diagnostic value (P < 0.05). Furthermore, the optimal cutoff value was 8.418%, with a diagnostic sensitivity of 0.819 and specificity of 0.947.
The serum levels of PMAs significantly increase in patients with sepsis and ARDS, which might have the potential to become a new biomarker for clinically diagnosing sepsis complicated by ARDS.
Our study provides a new method for the early diagnosis of sepsis combined with ARDS, which is the detection of serum PMAs. More samples should be enrolled to confirm this method in the future study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Nepal S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Hu Q, Hao C, Tang S. From sepsis to acute respiratory distress syndrome (ARDS): emerging preventive strategies based on molecular and genetic researches. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 2. | Li X, Jamal M, Guo P, Jin Z, Zheng F, Song X, Zhan J, Wu H. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed Pharmacother. 2019;118:109363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Chen Y, Qiu C, Cai W. Identification of key immune genes for sepsis-induced ARDS based on bioinformatics analysis. Bioengineered. 2022;13:697-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Le Joncour A, Biard L, Vautier M, Bugaut H, Mekinian A, Maalouf G, Vieira M, Marcelin AG, Rosenzwajg M, Klatzmann D, Corvol JC, Paccoud O, Carillion A, Salem JE, Cacoub P, Boulaftali Y, Saadoun D. Neutrophil-Platelet and Monocyte-Platelet Aggregates in COVID-19 Patients. Thromb Haemost. 2020;120:1733-1735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA, Bozza PT. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 579] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 6. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3352] [Cited by in RCA: 4008] [Article Influence: 501.0] [Reference Citation Analysis (0)] |
| 7. | ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 4296] [Article Influence: 330.5] [Reference Citation Analysis (0)] |
| 8. | Chiu C, Legrand M. Epidemiology of sepsis and septic shock. Curr Opin Anaesthesiol. 2021;34:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 9. | Huang M, Cai S, Su J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 484] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 10. | Li W, Li D, Chen Y, Abudou H, Wang H, Cai J, Wang Y, Liu Z, Liu Y, Fan H. Classic Signaling Pathways in Alveolar Injury and Repair Involved in Sepsis-Induced ALI/ARDS: New Research Progress and Prospect. Dis Markers. 2022;2022:6362344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Liu D, Huang SY, Sun JH, Zhang HC, Cai QL, Gao C, Li L, Cao J, Xu F, Zhou Y, Guan CX, Jin SW, Deng J, Fang XM, Jiang JX, Zeng L. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 223] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 12. | Akinosoglou K, Kapsokosta G, Mouktaroudi M, Rovina N, Kaldis V, Stefos A, Kontogiorgi M, Giamarellos-Bourboulis E, Gogos C; Hellenic Sepsis Study Group. Diabetes on sepsis outcomes in non-ICU patients: A cohort study and review of the literature. J Diabetes Complications. 2021;35:107765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |