Published online Feb 16, 2024. doi: 10.12998/wjcc.v12.i5.880
Peer-review started: October 16, 2023
First decision: November 28, 2023
Revised: December 12, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: February 16, 2024
Processing time: 106 Days and 19.5 Hours
Clinical studies have reported that patients with gastroesophageal reflux disease (GERD) have a higher prevalence of hypertension.
To performed a bidirectional Mendelian randomization (MR) analysis to investi
Eligible single nucleotide polymorphisms (SNPs) were selected, and weighted median, inverse variance weighted (IVW) as well as MR egger (MR-Egger) re
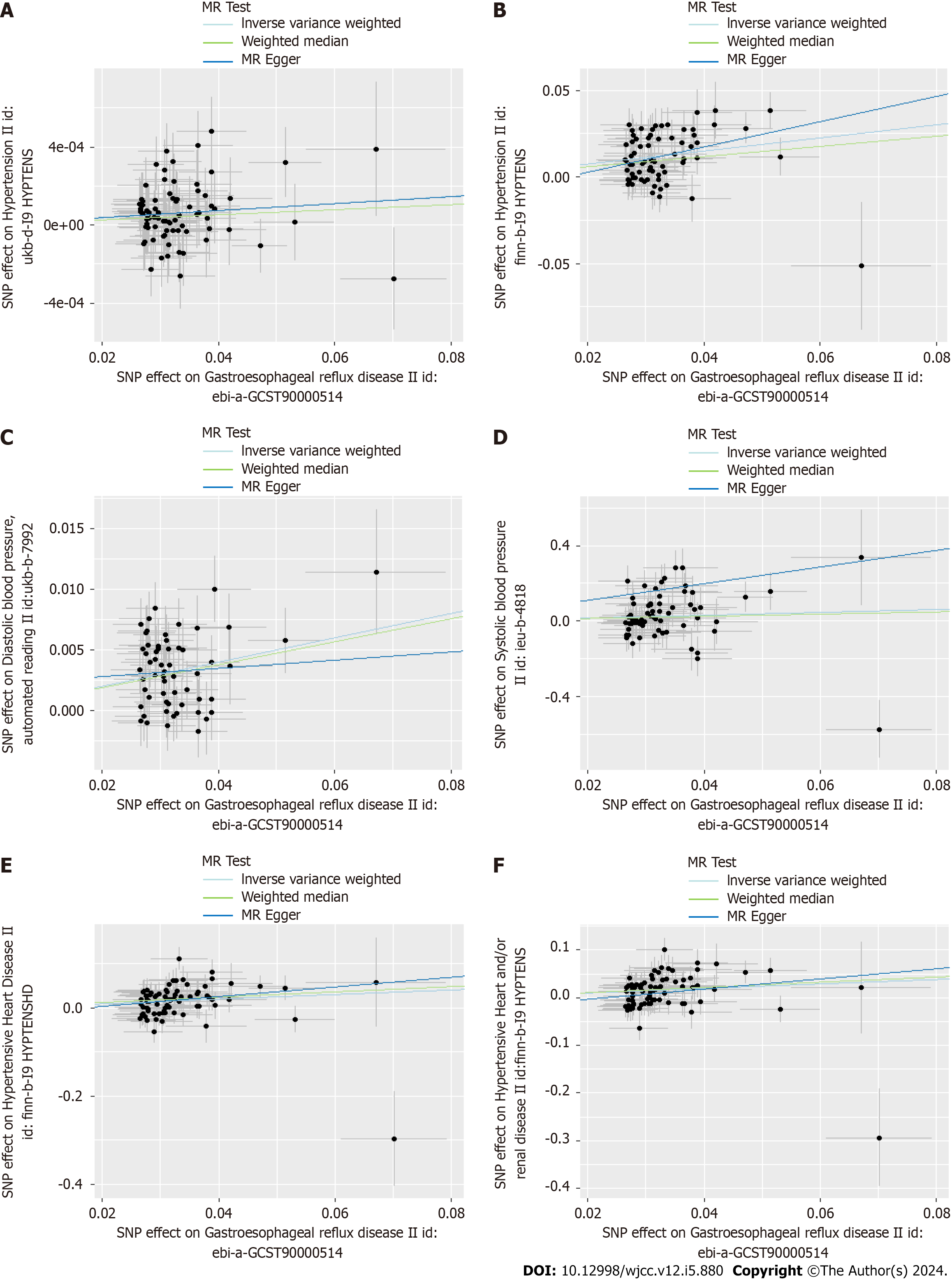
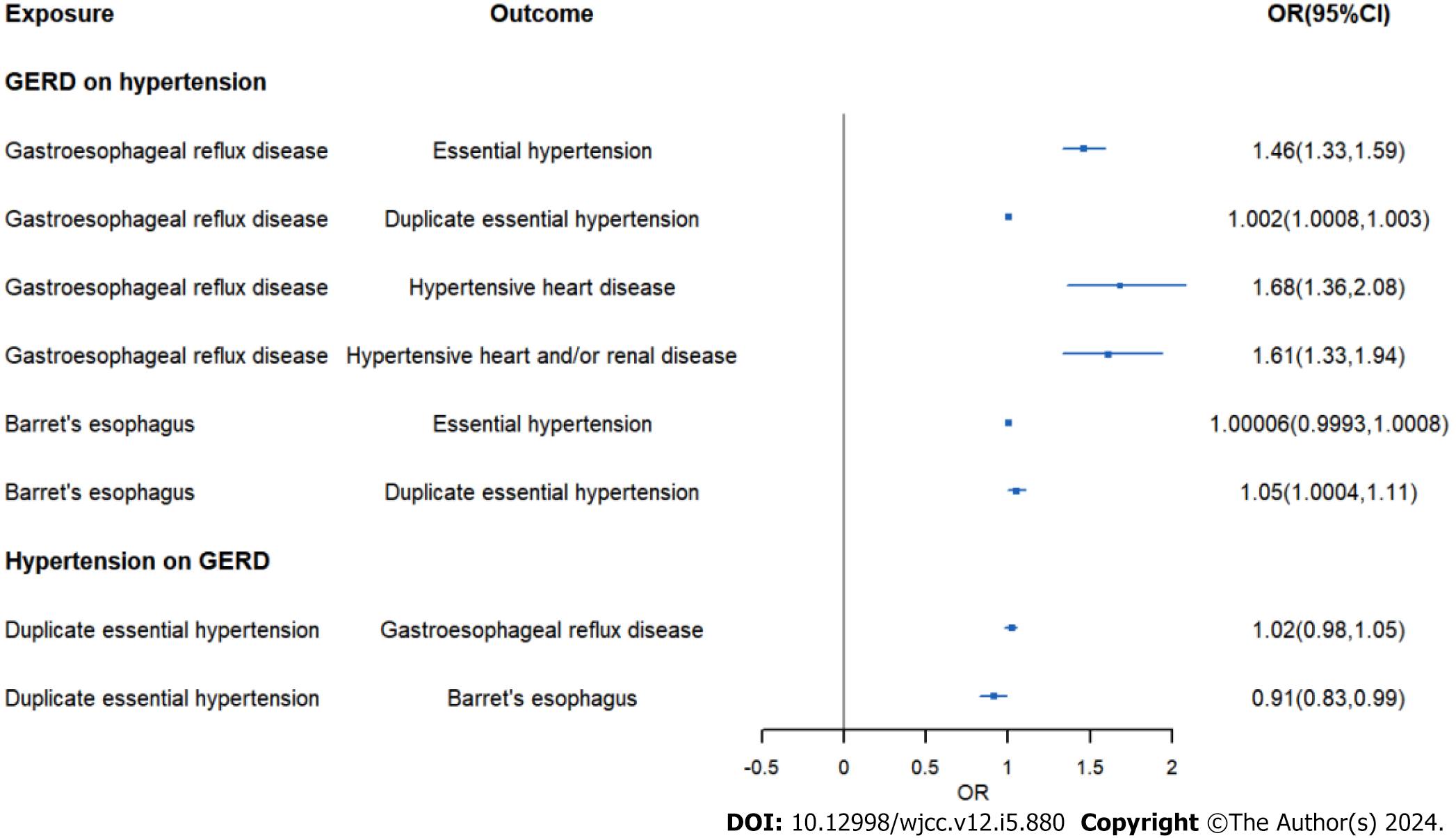
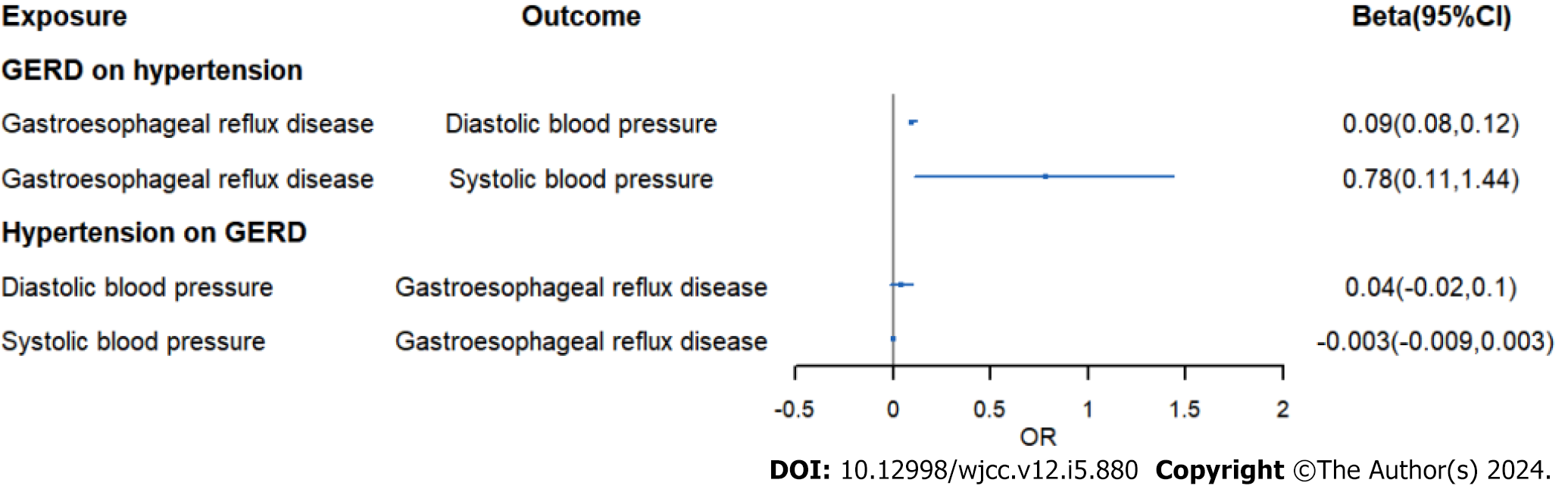
IVW analysis exhibited an increased risk of hypertension (OR = 1.46, 95%CI: 1.33-1.59, P = 2.14E-16) in GERD patients. And the same result was obtained in replication practice (OR = 1.002, 95%CI: 1.0008-1.003, P = 0.000498). Meanwhile, the IVW analysis showed an increased risk of systolic blood pressure (β = 0.78, 95%CI: 0.11-1.44, P = 0.021) and hypertensive heart disease (OR = 1.68, 95%CI: 1.36-2.08, P = 0.0000016) in GERD patients. Moreover, we found an decreased risk of Barrett's esophagus (OR = 0.91, 95%CI: 0.83-0.99, P = 0.043) in essential hypertension patients.
We found that GERD would increase the risk of essential hypertension, which provided a novel prevent and therapeutic perspectives of essential hypertension.
Core Tip: This study used a method of bidirectional Mendelian randomization, and its results highlighted that gastroesophageal reflux disease (GERD) was positively associated with the risk of essential hypertension, suggesting a new prevent strategy and therapeutic perspectives of essential hypertension in patients with GERD.
- Citation: Wei N, Liu MH, Song YH. Causal associations between gastroesophageal reflux disease and essential hypertension: A bidirectional Mendelian randomization study. World J Clin Cases 2024; 12(5): 880-890
- URL: https://www.wjgnet.com/2307-8960/full/v12/i5/880.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i5.880
Gastroesophageal reflux disease (GERD) is a disease in which gastric acid, bile acids and other gastric contents reflux into the esophagus for etiologies like hiatal hernia or abnormal movement of the lower esophagus[1,2]. Even in East Asia, where the prevalence is relatively low, GERD has an prevalence of 5%-10%, while in Europe and the United States, that could be as high as 15%-30%[3-5]. Gastroesophageal reflux can not only lead to esophagitis, Barrett's esophagus (BE), but also a risk factor for esophageal cancer. GERD is also closely linked to heart disease[6]. A Mendelian randomized study showed that GERD can lead to heart diseases such as myocardial infarction and atrial fibrillation[7]. As another common disease, essential hypertension can damage the heart, kidneys, and increase the risk of cerebral hemorrhage, but the cause of essential hypertension remains unclear[8].
Previous clinical studies showed that patients with GERD may have a higher prevalence of essential hypertension, but the results might be influenced by sample size and potentially confounding factors such as lifestyle, socioeconomic status, and underlying medical conditions, and that conclusions may not be accurate[9-11]. There were a few studies on this topic and little attention was paid. Mendelian randomization (MR) is an increasingly popular clinical research method that applies instrumental variable (IV) techniques to assess causal relationships between risk factors and complex human characteristics[12,13]. For exposed IV randomly assigned during conception and was not affected by disease state, MR studies can rule out the influence of confounding factors and reverse causation on causation between exposure and outcome[14].
Our study used the MR method to investigate the causal role of GERD and BE in the development of essential hypertension, and then studied the relationship between GERD and hypertensive heart failure, and further explored the protective effect of gastroesophageal reflux treatment on essential hypertension.
In order to examine the causal connection between GERD/BE and essential hypertension, we used data from two different genome-wide association studies (GWAS) to perform this MR analysis. Data of GERD and BE were obtained from the largest and latest GWAS conducted by Ong et al[15]. They applied multitrait GWAS models combining 129080 cases and 473524 controls to identify risk loci of GERD and BE. GERD and BE cases were defined through the International Classification of Disease, tenth version code [for GERD Multi-trait Analysis of GWAS (MTAG)] and confirmed BE diagnosis pathologically (for BE MTAG).
GWAS of essential hypertension (55917 cases and 162837 controls), hypertensive heart disease (3938 cases and 162837 controls), and hypertensive heart and/or renal disease (4363 cases and 162837 controls) were obtained from FinnGen R7 study. Summary statistics for replication practice of essential hypertension (1237 cases and 359957 controls) and diastolic blood pressure (436424 individuals) were obtained from the United Kingdom Biobank. Summary statistics for systolic blood pressure (97656 individuals) were obtained from the IEU study in 2022.
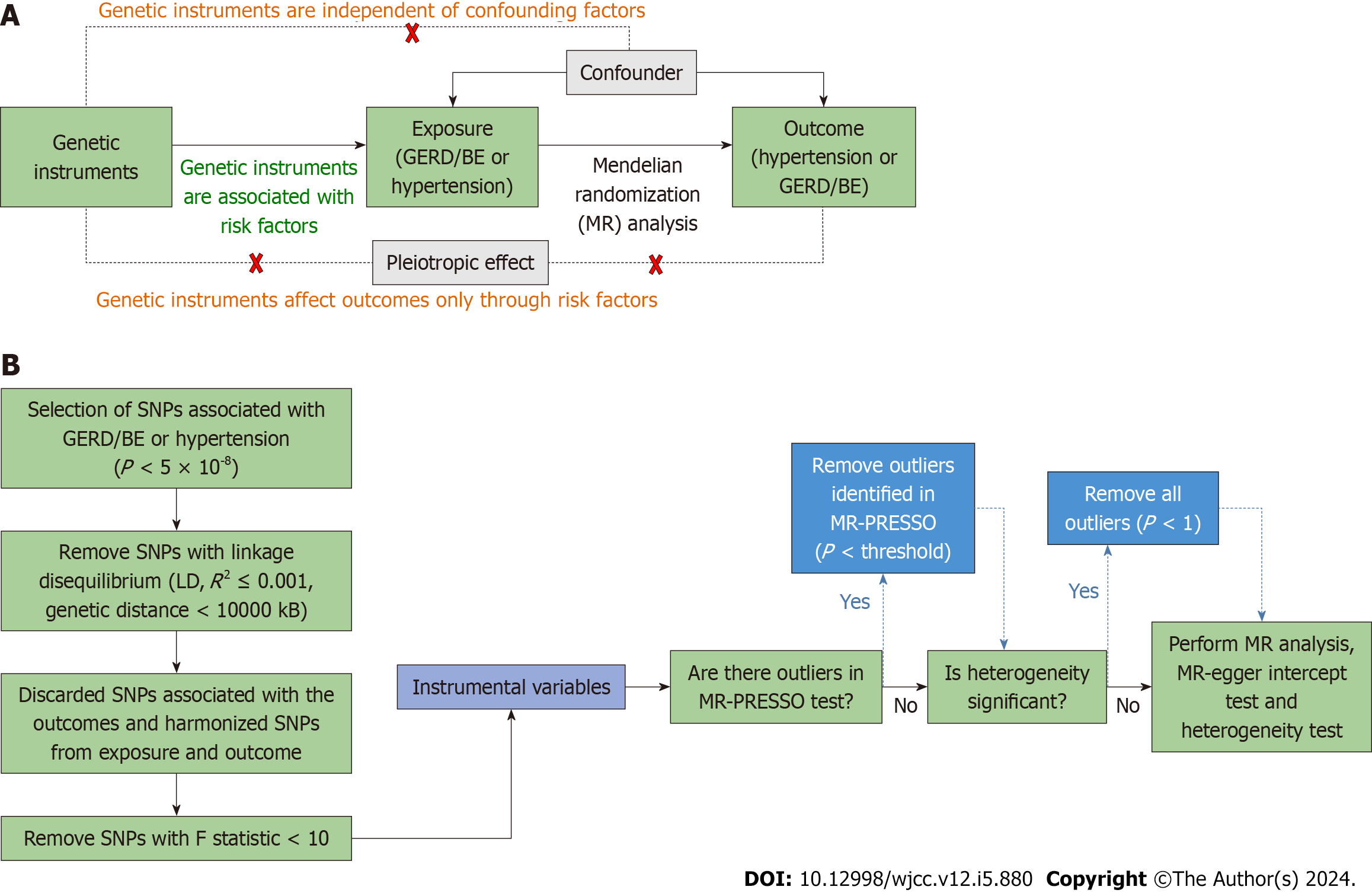
Schematic diagram of the bidirectional MR study on the causal relationship between GERD and hypertension was shown in Figure 1. In our study, we firstly performed MR analysis with all eligible single nucleotide polymorphisms (SNPs). The outlier variants were eliminated if the MR-Pleiotropy RESidual Sum and Outlier (MR-PRESSO) analysis identified a significant horizontal pleiotropy (with a P value smaller than the cutoff in the MR-PRESSO outlier test). After detecting heterogeneity with Cochran's Q test, we eliminated all the SNPs whose P value in the MR-PRESSO outlier test was less than 1 if the heterogeneity was still significant. At last, we performed MR PRESSO and Cochran's Q test again, MR analysis, “leave-one-out” sensitivity analysis and MR-Egger intercept test to draw the conclusion with caution.
SNPs are used in MR analyses to assess the causal relationship; the SNPs chosen should meet three key assumptions: (1) Genetic instruments predict the exposure (P < 5 × 10-8); (2) genetic instruments are not associated with potential confounders; and (3) genetic instruments affect the outcome only through the exposure[16]. We undertook a number of procedures to choose eligible SNPs.
First, P < 5 × 10-8, linkage disequilibrium (R2 ≤ 0.001), Hardy-Weinberg equilibrium, and genetic distance 10000 kB were necessary for SNPs related with GERD/BE. The effect alleles, allele frequencies, P values, SEs, and P values for each SNP were then gathered. The exposure SNPs were then retrieved from the selected outcome data, and SNPs that were substantially (P < 5 × 10-8) linked with the outcomes were excluded. Thirdly, the palindromic and incompatible SNPs were deleted while harmonizing the exposure and result SNPs to maintain the concordance of the effect alleles. The F-statistic was determined in order to avoid bias brought on by weak proxies, although no IV had a F statistic of less
In this investigation, various techniques were utilized to determine whether there was a causal relationship between GERD/BE and essential hypertension. These techniques included inverse variance weighted (IVW), weighted median (WM), and MR-Egger regression. For SNPs, which showed the greatest power but was subject to biases, IVW computed a weighted average of the Wald ratio on the premise that all the instruments were valid[18]. Because the random-effect model maintains conservative estimates even when heterogeneity is identified, it was used in this work for IVW. When at least half of the IVs were valid, WM investigated the median effects of all instrumental SNPs, which made it harder to create biases[19]. Independent of the validity of IVs, the MR-Egger regression model yielded a reasonably reliable estimate. But the MR-Egger approach was susceptible to being influenced by outliers[20].
In this study, the Cochran's Q test P value was utilized to determine whether there was heterogeneity in the MR analysis. When P ≥ 0.05, it was decided that there was no heterogeneity in the analysis. A symmetry plot showed that there was no heterogeneity, and the funnel plot was also utilized to find it.
Pleiotropy was discovered using the intercept term in MR-Egger regression and MR-PRESSO[21]. The MR-Egger intercept test with P < 0.05 indicated the existence of directional horizontal pleiotropy[22]. The MR-PRESSO analysis detected and attempted to reduce horizontal pleiotropy by removing significant outliers. Global test in MR-PRESSO with P < 0.05 indicated the existence of horizontal pleiotropy and outlier test P value was used to correct the results, which can eliminate horizontal pleiotropy by removing outlier SNPs. The total effect of each remaining SNP was also estimated using the leave-one-out method in order to evaluate the impact of each SNP. All statistical tests were performed by the “TwoSampleMR” package for the R program (version 4.2.1).
We used publicly accessible GWAS summary data or published trial data for our analyses. For this manuscript, no original data were gathered, and no ethics committee permission was needed. The institutional ethics review committees for each of the included studies gave their approval, and all participants gave their written informed permission.
As shown in Table 1, the result of IVW demonstrated that the strong causal link of GERD and essential hypertension
| Exposure | Outcome | Step | Nsnp | IVW | WM | MR-Egger | ||||||
| OR or beta | 95%CI | P value | OR or beta | 95%CI | P value | OR or beta | 95%CI | P value | ||||
| Gastroesophageal reflux disease | Essential hypertension | 3 | 69 | 1.46 | 1.33, 1.59 | 2.14E-16 | 1.34 | 1.19, 1.50 | 6.80E-07 | 2.073 | 1.23, 3.50 | 0.0082 |
| Duplicate essential hypertension | 1 | 77 | 1.002 | 1.0008, 1.003 | 4.98E-04 | 1.0013 | 0.9998, 1.0028 | 0.084 | 1.0018 | 0.996, 1.0076 | 0.54 | |
| Diastolic blood pressure1 | 3 | 58 | 0.09 | 0.08, 0.12 | 1.2E-17 | 0.095 | 0.066, 0.12 | 7.8E-11 | 0.034 | -0.12, 0.19 | 0.66 | |
| Systolic blood pressure1 | 3 | 61 | 0.78 | 0.11, 1.44 | 0.021 | 0.59 | -0.36, 1.53 | 0.23 | 4.42 | 0.28, 8.55 | 0.04 | |
| Hypertensive heart disease | 1 | 75 | 1.68 | 1.36, 2.08 | 1.60E-06 | 1.82 | 1.38, 2.42 | 2.90E-05 | 2.99 | 0.85, 10.48 | 0.09 | |
| Hypertensive heart and/or renal disease | 2 | 73 | 1.61 | 1.33, 1.94 | 1.00E-06 | 1.72 | 1.31, 2.26 | 8.91772E-05 | 2.89 | 0.96, 8.75 | 0.064 | |
| Barret's esophagus | Essential hypertension | 1 | 16 | 1.00006 | 0.9993, 1.0008 | 0.88 | 1.000033 | 0.999, 1.001 | 0.95 | 1.002 | 0.997, 1.0067 | 0.4 |
| Duplicate essentialhypertension | 1 | 16 | 1.05 | 1.0004, 1.11 | 0.048 | 1.078 | 1.001, 1.16 | 0.046 | 1.1 | 0.75, 1.61 | 0.62 | |
Moreover, we assessed causal relationship of GERD and blood pressure. The IVW analysis exhibited an increased risk of systolic blood pressure in GWRD patients (β = 0.78, 95%CI: 0.11-1.44, P = 0.021) and an increased risk of diastolic blood pressure in GWRD patients (β = 0.09, 95%CI: 0.08-0.12, P = 1.2E-17), but heterogeneity and horizontal pleiotropy were detected in diastolic blood pressure, making the result doubtful. Meanwhile, the IVW analysis exhibited an increased risk of hypertensive heart disease (OR = 1.68, 95%CI: 1.36-2.08, P = 0.0000016) in GERD patients and an increased risk of hypertensive heart and/or renal disease in GERD patients (OR = 1.61, 95%CI: 1.33-1.94, P = 0.000001), indicating a strong causal relationship between GERD and hypertensive heart/renal disease.
As the results mentioned above, we could conclude the causal effect of genetically predicted GERD on hypertension, hypertensive heart/renal disease, and systolic blood pressure.
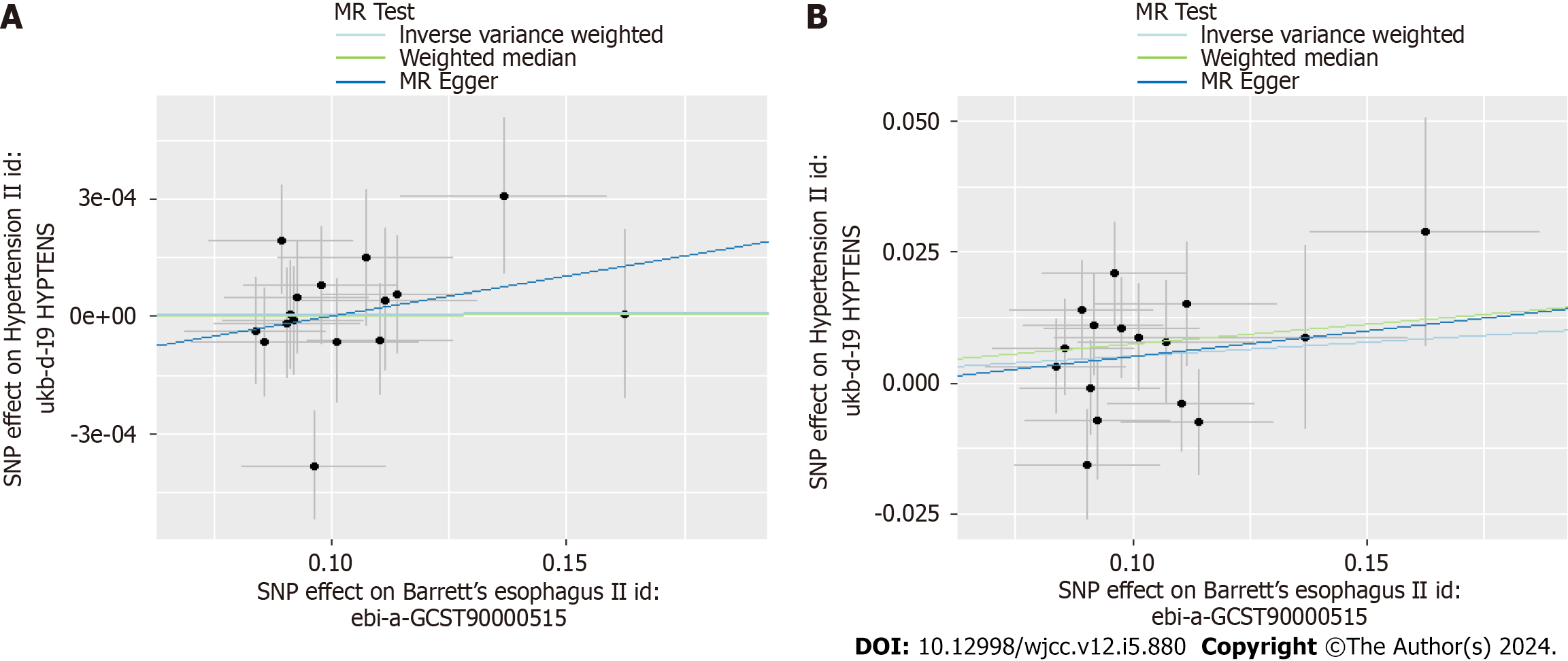
The results of Table 1 showed no causal link of BE and essential hypertension (OR = 1.000058, 95%CI: 0.9993-1.00079, P = 0.88). However, in replication practice, there was strong causal link of BE and essential hypertension (OR = 1.054,
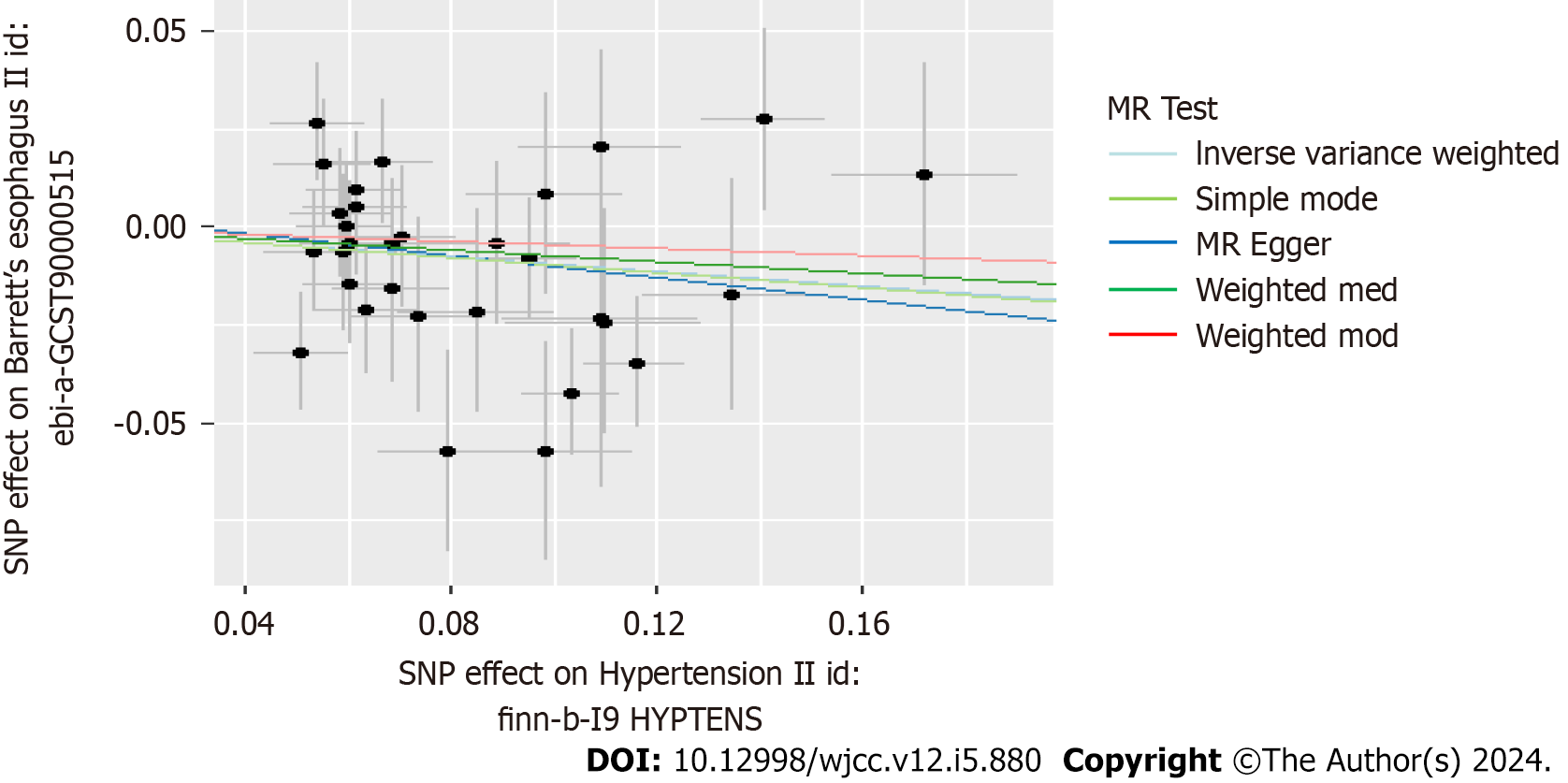
Scatter plots were used to display the individual SNP effects and combined effects from each MR approach for each outcome database (Figures 2-5).
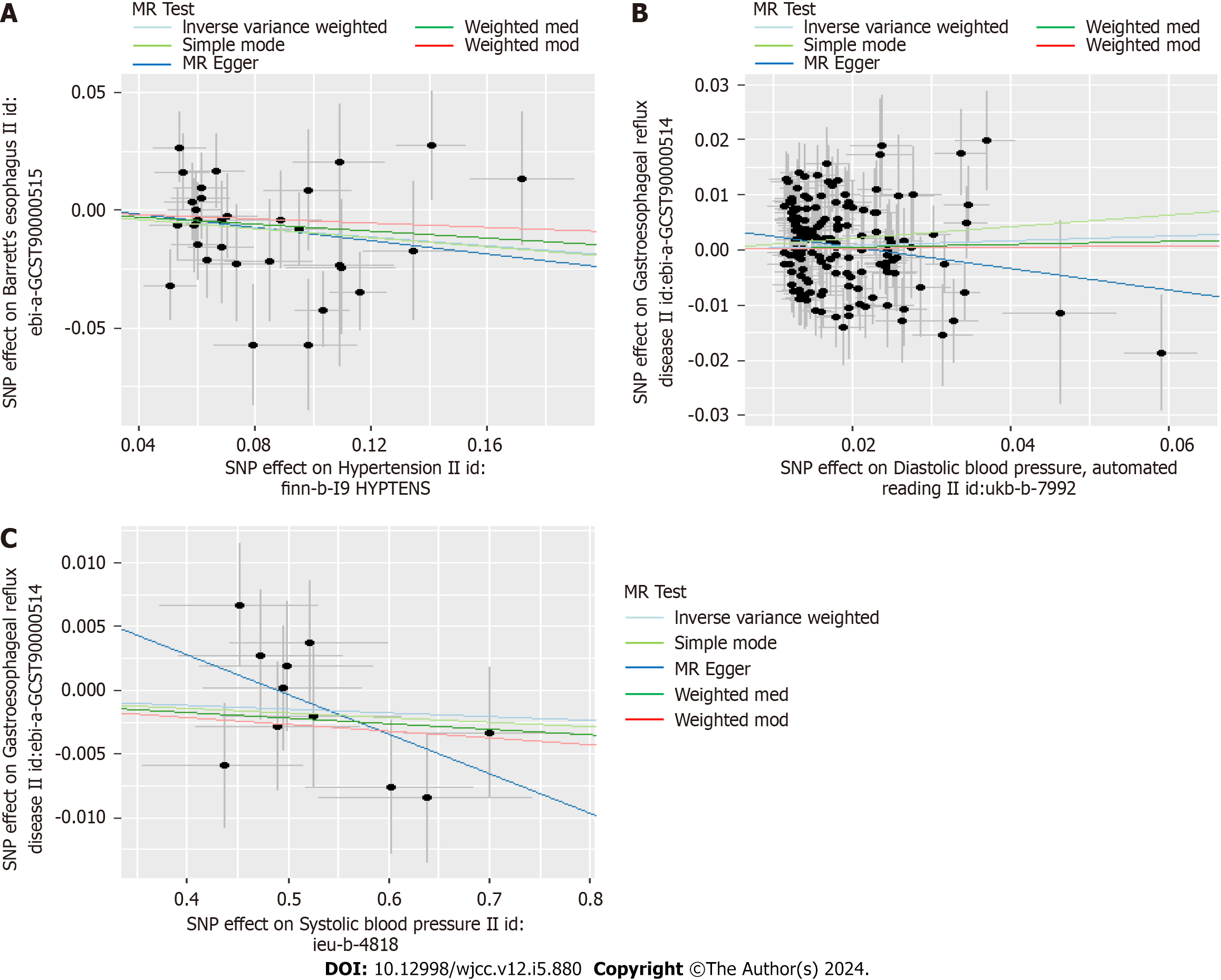
In Table 2, we displayed the relationship between hypertension and GERD/BE and the credibility of results was judged using heterogeneity test and pleiotropy test (Supplementary Table 2). There was no causal relationship between essential hypertension and GERD (OR = 1.02, 95%CI: 0.98-1.05, P = 0.344) (Figure 6). Similarly, diastolic blood pressure and systolic blood pressure are not related to the prevalence rate of GERD, with IVW as (β = 0.04, 95%CI: -0.02-0.1, P = 0.179) and (β = -0.003, 95%CI: -0.009-0.003, P = 0.311) respectively (Figure 7). However, we found an decreased risk of BE (OR = 0.91, 95%CI: 0.83-0.99, P = 0.043) in essential hypertension patients, and there were no heterogeneity or horizontal pleiotropy, proving the reliability of this result.
| Exposure | Outcome | Step | Nsnp | IVW | WM | MR-Egger | ||||||
| OR or beta | 95%CI | P value | OR or beta | 95%CI | P value | OR or beta | 95%CI | P value | ||||
| Duplicate essential hypertension | Gastroesophageal reflux disease | 3 | 31 | 1.015 | 0.98, 1.05 | 0.344 | 1.027 | 0.99, 1.07 | 0.202 | 1.038 | 0.94, 1.15 | 0.471 |
| Diastolic blood pressure1 | 3 | 154 | 0.042 | -0.02, 0.104 | 0.179 | 0.026 | -0.05, 0.103 | 0.518 | -0.194 | -0.39, 0.003 | 0.056 | |
| Systolic blood pressure1 | 3 | 11 | -0.003 | -0.009, 0.003 | 0.311 | -0.004 | -0.01, 0.003 | 0.274 | -0.031 | -0.07, 0.007 | 0.148 | |
| essentialhypertension | Barret's esophagus | 3 | 31 | 0.911 | 0.83, 0.997 | 0.043 | 0.929 | 0.82, 1.05 | 0.254 | 0.869 | 0.65, 1.16 | 0.345 |
Funnel plots indicated the locations of each outcome's heterogeneity, and leave-one-out plots revealed that the relationships were unlikely to be caused by specific extreme SNPs (Supplementary Figures 1-4).
Clinical and mendelian randomized studies had shown that gastroesophageal reflux was a risk factor for heart diseases such as atrial fibrillation and coronary heart disease[9,23-25]. Proton-pump inhibitors (PPI) used to treat gastroesophageal reflux may also relieve pain due to cardiovascular disease[26,27]. The β-blockers used to treat hypertension can also reduce the tone of the lower esophageal sphincter while lowering blood pressure, resulting in aggravation of gastroesophageal reflux symptoms in some hypertensive patients at the beginning of medication[28].
The prevalence of GERD in East Asia is low, ranging from 5 to 10 percent[29]. However, after studying some populations in central China, Li et al[27] found that 44.2% (38/86) of essential hypertensive patients had gastroesophageal reflux. Suyu et al[11] also found that the proportion of patients with hypertension with GERD was as high as 31.4% (137/436). Our findings clearly suggest that gastroesophageal reflux can lead to elevated blood pressure and essential hypertension.
Gudlaugsdottir et al[30] concluded that hypertension was more prevalent in patients with BE (OR = 5.1, P < 0.0001) and also had a higher prevalence in patients with reflux esophagitis (OR = 3.8, P < 0.001). But our study did not clarify the role of BE in hypertension. PPI therapy, anti-reflux mucosectomy (ARMS), and fundoplication are other treatments for gastroesophageal reflux, which may play a protective role against hypertension by relieving gastroesophageal reflux[31]. Some clinical studies have found that the hypertension was well controlled in some patients after the treatment of gastroesophageal reflux by fundoplication[10]. We were failed to determine the possible protective effects of PPI/ARMS/fundoplication on hypertension due to insufficient SNP/databases. In addition, our study suggested that gastroesophageal reflux can also lead to hypertensive heart failure.
The anterior wall of the esophagus is closely adjacent to the posterior wall of the heart, and the autonomic nerves of the esophagus and heart also overlap and cross[32,33]. Some studies believe that the presence of gastroesophageal reflux is often accompanied by pain, which would stimulate the patient's sympathetic nerve excitation, resulting in increased blood pressure[34]. In addition, gastroesophageal reflux can lead to arrhythmias, and arrhythmias such as bradycardia can also lead to hypertension[35,36]. Gastroesophageal reflux may also cause hypertension by affecting the level of mediators in plasma that regulate hypertension. Some studies found that plasma concentrations of nitric oxide metabolites increased significantly after 8 wk of inhibition of gastric acid secretion[37,38].
Several limitations should be considered in our MR analysis. Firstly, the summary GWAS data only concern individuals of European, so results may not be representative of the whole population. Secondly, although we took steps to exclude outlier SNPs, horizontal pleiotropy and heterogeneity still exited in our analysis. However, we used different methods to draw a conclusion to eliminate the impact of pleiotropy and heterogeneity.
Gastroesophageal reflux can lead to increased blood pressure, hypertension, and hypertensive heart failure. Patients with essential hypertension should be examined and treated for gastroesophageal reflux, and patients with gastroesophageal reflux should also be monitored for hypertension.
Some clinical studies have suggested that gastroesophageal reflux disease (GERD) may have a causal relationship with essential hypertension, but the relevant conclusions may be affected by confounding factors and small sample sizes.
Determining the causal relationship between GERD and essential hypertension could provide new perspectives for the treatment of patients with GERD and hypertension.
We would perform a bidirectional Mendelian randomization (MR) analysis to investigate the causal link between GERD and essential hypertension.
A series of steps were conducted to select eligible single nucleotide polymorphisms, and inverse variance weighted (IVW), weighted median and MR egger regression were used to examine whether there was a causal association between GERD and hypertension.
IVW analysis exhibited an increased risk of hypertension (OR = 1.46, 95%CI: 1.33-1.59, P = 2.14E-16) in GERD patients. Meanwhile, the IVW analysis showed an increased risk of systolic blood pressure and hypertensive heart disease in GERD patients.
GERD was positively associated with the risk of essential hypertension, suggesting a new prevent strategy and therapeutic perspectives of essential hypertension in patients with GERD.
The specific mechanisms associated with GERD and essential hypertension need to be further clarified.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kreisel W, Germany; Skrypnyk I, Ukraine S-Editor: Qu XL L-Editor: A P-Editor: Zheng XM
| 1. | Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Zheng Z, Shang Y, Wang N, Liu X, Xin C, Yan X, Zhai Y, Yin J, Zhang J, Zhang Z. Current Advancement on the Dynamic Mechanism of Gastroesophageal Reflux Disease. Int J Biol Sci. 2021;17:4154-4164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal Reflux Disease. JAMA. 2020;324:2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 4. | Sandhu DS, Fass R. Current Trends in the Management of Gastroesophageal Reflux Disease. Gut Liver. 2018;12:7-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1264] [Article Influence: 114.9] [Reference Citation Analysis (2)] |
| 6. | Clarrett DM, Hachem C. Gastroesophageal Reflux Disease (GERD). Mo Med. 2018;115:214-218. [PubMed] |
| 7. | Sun X, Chen L, Zheng L. A Mendelian randomization study to assess the genetic liability of gastroesophageal reflux disease for cardiovascular diseases and risk factors. Hum Mol Genet. 2022;31:4275-4285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 8. | Elliott WJ. Systemic hypertension. Curr Probl Cardiol. 2007;32:201-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Chen CH, Lin CL, Kao CH. Association between gastroesophageal reflux disease and coronary heart disease: A nationwide population-based analysis. Medicine (Baltimore). 2016;95:e4089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Hu Z, Chen M, Wu J, Song Q, Yan C, Du X, Wang Z. Improved control of hypertension following laparoscopic fundoplication for gastroesophageal reflux disease. Front Med. 2017;11:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Suyu H, Liu Y, Jianyu X, Luo G, Cao L, Long X. Prevalence and Predictors of Silent Gastroesophageal Reflux Disease in Patients with Hypertension. Gastroenterol Res Pract. 2018;2018:7242917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89-R98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2280] [Cited by in RCA: 2911] [Article Influence: 264.6] [Reference Citation Analysis (0)] |
| 13. | Birney E. Mendelian Randomization. Cold Spring Harb Perspect Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 352] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 14. | Sekula P, Del Greco M F, Pattaro C, Köttgen A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol. 2016;27:3253-3265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 1398] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 15. | Ong JS, An J, Han X, Law MH, Nandakumar P; 23andMe Research team; Esophageal cancer consortium, Schumacher J, Gockel I, Bohmer A, Jankowski J, Palles C, Olsen CM, Neale RE, Fitzgerald R, Thrift AP, Vaughan TL, Buas MF, Hinds DA, Gharahkhani P, Kendall BJ, MacGregor S. Multitrait genetic association analysis identifies 50 new risk loci for gastro-oesophageal reflux, seven new loci for Barrett's oesophagus and provides insights into clinical heterogeneity in reflux diagnosis. Gut. 2022;71:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 16. | Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. 2015;44:496-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 380] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 17. | Burgess S, Thompson SG; CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2753] [Cited by in RCA: 2330] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 18. | Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 3808] [Article Influence: 317.3] [Reference Citation Analysis (1)] |
| 19. | Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4015] [Cited by in RCA: 5723] [Article Influence: 635.9] [Reference Citation Analysis (0)] |
| 20. | Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 2880] [Article Influence: 360.0] [Reference Citation Analysis (0)] |
| 21. | Verbanck M, Chen CY, Neale B, Do R. Publisher Correction: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 22. | Burgess S, Thompson SG. Erratum to: Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:391-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Mohamed A, Ochoa Crespo D, Kaur G, Ashraf I, Peck MM, Maram R, Malik BH. Gastroesophageal Reflux and Its Association With Atrial Fibrillation: A Traditional Review. Cureus. 2020;12:e10387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Maruyama T, Fukata M, Akashi K. Association of atrial fibrillation and gastroesophageal reflux disease: Natural and therapeutic linkage of the two common diseases. J Arrhythm. 2019;35:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Chang CS, Chen HJ, Liao CH. Patients with Cerebral Stroke Have an Increased Risk of Gastroesophageal Reflux Disease: A Population-Based Cohort Study. J Stroke Cerebrovasc Dis. 2018;27:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Dong H, Li X, Cai M, Zhang C, Mao W, Wang Y, Xu Q, Chen M, Wang L, Huang X. Integrated bioinformatic analysis reveals the underlying molecular mechanism of and potential drugs for pulmonary arterial hypertension. Aging (Albany NY). 2021;13:14234-14257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Li ZT, Ji F, Han XW, Wang L, Yue YQ, Wang ZG. The Role of Gastroesophageal Reflux in Provoking High Blood Pressure Episodes in Patients With Hypertension. J Clin Gastroenterol. 2018;52:685-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Lazebnik LB, Komissarenko IA, Mikheeva OM. [Cardiovascular pathology associated with digestive system diseases]. Eksp Klin Gastroenterol. 2011;69-74. [PubMed] |
| 29. | Zhang D, Liu S, Li Z, Wang R. Global, regional and national burden of gastroesophageal reflux disease, 1990-2019: update from the GBD 2019 study. Ann Med. 2022;54:1372-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 30. | Gudlaugsdottir S, Verschuren W, Dees J, Stijnen T, Wilson J. Hypertension is frequently present in patients with reflux esophagitis or Barrett's esophagus but not in those with non-ulcer dyspepsia. Eur J Intern Med. 2002;13:369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 32. | Sánchez-Quintana D, Cabrera JA, Climent V, Farré J, Mendonça MC, Ho SY. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation. 2005;112:1400-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Celebi OO, Celebi S, Aydogdu S. A dangerous and risky relationship: Esophagus and left atrium. Pacing Clin Electrophysiol. 2019;42:568-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Blackshaw LA, Haupt JA, Omari T, Dent J. Vagal and sympathetic influences on the ferret lower oesophageal sphincter. J Auton Nerv Syst. 1997;66:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Bayés-Genís A, Guindo J, Viñolas X, Tomás L, Elosua R, Duran I, Bayés de Luna A. Cardiac arrhythmias and left ventricular hypertrophy in systemic hypertension and their influences on prognosis. Am J Cardiol. 1995;76:54D-59D. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Afzal MR, Savona S, Mohamed O, Mohamed-Osman A, Kalbfleisch SJ. Hypertension and Arrhythmias. Heart Fail Clin. 2019;15:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Kato S, Kitamura M, Korolkiewicz RP, Takeuchi K. Role of nitric oxide in regulation of gastric acid secretion in rats: effects of NO donors and NO synthase inhibitor. Br J Pharmacol. 1998;123:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Takeuchi K, Sugamoto S, Yamamoto H, Kawauchi S, Tashima K. Interactive roles of endogenous prostaglandin and nitric oxide in regulation of acid secretion by damaged rat stomachs. Aliment Pharmacol Ther. 2000;14 Suppl 1:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (1)] |