Published online Feb 16, 2024. doi: 10.12998/wjcc.v12.i5.1004
Peer-review started: October 27, 2023
First decision: November 28, 2023
Revised: December 27, 2023
Accepted: January 17, 2024
Article in press: January 17, 2024
Published online: February 16, 2024
Processing time: 95 Days and 17.5 Hours
Non-ketotic hyperglycaemic (NKH) seizures are a rare neurological complication of diabetes caused by hyperglycaemia in non-ketotic and non-hyperosmotic states. The clinical characteristics of NKH seizures are atypical and lack unified diagnostic criteria, leading to potential misdiagnoses in the early stages of the disease.
This report presents a rare case of NKH seizures in a 52-year-old male patient with a history of type 2 diabetes mellitus. We performed comprehensive magnetic resonance imaging (MRI) studies at admission, 12 d post-admission, and 20 d post-discharge. The imaging techniques included contrast-enhanced head MRI, T2-weighted imaging (T2WI), fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging, susceptibility-weighted imaging, magnetic reso
This case study provides valuable insights into the potential pathogenesis, diagnosis, and treatment of NKH seizures. The comprehensive MRI findings highlight the potential utility of various MRI sequences in diagnosing and characterizing NKH seizures.
Core Tip: This study presents a rare case of non-ketotic hyperglycaemic (NKH) seizures in a patient with type 2 diabetes. These seizures are a complication of diabetes, though they lack specific diagnostic criteria and are often misdiagnosed. In particular, the magnetic resonance imaging (MRI) findings of these manifestation have rarely been described in the literature, and previous reports are inconsistent. In this report, we describe the comprehensive findings in several MRI sequences, including T2-weighted images, fluid-attenuated inversion recovery, diffusion-weighted imaging, susceptibility-weighted imaging, and magnetic resonance spectroscopy and venography. We believe that our study makes a significant contribution to the literature because these findings can help elucidate the pathogenesis, diagnosis, and treatment of this rare condition. Further, we believe that this paper will be of interest to the readership of your journal because this case report expands the clinical knowledge on NKH seizures, a rare but severe complication of diabetes.
- Citation: Wu J, Feng H, Zhao Y, Li J, Li T, Li K. Neuroimaging features in a patient with non-ketotic hyperglycaemic seizures: A case report. World J Clin Cases 2024; 12(5): 1004-1009
- URL: https://www.wjgnet.com/2307-8960/full/v12/i5/1004.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i5.1004
Non-ketotic hyperglycaemia (NKH) is a rare clinical syndrome characterised by hyperglycaemia, serum hyperosmolarity, and intracellular dehydration with no ketoacidosis. NKH mainly affects patients with type 2 diabetes over 50 years of age and is often considered a complication of this metabolic disease in patients with poor glycaemic control or in undia
NKH seizures are associated with various neurological manifestations, including epilepsy, headache, consciousness and vision alterations, and movement disorders. Magnetic resonance imaging (MRI) is a valuable tool for the accurate diagnosis of NKH seizures; however, findings from head imaging studies are inconsistent. Some researchers have suggested that unilateral subcortical hypointensities in T2-weighted imaging (T2WI) or fluid-attenuated inversion recovery (FLAIR) sequences are a characteristic MRI feature of NKH seizures, whereas others did not observe these findings[2,3]. Other MRI features reported in the literature include subcortical hypointense shadows on susceptibility-weighted imaging (SWI), focal enhancement of the pia mater and local cortex, and in arterial spin labelling sequences, enhancement of focal cerebral parenchyma perfusion[3-5]. Nevertheless, more extensive MRI evidence with multiple sequences is necessary to characterise and comprehensively understand the pathogenesis of NKH seizures.
Here, we present a rare case of NKH seizures in a patient. We also review the comprehensive MRI characteristics of this patient, including contrast-enhanced head MRI, T2WI, FLAIR, diffusion-weighted imaging (DWI), SWI, magnetic resonance spectroscopy (MRS), and magnetic resonance venography (MRV).
Dizziness for three days, aggravated with general convulsions for two hours.
A 52-year-old man with a one-year history of type 2 diabetes mellitus developed dizziness of unknown aetiology, accompanied by lethargy, poor mental status, and delayed reactions. The patient self-administered ‘cold and flu capsules’, although the dizziness symptoms persisted. On the second day, the patient experienced weakness and numbness in the right upper limb during work, accompanied by dizziness. This right upper extremity symptom manifested as weakness/difficulty in fine movements, such as holding chopsticks, with conserved lifting ability. The upper extremity and dizziness symptoms were not accompanied by altered consciousness or convulsions and resolved spontaneously after approximately one hour.
Three days later, the patient presented to the emergency room with worsening symptoms. During the consultation, the patient was in and out of consciousness, with delayed and inappropriate responses, subsequent involuntary lifting of the right upper limb, flexion and tonicity of the limbs with clonus, mouth foaming, upward gaze, and clenched teeth for more than 10 min. The symptoms were relieved with an intramuscular injection of sodium phenobarbital. Upon regaining full consciousness, the patient was able to communicate and answer questions correctly.
The patient was admitted to the neurology department for further treatment. Admission vital signs and neurological examination were normal.
History of "diabetes mellitus" for 1 year, complaining of fair glycaemic control.
No special notes.
Admission vital signs and neurological examination were normal.
His admission blood glucose level was 329.4 mg/dL, and a cerebrospinal fluid sample showed a glucose level of 138.2 mg/dL, with negative tests for antibodies associated with autoimmune encephalitis and paraneoplastic syndrome. Blood glucose levels fluctuated between 129.6 and 351.0 mg/dL after admission.
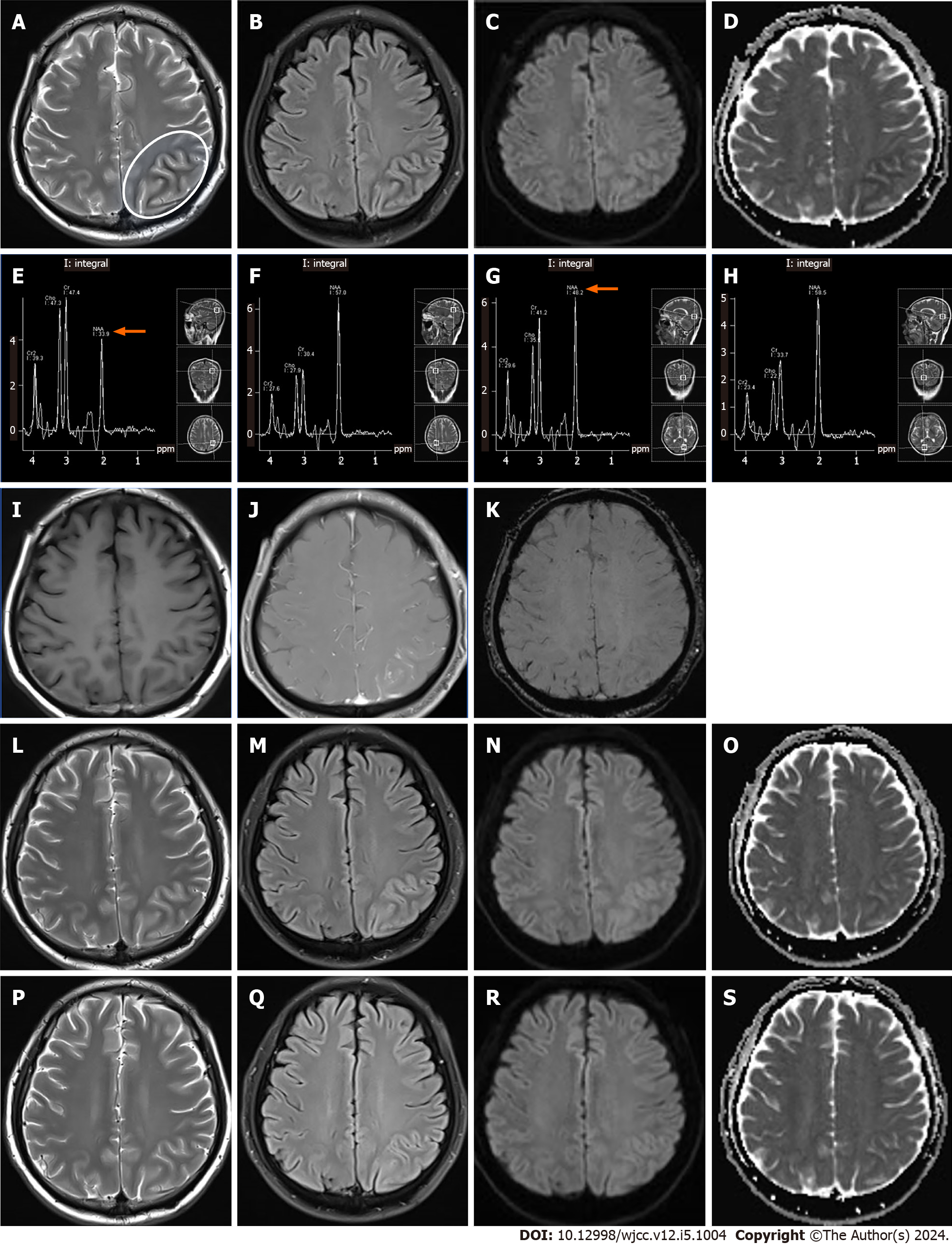
A head MRI at admission showed a slight hyperintensity in the left parieto-occipital cortex and a subcortical hypointense line in T2WI and FLAIR images (Figure 1A and B). DWI revealed a limited diffusion-restricted signal in the same area and a reduced apparent diffusion coefficient (ADC map) (Figure 1C and D). MRS revealed a reduced N-acetylaspartate (NAA) peak and increased creatine and choline peaks in the left parietal-occipital cortex and subcortical areas, suggesting neuronal damage and metabolic encephalopathy (Figure 1E–H). Contrast-enhanced MRI showed swelling of the left parieto-occipital gyrus and localized soft meningeal enhancement in the parietal lobe (Figure 1I and 1J). No significant alterations were noted on SWI (Figure 1K) and MRV. Non-ketotic hyperglycaemia-associated epilepsy is difficult to identify and is even misdiagnosed in the emergency medical setting. It is distinguished from major acute infarction stroke, reversible posterior encephalopathy syndrome, encephalitis, and meningitis by the richness of cranial nuclear magnetic sequence manifestations in conjunction with clinical test indices. On day 5 of hospitalization, an electroencephalogram (EEG) revealed bilateral spike emanations in the frontal and central regions, asynchronous left and right sides, and multiple slow-wave activities in the left parietal, occipital, and temporal regions. These EEG alterations were consistent with the cortical swelling seen on MRI.
Non-ketotic hyperglycaemia-associated epilepsy.
Following admission, the patient was treated with antiepileptic drugs via an intramuscular injection of 200 mg of phenobarbital sodium and antidiabetic therapy consisting of 10 mg of dapagliflozin taken orally once a day, 0.5 g of metformin hydrochloride extended-release tablets taken twice a day, and 4–6 IU of subcutaneous insulin given once a day in case of poor glycaemic control, as well as fluid supplementation to improve blood circulation.
MRI after 12 d of treatment showed alleviation of the subcortical hypointense T2 signal (Figure 1L). The hyperintensity observed on FLAIR images in the left parieto-occipital cortex was more pronounced than that seen on the MRI upon admission (Figure 1M). The performance of DWI and ADC did not change significantly from the images taken on admission (Figure 1N and O).
The patient did not experience any further seizures after admission and was discharged after 2 wk, continuing the diabetes treatment and blood glucose monitoring at home. A follow-up MRI 20 d later showed disappearance of the swelling and hyperintensity in the left parieto-occipital cortex and of the subcortical hypointensities seen on T2WI and FLAIR images (Figure 1P and Q).
Currently, no diagnostic criteria for NKH seizures have been established. Previous case studies show that blood glucose, osmolality, and haemoglobin A1c levels are often elevated in patients with NKH seizures. However, the degree of elevation varies from case to case, with the majority of patients having only moderate hyperglycaemia, without significant hyperosmolality, and not meeting the diagnostic criteria for hyperosmolar hyperglycaemia syndrome (plasma glucose level of ≥ 600 mg/dL, serum osmolality of ≥ 320 mOsm/kg)[6]. This suggests that prolonged hyperglycaemia may lead to seizures rather than extreme hyperglycaemia, which is associated with an acute seizure[7].
In the present case, MRS revealed a decrease in NAA and an increase in creatine and choline in the affected cortical and subcortical regions compared to the contralateral regions. To our knowledge, these findings represent the first MRS report of non-ketotic hyperglycaemia-related epileptic brain lesions. NAA is a biomarker of neuronal density and sur
Reversible subcortical T2WI/FLAIR hypointense areas are a specific manifestation of non-ketotic hyperglycaemia-related epilepsy, often attributed to iron deposition or free radicals[4]. The exact aetiology of this manifestation remains unclear; however, some patients present hypointensities in SWI sequences related to astrocyte dysfunction, which leads to iron deposition due to astrocyte involvement in the regulation of iron molecule inflow and outflow[9]. In the present case, no mineral-related hypointense alterations were found in the SWI sequence, suggesting that the short-term accumulation of free radicals due to axonal damage in excitatory neurones caused the lesions. This accumulation can cause T2 shortening effects, resulting in subcortical T2/FLAIR undersignalling[10].
Limited cortical diffusion signal and T2WI/FLAIR hyperintensities are common imaging signs of seizures. Decreased cortical diffusion signals reflect cytotoxic oedema, whereas T2WI/FLAIR cortical hyperintensity is associated with vasogenic oedema resulting from increased blood–brain barrier permeability. Vasogenic cerebral oedema is caused by increased capillary permeability due to damage and disruption of the blood–brain barrier, increased water exudation, and accumulation in the perivascular and intercellular spaces. The cerebral pia mater enhancement confirmed the altered permeability in the present case.
The current treatment of NKH seizures focuses on glycaemic control and maintaining stable blood glucose in an effort to prevent, reduce, and control seizures. However, the use of antiepileptic drugs remains controversial. Available case reports have found that most patients are poorly treated with antiepileptic drugs alone and that antiepileptic drugs, such as phenytoin sodium, cause insulin resistance and elevated blood glucose levels, leading to decreased antiepileptic effects and NKH-associated exacerbation of epilepsy[11]. The disease has a favourable prognosis; poor glycaemic control is the main cause of its recurrence. NKH seizures are difficult to recognize and can be misdiagnosed in the emergency medical setting due to a lack of awareness, making early and accurate recognition and appropriate treatment important.
We have reported a case of NHK-related seizures, focusing on detailed characteristics derived from various MRI sequences. This case study provides valuable insights into the pathogenesis, diagnostic processes, and potential treatment strategies for this rare condition. Our findings underscore the importance of comprehensive MRI analysis in managing complex neurological presentations associated with metabolic disorders, paving the way for future research in this field.
We are grateful to the patient and his family for their collaboration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tuncyurek O, Cyprus; Yarmahmoodi F, Iran S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
| 1. | Hennis A, Corbin D, Fraser H. Focal seizures and non-ketotic hyperglycaemia. J Neurol Neurosurg Psychiatry. 1992;55:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Licchetta L, Ferri L, Morsillo F, Faustini-Fustini M, Toni F, Pondrelli F, Nonino F, Bisulli F, Tinuper P. Clinical characterization of non-ketotic hyperglycemia-related seizures: A systematic review and individual participant data meta-analysis. Seizure. 2023;106:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 3. | Peddawad D. Epileptic manifestations, pathophysiology, and imaging characteristics of non-ketotic hyperglycaemia: a review of the literature and a report of two cases with irreversible cortical vision loss. J Int Med Res. 2022;50:3000605221081429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Liu CJ, Tsai HH, Ko KY, Lu CC, Yen RF. Ictal Phase Perfusion SPECT of Nonketotic Hyperglycemia-Induced Parieto-occipital Seizure. Clin Nucl Med. 2017;42:e67-e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Sekar S, Vinayagamani S, Thomas B, Kesavadas C. Arterial spin labeling hyperperfusion in seizures associated with non-ketotic hyperglycaemia: is it merely a post-ictal phenomenon? Neurol Sci. 2021;42:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:2739-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 7. | Hung WL, Hsieh PF, Lee YC, Chang MH. Occipital lobe seizures related to marked elevation of hemoglobin A1C: report of two cases. Seizure. 2010;19:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Najm IM, Wang Y, Hong SC, Lüders HO, Ng TC, Comair YG. Temporal changes in proton MRS metabolites after kainic acid-induced seizures in rat brain. Epilepsia. 1997;38:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Tsai JP, Sheu JJ, Hsieh KL. Unusual Magnetic Resonance Imaging Abnormality in Nonketotic Hyperglycemia - related Epilepsia Partialis Continua. Ann Indian Acad Neurol. 2018;21:225-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Paoletti M, Bacila A, Pichiecchio A, Farina LM, Rognone E, Cremascoli R, Fanucchi S, Manni R, Bastianello S. Atypical postictal transient subcortical T2 hypointensity in a newly diagnosed diabetic patient with seizures. Epileptic Disord. 2018;20:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Tosur M, Viau-Colindres J, Astudillo M, Redondo MJ, Lyons SK. Medication-induced hyperglycemia: pediatric perspective. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |