Published online Feb 6, 2024. doi: 10.12998/wjcc.v12.i4.688
Peer-review started: November 22, 2023
First decision: December 8, 2023
Revised: December 15, 2023
Accepted: January 8, 2024
Article in press: January 8, 2024
Published online: February 6, 2024
Processing time: 63 Days and 23.3 Hours
Cerebral ischemia-reperfusion is a process in which the blood supply to the brain is temporarily interrupted and subsequently restored. However, it is highly likely to lead to further aggravation of pathological damage to ischemic tissues or the nervous system., and has accordingly been a focus of extensive clinical research. As a traditional Chinese medicinal formulation, Sanhua Decoction has gradually gained importance in the treatment of cerebrovascular diseases. Its main constituents include Citrus aurantium, Magnolia officinalis, rhubarb, and Qiangwu, which are primarily used to regulate qi. In the treatment of neurological diseases, the therapeutic effects of the Sanhua Decoction are mediated via different pathways, including antioxidant, anti-inflammatory, and neurotransmitter regu
Core Tip: As a traditional Chinese medicinal formulation, Sanhua Decoction are mediated via different pathways, including antioxidant, anti-inflammatory, and neurotransmitter regulatory pathways, as well as through the protection of nerve cells and a reduction in cerebral edema. In this paper, we describe the pathogenesis of cerebral ischemia-reperfusion injury and review the current status of its treatment to examine the therapeutic mechanisms of action of the Sanhua Decoction.
- Citation: Wang YK, Lin H, Wang SR, Bian RT, Tong Y, Zhang WT, Cui YL. Application and mechanisms of Sanhua Decoction in the treatment of cerebral ischemia-reperfusion injury. World J Clin Cases 2024; 12(4): 688-699
- URL: https://www.wjgnet.com/2307-8960/full/v12/i4/688.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i4.688
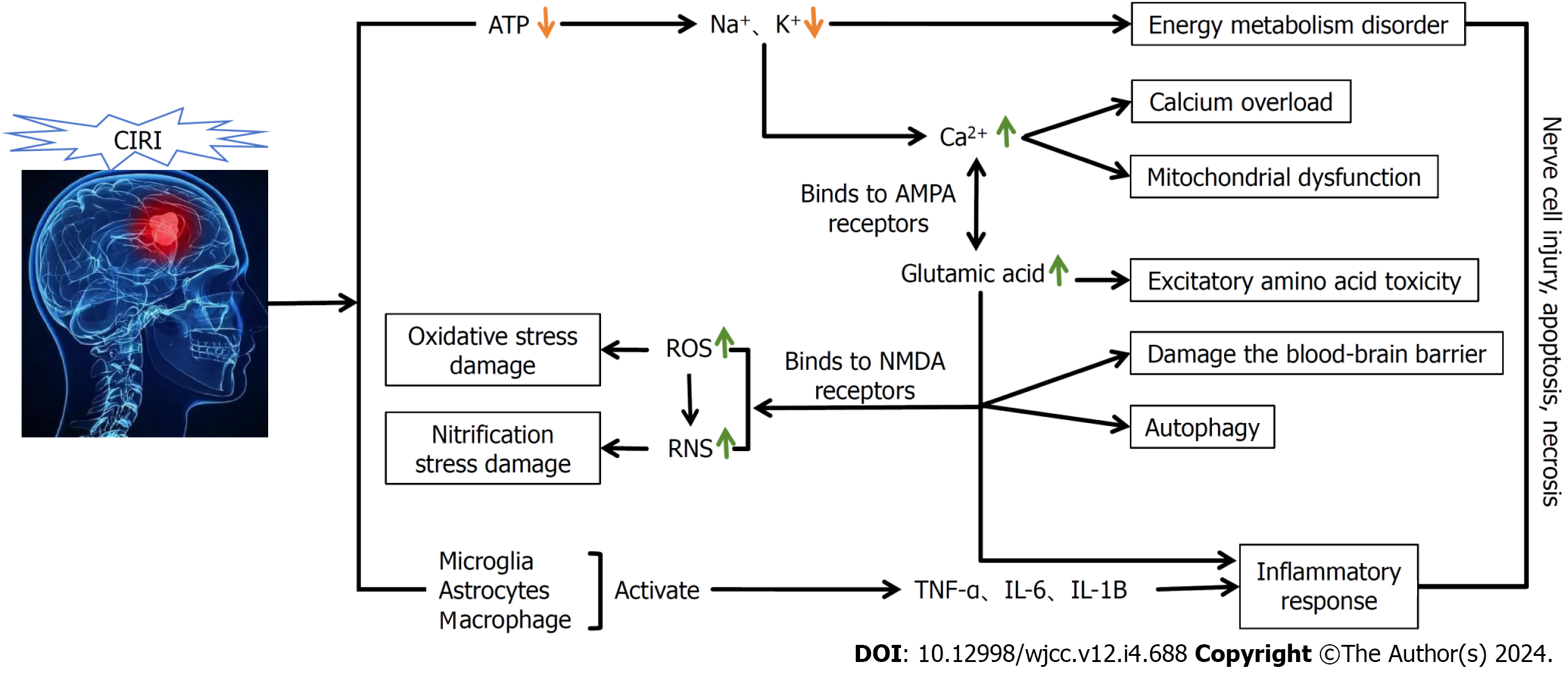
Cerebrovascular diseases are relatively commonly encountered in clinical practice and have clear harmful effects on human health and safety. Among these diseases, the most common, cerebral ischemia, is associated with high rates of morbidity and disability. The disease is mainly caused by vascular embolism, resulting in reduced cerebral blood flow and insufficient supplies of blood and oxygen to the brain tissue, resulting in damage. Typically, the duration of this process and the degree of brain damage in patients show a certain positive correlation[1]. In clinical practice, treatment of this disease is often based on thrombolysis or mechanical recanalization, which are performed to restore blood reperfusion to the ischemic area as soon as possible. Cerebral ischemic reperfusion is the process whereby the blood supply to the brain is restored after a temporary interruption. However, this process often leads to further aggravation of pathological damage to ischemic tissues or the nervous system, thereby contributing to an exacerbation of clinical symptoms and, to a certain extent, cerebral ischemia-reperfusion injury. The primary pathogenesis of this disease involves impaired energy metabolism, inflammatory responses, and the production of oxidative free radicals (Figure 1). According to epidemiological reports, the incidence of cerebral ischemia-reperfusion injury is increasing annually worldwide, and the probability of patients developing this type of injury increases significantly with age[2]. Moreover, patients are often diagnosed with one or more underlying diseases at the onset of the disease, which can also contribute to the complexity of clinical treatment. Consequently, the identification of effective treatment modalities for cerebral ischemia-reperfusion has become a key focus in clinical research.
In Chinese medicinal theory, cerebral ischemia-reperfusion injury is classified in the category “stroke,” which is mainly caused by a decline of qi and blood, and the blockage of cerebral orifices. The Sanhua Decoction is a traditional Chinese medicinal formulation, which has the function of passing qi and blood, regulating qi, and opening the metaphysical system. It is used for treating stroke that enters the viscera; evil qi is solid inside, and the heat is extremely strong[3]. This formulation is derived from “Suwen Zhiqi Qi Yi Baosheng Ji” which consists of four Chinese medicines, including rhubarb and Houpu. The Sanhua Decoction is widely used clinically, particularly for acute cerebral hemorrhage, hemorrhagic stroke, and other diseases, with significant therapeutic effects. Furthermore, Yang et al[4] have confirmed that the Sanhua Decoction has a better protective effect against neurological diseases, and on the basis of network pharmacological analysis, these authors found that the treatment of stroke with the Sanhua Decoction is closely associated with its anti-inflammatory effects. In addition, Gou et al[5] have found that intervention with the Sanhua Decoction can significantly ameliorate the abnormal changes in saturated fatty acid, monounsaturated fatty acid, and trans-unsaturated fatty acid contents in the serum of rats with ischemic stroke and restore the disordered fatty acid profiles, thereby effectively ameliorating fatty acid metabolism disorders in rats.
In this paper, with a view toward clarifying the application of the Sanhua Decoction in the treatment of ischemia-reperfusion, we review the pathogenesis of ischemia-reperfusion, Chinese medicine’s understanding of ischemia-reperfusion and its treatment, and the effects of the Sanhua Decoction on ischemia-reperfusion, which we believe will provide a useful reference for clinical ischemia-reperfusion-related research.
The pathogenesis of cerebral ischemia-reperfusion injury mainly involves a series of “waterfall” cascade reactions, including impaired energy metabolism, elevated oxidative free radical generation, and release of inflammatory factors and mediators. In previous studies, as treatments, researchers have mainly used chemical drugs that can act directly on certain receptors. However, commonly used clinical drugs generally have a single target and link, and thus tend to be ineffective in treating the multi-link and multi-level pathogenesis of cerebral ischemia-reperfusion. Consequently, in-depth studies of the etiology and pathogenesis of cerebral ischemia-reperfusion are of considerable significance for the discovery of new intervention targets for the prevention and treatment of cerebrovascular diseases.
The development of cerebral ischemic injury is mainly attributable to disordered energy metabolism. In the process of cerebral ischemia and hypoxia, there is a reduction in ATP synthesis, with a concomitant increase in its consumption. These changes are accompanied by reductions in the activities of Na+ -K+ -ATPase and Mg2+ -ATPase in the brain tissue, thereby leading to a loss of Na+, K+ transport capacity. As a consequence, there is a reduction in cell membrane resting potential, which can result in the accumulation of large number of Ca2+ ions and aggravate pre-existing brain damage, leading in turn to the occurrence of brain cell edema. In this regard, it has been found that under normal physiological conditions, the concentrations of extracellular Ca2+ are tens of thousands of times higher than those of intracellular Ca2+[6]. In patients suffering from cerebral ischemia-reperfusion injury, oxidative stress injury, along with other conditions, will contribute to promoting changes in the permeability of cell membrane ion channels, which is accompanied by a large influx of Ca2+, further contributing to an overload of intracellular Ca2+, and thus inducing DNA cleavage and leading to the occurrence of edema. This in turn blocks the transmission of information to axons, leading to cerebrovascular dysfunction. In addition, excessive intracellular Ca2+ can induce mitochondrial dysfunction, resulting in an inability to provide a source of energy for metabolic processes in brain tissue, and thereby contributing to an exacerbation of the damage caused by cerebral ischemia and reperfusion.
Inflammation plays an important role in the development of cerebral ischemia-reperfusion injury. In this regard, it has been found that after cerebral ischemia-reperfusion, inflammatory cell infiltration and inflammatory factor elevation occur in both brain tissue and peripheral blood, which exacerbates the damage caused by cerebral ischemia-reperfusion[7]. Moreover, Köseoğlu et al[8]. have found that the tumor necrosis factor-α (TNF-α) secreted by macrophages can activate inflammatory cells, which can contribute to increases in the adherence of inflammatory cells to the walls of capillaries and small blood vessels, and also increase the permeability of blood vessel walls. Moreover, the intervention of cerebral ischemia/reperfusion injuries has been found to be associated with a significant reduction in the expression of TNF-α. In addition, the findings of further studies have indicated that IL-1β is expressed earlier in the pathogenesis of cerebral ischemia-reperfusion[9], and it has been shown that the levels of plasma and cerebrospinal fluid IL-1β are significantly elevated in rats with ischemia-reperfusion[10].
Oxidative stress has been established to play an important role in the pathogenesis of cerebral ischemia-reperfusion injury. Under conditions in which the brain is deprived of oxygen and nutrients, the oxidative processes associated cellular metabolism are disrupted. During ischemia, a lack of intracellular oxygen supply leads to mitochondrial dysfunction and the release of large amounts of free radicals. Moreover, when blood is re-perfused, the concomitant re-oxygenation promotes the oxidative generation of further free radicals. These free radicals can contribute to the development of oxidative stress, including that associated with lipid peroxidation, protein oxidation, and nucleic acid damage. In this regard, it has been found that free radicals can attack lipid molecules within the cell membranes, thereby leading to the peroxidation of these molecules and the formation of single electron-rich lipid radicals[11]. These peroxidized lipid molecules subsequently undergo chain reactions, during which the structure and function of the cell membrane are disrupted, thereby increasing membrane permeability, which in turn leads to the leakage of intracellular contents and, eventually, cell death. Similarly, free radicals can attack proteins and nucleic acids, thereby triggering oxidative stress, and leading to cerebral ischemia-reperfusion injury.
Other key factors contributing to the development of cerebral ischemia-reperfusion injury include endoplasmic reticulum stress, cellular autophagy, disruption of the blood–cerebrospinal fluid barrier (BBB), gene activation, and disruption of the expression of heat shock proteins. Relevant studies have found that in response to the development of cerebral ischemia-reperfusion, there are significant increases in levels of the GRP78 and CHOP proteins, which activates the endoplasmic reticulum stress response, thereby contributing to a restoration of the structure of unfolded or misfolded proteins[12]. Moreover, in a related study, autophagy-related protein expression levels were found to be significantly reduced in a rat model of cerebral ischemia-reperfusion injury, thereby further indicating that autophagy plays a role in neuronal damage during cerebral ischemia-reperfusion injury[13]. In subsequent studies, it was found that that the development of cerebral ischemia and hypoxia is associated with pronounced leukocyte infiltration and the production of large amounts of protein hydrolases, accompanied by notable ATP deficiency, which also exacerbated disruption of the BBB[14]. In addition, Fu et al[15] have suggested that among the genes expressed in response to cerebral ischemia and reperfusion, a majority are associated with apoptosis.
On the basis of the findings of the aforementioned studies, it has thus been established that a diverse range of processes, including disordered energy metabolism, inflammation, oxidative stress-induced damage, and apoptosis, contribute to the development of cerebral ischemia-reperfusion injury. Consequently, it can be reasoned that effective intervention for these pathogenic mechanisms will improve the therapeutic outcome for patients. However, the therapeutic effects of the currently applied clinical treatments for this disease remain unsatisfactory. As an alternative approach to treating this disorder, Chinese traditional medicine has in recent years been established to have unique advantages and potential for the treatment of cerebral ischemia-reperfusion injury, and has accordingly received increasing attention in the study of this condition. Consequently, it is anticipated that an in-depth assessment of the therapeutic mechanisms of traditional Chinese medicines will provide a valuable reference for the treatment of patients suffering from cerebral ischemia-reperfusion injury.
In traditional Chinese medicine, cerebral ischemia-reperfusion is considered a deficiency syndrome, in which deficiency of the liver and kidney yin and deficiency of qi and blood are assumed to be the root causes of the disease. Moreover, silt, toxicity, heat, and phlegm are believed to play important roles in the development cerebral ischemia–reperfusion. Therefore, understanding the pathogenesis of cerebral ischemia-reperfusion will provide a strong basis for patient treatment.
In Chinese medicinal theory, it is believed that the pathogenesis of cerebral ischemia-reperfusion injury mainly involves the following four aspects: (1) Qi stagnation and blood stasis: After cerebral ischemia, blood circulation is impaired, and the local operation of qi and blood is poor, which can readily lead to a local stagnation of blood, thus aggravating the degree of injury; (2) Dampness-heat stasis: Dampness-heat and stagnant blood in the body will interact, thereby perturbing the functions of "qi" and "blood" inside the body and disrupting the supply of oxygen to the brain. Under these conditions, dampness-heat and blood stasis hinder the patient’s normal metabolism and exacerbate the ischemic and hypoxic states of the brain tissue; (3) Weakness of kidney yang: Kidney yang is considered one of the basic energies for human life activities, a weakness of which may lead to metabolic dysfunction in the human body, thereby rendering it unable to produce sufficient heat and thus adversely influencing the normal functioning of the brain; and (4) Disorders of the spleen and stomach: In Chinese medicine, the spleen and stomach are regarded as the digestive system of the human body, the disorders of which may cause indigestion, the malabsorption of nutrients, and other symptoms, thus affecting the supply of nutrients to the body and leading to brain dysfunction.
Cerebral ischemic injury is the pathophysiological manifestation of cerebral ischemic disease, and treatment aims to restore reperfusion and protect the brain tissue. Traditional Chinese medicine is considered to offer a more effective treatment approach for this disease by activating blood and muscles, promoting qi and blood circulation, and tonifying qi and blood. In Chinese medicinal theory, the principles underpinning the treatment for cerebral ischemia-reperfusion can be divided into the following three main aspects.
(1) Dredging meridians and collaterals, activating blood, and relieving pain: During the development of cerebral ischemia-reperfusion injury, blood circulation is blocked, and local bruises and stagnation contribute a degree of obstruction, thereby preventing nerves impulses, qi, and blood from flowing freely. Consequently, by dredging the meridians and channels, activating blood circulation, and relieving pain, patients’ symptoms can be relieved, local circulation can be promoted, and body function can be improved.
(2) Harmonizing the spleen and stomach and strengthening the body: In Chinese medicine, it is believed that harmonizing the spleen and stomach and strengthening the body are indispensable facets of cerebral ischemia-reperfusion treatment, which can thus enhance the body's ability to resist disease and promote recovery and rehabilitation.
(3) Clearing heat and dampness and detoxifying and dispelling wind: Clearing heat and dampness and detoxifying and dispelling wind can assist the body in eliminating toxins and waste, improve the internal environment of the body, and alleviate liver and kidney injuries caused by cerebral ischemia-reperfusion.
Traditional Chinese medicine compound treatment: (1) Treatment of cerebral ischemia-reperfusion by Tonifying Yang and restoring Wu Tang. In order to investigate the protective effect of Tonifying Yang and returning five soups in cerebral ischemia-reperfusion injury and its mechanisms, Li et al[16] selected a total of 75 rats for random group intervention. Examination of the neurological functions and cerebral infarcts of the rats after seven consecutive days of drug administration revealed that the neurological functions of rats in the cerebral ischemia-reperfusion model group were significantly deficient compared with that of rats in the sham-operated group. Moreover, they detected increase in the volume of cerebral infarcts. However, after treatment based on tonifying Yang and returning five soups, the neurological deficits of rats were significantly ameliorated. In addition, the treatment effectively reduced the hip
Acupuncture and moxibustion: (1) Electroacupuncture for cerebral ischemia-reperfusion. Mei et al[20] discussed the role of electroacupuncture in improving cerebral ischemia-reperfusion injury in rats based on SIRT1-FOXO1 signaling pathway. In the experiment, the researchers used the suture method to prepare cerebral ischemia-reperfusion model, and divided it into groups with different intervention methods, and found that compared with the model group, Electroacupuncture reduced the ratio of LC3-II/LC3-I, the levels of Ac-FOXO1 and Atg7, and the interaction between Ac-FOXO1 and Atg7 in the ischemic peripheral cortex of rats. Meanwhile, the SIRT1 inhibitor EX527 could eliminate the above effects. These results indicate that electroacupuncture can inhibit autophagy by activating SIRT1-FOXO1 signaling pathway, and thus produce neuroprotective effect on CIR injury. Similarly, Ye et al[21] have shown that electroacupuncture can be used to induce an accelerated activation of the JAK/STAT signaling pathway, promote the expression of P-JAK2 and P-STAT3 in the semi-dark band, and further increase the Bcl2/Bax ratio, which is associated with the immu
The classic formulation of the Sanhua Decoction is that San Hua Tang, which primarily comprises Hovenia, Hou Pu, Rhubarb, and Qiang Wu. The efficacy of this decoction is proposed to be based on the regulation qi, and is a representative prescription for the treatment of stroke via internal organ circulation. In their study using an animal model, Wang et al[24] found that the Sanhua Decoction could enhance the motor ability of rats with middle cerebral artery occlusion and alleviate the symptoms of neurological deficits to a greater extent than the conventional drug nimodipine. Similarly, in their clinical observational study, Luo et al[25] found in the observation of animal experiments that using Sanhua Decoction to treat ischemia/reperfusion could better reduce nerve injury and cerebral infarction volume. These findings accordingly imply that the Sanhua Decoction has potential clinical application in the treatment and prevention of cerebral ischemia-reperfusion.
Citrus aurantium (C. aurantium) is commonly used for its medicinal properties in traditional Chinese medicine, and has been established to have specific effects and mechanisms of action in the treatment of cerebral ischemia-reperfusion.
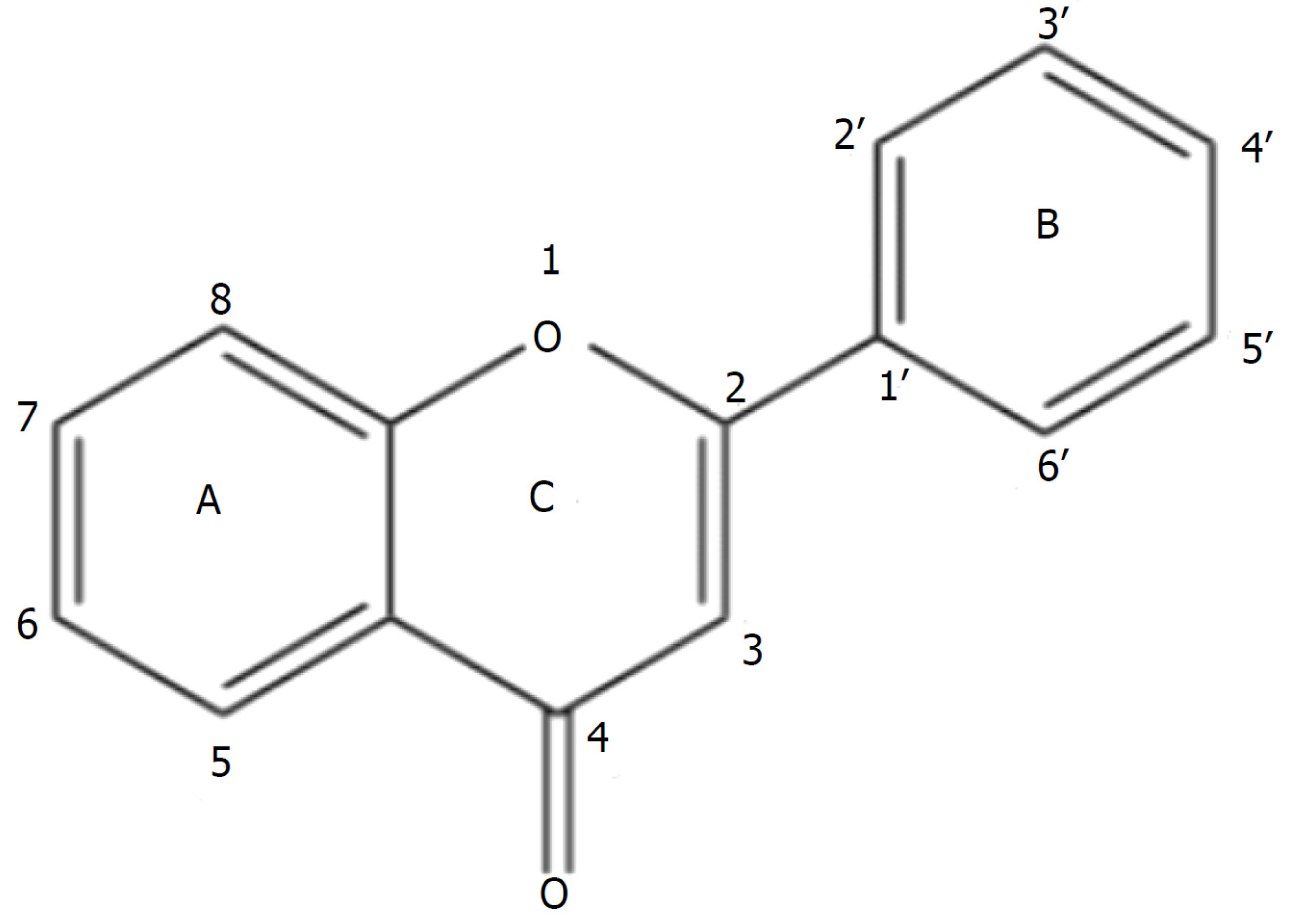
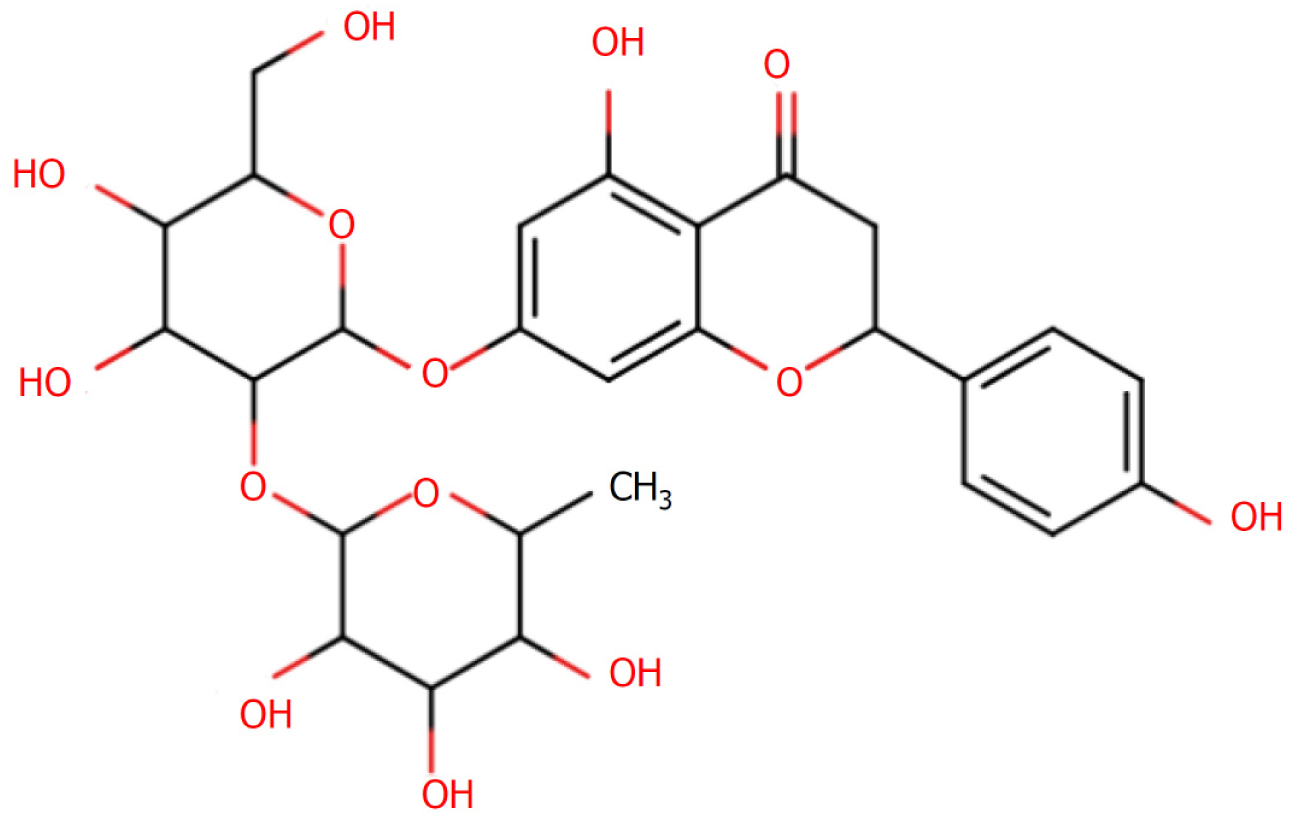
Flavonoids in Citrusaurantium exert antioxidant effects: The findings of a study by Zhao et al[26] have revealed that C. aurantium has antioxidant effects in the treatment of cerebral ischemia. In the development of cerebral ischemia-reperfusion injury, brain cells are damaged by hypoxia and ischemia, leading to the excessive production of free radicals, which trigger oxidative stress, thereby adversely affecting cells. Hovenia citriodora has been established to be rich in flavonoids (Figure 2), many of which are characterized by strong antioxidant properties, and accordingly have the capacity to neutralize free radicals. Moreover these flavonoids can contribute to inhibiting the production of free radicals derived from metabolic processes and promote the activity of enzyme systems for scavenging free radicals , which can in turn contribute to alleviating the oxidative stress-induced damage to brain cells.
Anti-inflammatory effects of Citrusaurantium-derived chorophyllin: To assess the in vitro and in vivo anti-inflammatory mechanisms of an extract of Hovenia quinquefolium (H. quinquefolium), Li et al[27] constructed a mouse model of acute lung injury, in which they found that the levels of the pro-inflammatory cytokines TNF, IL-6, and IL-1β were suppressed in response to pre-treatment with H. quinquefolium extract, whereas levels of the anti-inflammatory cytokine IL-10 were elevated. Simultaneously, they detected significant reductions in the numbers of neutrophils and macrophages in bronchoalveolar lavage fluid, which explains the significant anti-inflammatory effects of C. aurantium. Using a web-based pharmacological system, Jin et al[28] succeeded in resolving the pharmacological mechanism of C. aurantium and found that chuanpianin in C. aurantium has certain anti-inflammatory effects. Furthermore, Güvenç et al[29] examined the effects of tangerine in trifoliate aurantium (Figure 3) on the renal tissue of rats with renal ischemia-reperfusion injury and accordingly found that after the intervention with tangerine, there was a significant reduction in the levels of TNF-α and IL-1 expression in these rats, which were comparable to those detected in the control group rats. These findings thus indicated that tangerine has certain protective effect against kidney damage in rats by reducing the inflammatory response in these animals.
Houpu, also referred to as Sumac and White Houpu, is a commonly used Chinese herbal medicine that has a range of pharmacological effects, among which, recent studies have indicated protective effects against cerebral ischemia-reperfusion injury[30].
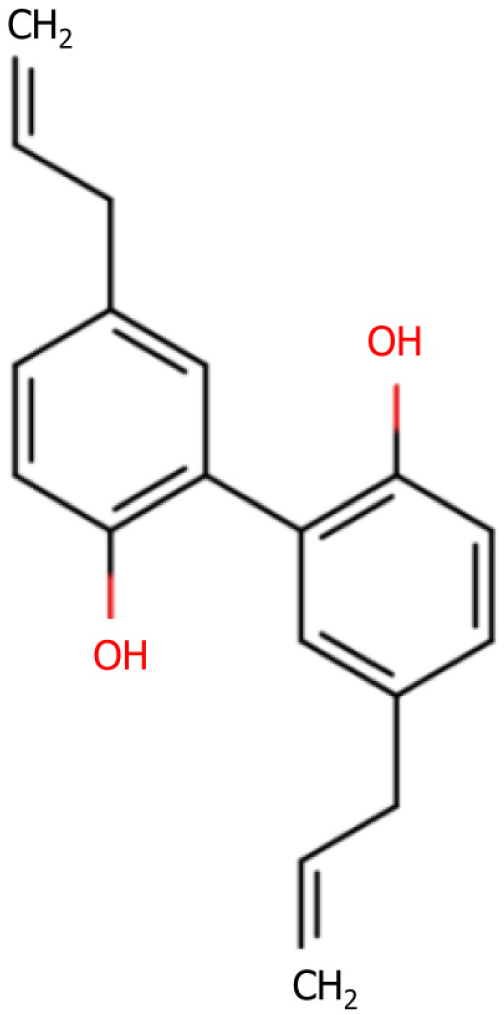
The anti-inflammatory effects: In their study of the anti-inflammatory effect of thujaplicin (Figure 4) on a lipopolysaccharide-induced mouse model of inflammation and its effect on the NF-κB pathway, Mo et al[31] found that thujaplicin promoted a significant reduction in the serum levels of TNF-α, IL-17, and IL-22 in the drug group. In addition, the levels of IL-17, TNF-α, and NF-κB p65 protein expression in the thymus tissues of these mice were observed to be more markedly reduced compared with those of the model group. Furthermore, these authors demonstrated the inhibitory effects of thujaplicin on lipopolysaccharide-induced inflammation, and established that the anti-inflammatory effects produced were associated with a down-regulated expression of TNF-α and IL-17 proteins in the NF-κB p65 inflammatory pathway.
Huperzolol in Huperzia has a neurotransmitter-modulating effect in Parkinson's disease: In their study in which they examined the mechanisms underlying the effects of Houpu extract on dopaminergic neurons in the substantia nigra region of Parkinson's mice, Wu et al[32] found that the numbers of α-synuclein-, E3 Ligase-, and ubiquitin-positive cells were reduced in the model group mice compared with those in a Parkinson’s model group. In addition, compared with the normal group mice, these authors detected no significant differences in the expression of TH in the substantia nigra region of mice in the Houpu extract-intervention group, which indicated that the Chinese herbal medicine Houpu extract can contribute to protecting dopaminergic neurons in the substantia nigra region, and the underlying mechanisms may be associated with a reduction in cell apoptosis. Furthermore, the findings of pharmacological studies have indicated that houpulol has anti-apoptotic and neuroprotective effects in neurological diseases[33], whereas Xian et al[34] have demonstrated that houpalol reduces the activity of acetylcholinesterase, thereby promoting a significant increase in the levels of acetylcholine in the brains of treated mice, which in turn ameliorated scopolamine-induced learning and memory deficits in these mice.
Rhubarb is used in traditional Chinese medicine to clear heat, remove toxins, and facilitate the flow of water, and has also been established to have therapeutic effects against cerebral ischemia-reperfusion.
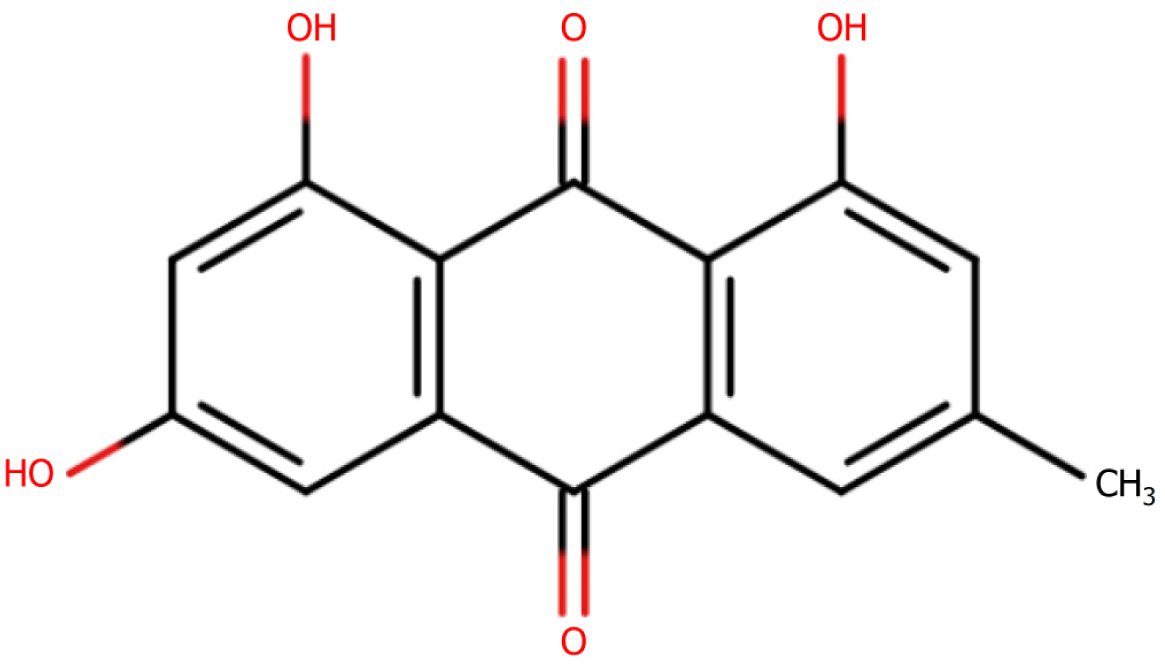
Rhubarb protects nerve cells: Sun et al[35] have previously demonstrated that treatment with an extract of rhubarb promoted increases in the expression of nerve growth factor and brain-derived neurotrophic factor proteins, which in turn contributed to enhancing the ischemic lateral nerve and neuronal survival environment in a rat model of middle cerebral artery infarction, thus playing a neuroprotective role. Subsequently, Xu et al[36] found that in response to rehabilitation training and administration of rhodopsin (Figure 5) in rats with cerebral infarction, there were significant elevations in the expression of neuron-specific enolase and S100B, whereas in contrast, levels of NF-κB p65 expression in the hippocampal tissues on the ischemic side of the rats were significantly reduced. In addition, they detected significant increases in the expression of IκBα protein, which contributed to a reduction in the neurological function scores of the rats with cerebral infarction, and also had the effect of reducing the content of apoptotic molecules in brain cell tissues, and hence the degree of neurological damage.
Rhubarbic acid in rhubarb reduces cerebral edema: The findings of network pharmacological studies have provided evidence to indicate that rhubarb has beneficial effects in the treatment of cerebral edema, and that the associated mechanisms of action may be related to the inhibition of apoptosis, oxidative stress, and the inflammatory response[37]. Furthermore, in a study that examined the effects of rhubarbic acid on water-beating cerebral ischemia-reperfusion injury and the underlying mechanisms, Tian et al[38] found that both in vitro and in vivo, rhubarbic acid can effectively reduce cerebral ischemia-reperfusion injury in rats and can significantly reduce the proportion of cerebral edema.
Qiangwu is a Chinese herb that is commonly used to treat rheumatism, arthritis, and other diseases, and the findings of recent studies have indicated that extracts of this plant have a protective effect against cerebral ischemia-reperfusion injury[39].
Antioxidant effects: Liu et al[40] have established that coumarins found in Qiangwu have potent antioxidant activities, and that among these coumarins, furanocyclic coumarins, which are characterized by a polyolefin structure, have stronger antioxidant activities than other coumarin analogs. Furthermore, the findings of a study conducted by Wang et al[41], have indicated that qiangwuol in Qiangwu has a certain protective effect against cardiomyocyte injury, and that the underlying mechanisms may be associated with an enhancement of antioxidant enzyme activity. In order to study the protective effect of the classical Sanhua Decoction formulation on cerebral ischemia-reperfusion injury in rats with BBB and its mechanisms of grouping and compounding, Li et al[42] developed a rat model of cerebral ischemia-reperfusion injury using Longa's line bolus method. Compared with rats in the normal group, those in the model group that had been treated with a gavage of Sanhua Decoction were found to be characterized by significant reductions in the expression of KLF2 protein and mRNA within the brain, whereas the levels thrombomodulin and endothelial nitric oxide synthase (eNOS) protein and mRNA were significantly increased. Moreover, compared with the model group, there were significant reductions in the expression of thrombomodulin and eNOS protein and mRNA in the Sanhua Decoction group, and the expression of KLF2 protein and mRNA was significantly increased. These findings accordingly indicate that the Sanhua Decoction has a protective effect on the brain tissues of rats with cerebral ischemia-reperfusion injury, and can contribute to reducing damage to the BBB in these rats.
Gong et al[43] similarly used the Longa wire bolus method to generate a rat model cerebral ischemia-reperfusion injury, using which, they examined the protective effects of the Sanhua Decoction and its constituents on the brain tissues of rats with cerebral ischemia-reperfusion, which had been subjected to the gavage of Sanhua Decoction for 5 days. The results revealed that compared with the model group, the infarcted area of rat brain tissue in the Sanhua Decoction group was significantly reduced. Similarly, pathological injury was reduced and the expression levels of tight junction closure protein-5, occluder protein, and occluder bandlet protein-1 mRNA and protein in the brain tissue were significantly elevated. These findings thus indicate that the Sanhua Decoction can significantly reduce the infarcted area of brain tissue in ischemia-reperfusion rats and improve the pathology, morphology, and ultrastructure of the brain tissue.
The Sanhua Decoction is a traditional Chinese herbal formulation often used in Chinese medicine to treat cerebral ischemia-reperfusion injury. Several studies have shown that this formulation can be used to confer neuroprotective effects and reduce tissue inflammation and oxidative stress-induced damage based on its neuroprotective, anti-inflammatory, and antioxidant properties. However, there are still some shortcomings in the current study: (1) Lack of clinical evidence: Although the Sanhua Decoction has shown some efficacy in vitro and in animal experiments, there is still a lack of results from large-scale clinical trials to support its application in the treatment of cerebral ischemia-reperfusion. Accordingly, more high-quality clinical studies are needed to assess the efficacy and safety of the Sanhua Decoction; (2) Insufficient mechanistic studies: Although some studies have examined the mechanisms of action of the Sanhua Decoction in the treatment of cerebral ischemia-reperfusion, many questions remain unanswered. For example, the identity of the constituents that play key roles in neuroprotection, and how these interact with each other to achieve therapeutic effects, have yet to be sufficiently established. Accordingly, further mechanistic studies are required to gain a more detailed insight in this regard; and (3) Standardization issues: The Sanhua Decoction is a complex traditional Chinese medicinal formulation, and thus it is inevitable that differences in the composition and proportion of the constituents occur in different hands. The lack of a uniformly standardized production process and quality control standards has thus led to differences in the Sanhua Decoction preparations used in different studies, making the comparison and generalization of results difficult.
On the basis of anecdotal evidence and the findings of research studies conducted to date, the Sanhua Decoction would appear to have potential utility in the general treatment of cerebral ischemia-reperfusion injury. However, given the aforementioned complexity and uncertainty associated with traditional Chinese medicinal treatments, key research problems need to be resolved and further in-depth studies are necessary: (1) Among the outstanding problems, the safety and tolerability of the Sanhua Decoction in the treatment of cerebral ischemia-reperfusion injury is a primary concern. Accordingly studies are required to assess whether patients experience adverse reactions or side effects after receiving Sanhua Decoction treatment, and to provide corresponding safety indices and data; (2) With regards to assessments of the efficacy of the Sanhua Decoction, studies are needed to compare the differences in efficacy between the Sanhua Decoction and conventional treatments (e.g., antiplatelet drugs and thrombolytic therapy) in patients with cerebral ischemia-reperfusion injury. Of particular relevance in this respect are assessments of neurological function recovery, brain imaging indices (e.g., improvement in brain perfusion), and assessments of clinical symptoms; (3) The therapeutic mechanisms underlying the efficacy of the Sanhua Decoction are a key issue in the field of cerebral ischemia-reperfusion injury. Consequently, further in-depth studies examining the effects of the Sanhua Decoction on cerebrovascular, neuroprotective, and inflammatory responses are necessary to reveal the molecular mechanisms underlying its therapeutic effects; and (4) In view of the individualized characteristics of different patients and the differences in their conditions, studies are necessary to determine whether there are specific subgroups of patients that would be more responsive to treatment with the Sanhua Decoction. In this regard, individualized information, such as genotyping and clinical phenotypes, can be used to develop more precise treatment strategies for Sanhua decoctions.
The combined treatment of cerebral ischemia-reperfusion injury with Chinese and Western medicines is a comprehensive treatment strategy that can give full play to the respective advantages of Chinese and Western medicines, and thereby enhance the therapeutic effects. However, using a combination Chinese and Western medicines presents its own set of challenges: (1) The problem of standardization: The combination of Chinese and Western medicines needs to strictly follow standardized operation procedures and treatment guidelines. Given the differences and complexities of Chinese and Western medicinal therapies, it will be challenging to ensure the consistency and standardization of treatment; (2) Communication and cooperation issues: Treatments based on a combination of Chinese and Western medicine requires close cooperation and communication among physicians, including the joint development of treatment plans and the negotiation of drug selection and dosage. However, differences in the traditional education systems of Chinese and Western medicine, and the lack of mechanisms for medical teamwork, may lead to problems in communication and cooperation; (3) Differences in knowledge and philosophy: Differences in the understanding of disease mechanisms, diagnosis, and treatment methods between traditional Chinese medicine and Western medicine may lead to differences in the selection and use of treatment strategies.
Some of the potential solutions to these challenges are as follows: (1) Formulation of standardized guidelines: In order to improve the consistency and standardization of treatment, it will be necessary to formulate standardized guidelines for the treatment of cerebral ischemia-reperfusion injury using combining Chinese and Western medicine to clarify issues such as the treatment process, diagnostic criteria, and drug selection and dosage; (2) Establishment of interdisciplinary teams: To develop effective treatment strategies it will be essential to establish interdisciplinary medical teams comprising Chinese and Western doctors as the core, including neurologists, rehabilitation doctors, and Chinese medicine doctors, which will thereby contribute to strengthening communication and cooperation, enable the joint formulation of treatment plans, and provide timely feedback on treatment effects; and (3) Strengthening of medical education: For doctors and medical students, it will be imperative to strengthen the communication and understanding of knowledge between Chinese and Western medicinal practitioners, and thereby contribute to enhancing the recognition and application of combined Chinese and Western medicine treatment. Similarly, it will be important to strengthen scientific research and clinical practice by conducting more multi-center and large-sample clinical studies, thereby facilitating the collection of more evidence to support the efficacy of combined Chinese and Western medicinal treatment, and hence promoting its application in the field of cerebral ischemia-reperfusion injury.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dixit AB, India S-Editor: Wang JL L-Editor: A P-Editor: Zhang YL
| 1. | Dietz RM, Dingman AL, Herson PS. Cerebral ischemia in the developing brain. J Cereb Blood Flow Metab. 2022;42:1777-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Zhao Y, Zhang X, Chen X, Wei Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int J Mol Med. 2022;49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 257] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 3. | Wang J, Wang TH, Bai X. [Mechanism of Sanhua Decoction in the Treatment of Ischemic Stroke Based on Network Pharmacology Methods and The Xuan Fu Theory]. Zhongyiyao Linchuang Zazhi. 2022;34:1871-1882. [DOI] [Full Text] |
| 4. | Yang CH, Cheng F, Peng XJ, Liu F, Hu XZ, Xu G, Li H. [Connotative analysis of the formulation of Sanhua Decoction in the treatment of stroke based on network pharmacology]. Xibei Yaoxue Zazhi. 2022;37:37-44. [DOI] [Full Text] |
| 5. | Gou XJ, Sun MJ, Huang Y, Li WJ, Ren JH. [Effects of Sanhua Decoction on serum fatty acid profile of rats with ischemic stroke]. Zhongguo Yiyuan Yaoxue Zazhi. 2022;42:259-268. [DOI] [Full Text] |
| 6. | Ge L, Cao HL, Zhang J, Xu M, Zhu XF, Chen YF. [Effect of tetramethylpyrazine on oxidative stress, Ca2+-ATP activity and inflammatory factors after cerebral ischemia/reperfusion injury in rats]. International Journal of Laboratory Medicine, 2021; 42: 517-520. [DOI] [Full Text] |
| 7. | Pan HY, Zhang N, Zhou Y, Li XY. [Effect of MCC950 on NLRP3 inflammatory vesicle activation after cerebral ischaemia-reperfusion injury in rats]. Zhongguo Xinyao Yu Linchuang Zazhi. 2022;41:754-758. [DOI] [Full Text] |
| 8. | Köseoğlu Toksoy C, Sarıtaş ZK, Türk Börü Ü, Zeytin Demiral G, Görücü F, Bülbül A, Demirel HH, Koç Y. Investigation of the protective effect of anzer propolis in cerebral ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. 2023;27:8004-8012. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Luo L, Liu M, Fan Y, Zhang J, Liu L, Li Y, Zhang Q, Xie H, Jiang C, Wu J, Xiao X, Wu Y. Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFκB/NLRP3 signaling pathway in cerebral ischemic mice. J Neuroinflammation. 2022;19:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 174] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 10. | Luo J, Chen J, Yang C, Tan J, Zhao J, Jiang N, Zhao Y. 6-Gingerol protects against cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome and apoptosis via TRPV1 / FAF1 complex dissociation-mediated autophagy. Int Immunopharmacol. 2021;100:108146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 11. | Ma ZZ, Sun BJ, Fan XX, Sun Y. [Effects of inhaled formaldehyde at different concentrations on oxidative damage of brain tissues during my-ocardial ischemia-reperfusion]. Zhongguo Yaowu Yu Linchuang. 2021;21:2256-2258. [DOI] [Full Text] |
| 12. | Qu SB. [Preliminary study on the protective mechanism of chickpeasin A against cerebral ischaemia-reperfusion injury in rats]. M.Sc. Thesis, Guilin Medical College. 2018. [DOI] [Full Text] |
| 13. | Hu YQ, Chen W, Zhu MZ, Liang N, Wu L, Tang N. [Protective effect of Qingre Huayu prescription against cerebral ischemia-reperfusion injury by regulating autophagy-related gene P62/LC3 in rats]. Shaanxi Zhongyi. 2020;41:429-433. [DOI] [Full Text] |
| 14. | Tong X, Zhang QR, Zhao H, Cheng XX. [Effects of hyperbaric oxygen on the blood-brain barrier via the SIRT1/FoxO1 signaling pathway after cerebral ischemia and reperfusion]. Zhonghua Wuli Yixue Yu Kangfu Zazhi. 2022;44:13-15. [DOI] [Full Text] |
| 15. | Fu YL, Nie YQ, Liu ZQ, Lu M, Stefanie K, Yu J, Lei XM. [Mechanisms of inhibiting apoptosis in rats with cerebral ischemia-reperfusion injury by electroacupuncture]. Shaanxi Zhongyi. 2023;44:285-289. [DOI] [Full Text] |
| 16. | Li WY, Liu HS, Gao SY, Su ZQ. [Protective Effects of Buyang Huanwu Tangon Cerebral Ischemia-reperfusion Injury Based on Notch1/NF-κB Signaling Pathway and Its Mechanism]. Xibu Zhongyiyao. 2022;35:16-21. [DOI] [Full Text] |
| 17. | Zhang Y, Chen ZY, Liu Q, Song W. [Protective effect of Buyang Huanwu Tang on brain injury in rats following I/R]. Zhonghua Laonian Xinxueguanbing Zazhi. 2019;21:867-870. [DOI] [Full Text] |
| 18. | Zeng XJ, Zhou JF, Zhu MZ, Liu S, Qi JG, Mao DX. [Effect of Qinnao Yiyuan Decoction on TNF-α and IL-8 levels in Model rats with Cerebra Ischemia Reperfusion Injury]. Zhonghua Zhongyiyao Xuekan. 2019;37:1124-1127. [DOI] [Full Text] |
| 19. | Li XN, Hai Y. [Curative Effect of Qingnao Yiyuan Decoction in Treating Ischemic Stroke and Its Influence to Barthel Index and ADL Score]. Zhongyiyao Xuebao. 2020;48:46-49. [DOI] [Full Text] |
| 20. | Mei ZG, Huang YG, Feng ZT, Luo YN, Yang SB, Du LP, Jiang K, Liu XL, Fu XY, Deng YH, Zhou HJ. Electroacupuncture ameliorates cerebral ischemia/reperfusion injury by suppressing autophagy via the SIRT1-FOXO1 signaling pathway. Aging (Albany NY). 2020;12:13187-13205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 21. | Ye W, He JC, Xie WX, Cheng FF, Yang MY, Ma Y. [Effects of electroacupuncture on the expression of Bcl-2 and Bax in mice with cerebral ischemia-reperfusion injury]. Zhejiang Zhongyi Zazhi. 2021;56:379-380. [DOI] [Full Text] |
| 22. | Zhao YY, Zhang W, Wang Z. [Experimental Study on Mechanism of Eye Acupuncture Regulating Raf/MEK/Erk Pathway in Cerebral Penumbra Tissue of Cerebral Ischemia-Reperfusion Rats]. Zhonghua Zhongyiyao Xuekan. 2022;40:71-75. [DOI] [Full Text] |
| 23. | He SN, Han XW, Pan Q, Ma XD, Gao Y, Jing H, Xu C, Wang Z. [Eye acupuncture improves cerebral ischemia reperfusion injury by improving autophagy via ATF6 pathway in cerebral ischemia reperfusion injury rats]. Zhenci Yanjiu 2022; 47: 859-865. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Wang TH, Liu LZ, Yan FL, Luan H, Li YQ, Zhang YK, Li Y, Zhao HJ. [Evaluation of Effect of Sanhua Decoction on Middle Cerebral Artery Occlusion Rats Based on Catwalk Gait Analysis System]. Shandong Zhongyiyao Daxue Xuebao. 2022;46:731-737. [DOI] [Full Text] |
| 25. | Luo S, Chen Y, Zhao R, Ma D, Zhao Y, Zhang Y, Jiang J, Yu W. Application of omics technology to investigate the mechanism underlying the role of San Hua Tang in regulating microglia polarization and blood-brain barrier protection following ischemic stroke. J Ethnopharmacol. 2023;314:116640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Zhao N, Wang XZ, Mu JL, Pan J, Di JL, Feng YN, Jia HT. [Effect of Zhishi Xiebai Guizhi Decoction on Effects of Zhishi Xie Bai Guizhi Decoction on Rabbit's Myocardial Ischemia and Oxidative Stress Injury]. Zhongyiyao Daobao. 2021;27:49-53. |
| 27. | Li L, Chen J, Lin L, Pan G, Zhang S, Chen H, Zhang M, Xuan Y, Wang Y, You Z. Quzhou Fructus Aurantii Extract suppresses inflammation via regulation of MAPK, NF-κB, and AMPK signaling pathway. Sci Rep. 2020;10:1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Jin Q, Lu J, Gao R, Xu J, Pan X, Wang L. Systematically Deciphering the Pharmacological Mechanism of Fructus Aurantii via Network Pharmacology. Evid Based Complement Alternat Med. 2021;2021:6236135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Güvenç M, Cellat M, Uyar A, Özkan H, Gokcek İ, İsler CT, Yakan A. Nobiletin Protects from Renal Ischemia-Reperfusion Injury in Rats by Suppressing Inflammatory Cytokines and Regulating iNOS-eNOS Expressions. Inflammation. 2020;43:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Zhang MF, Shen YQ. [Research Progress in the Protective Effect of Magnolol and Honokiol on Brain Injury and Their Mechanisms]. Kangganran Yaoxue. 2022;19:621-626. [DOI] [Full Text] |
| 31. | Mo SY, Chung QB, Cai ZY, Lu SJ, Zang LQ. [Preliminary study on the anti-inflammatory effect of magnolol on LPS-induced mice]. Hainan Yixueyuan Xuebao. 2019;25:335-338, 342. [DOI] [Full Text] |
| 32. | Wu AM, Sun KH, Huang FJ, Li YH, Wu ZZ. [Effects of Houpu extract on nigrostriatal dopaminergic neurons in a mouse model of Parkinson's disease]. Shenzhen Zhongxiyi Jiehe Zazhi. 2019;29:1-3. [DOI] [Full Text] |
| 33. | Wang HH, Wu HW, Li X, Zhang X, Xu J, Guo FF, Zhang H, Jian WF, Yang HJ. [Effective Constituents and Mechanism of Magnoliae Officinalis Cortex for Depressive Disorder Based on Network Pharmacology]. Zhongguo Shiyan Fangjixue Zazhi. 2019;25:162-169. [DOI] [Full Text] |
| 34. | Xian YF, Ip SP, Mao QQ, Su ZR, Chen JN, Lai XP, Lin ZX. Honokiol improves learning and memory impairments induced by scopolamine in mice. Eur J Pharmacol. 2015;760:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Sun CX, Huang JH, Qiao CL, Liu JJ, Tang YH, Xu AJ, Nie JW, Huang SY, Luo R, Yang ZL, Lai WF, Hong GZ. [The neuroprotective effect of rhubarb on MCAO model rats]. Zhongguo Yaolixue Tongbao. 2021;37:584-589. [DOI] [Full Text] |
| 36. | Xu J, Liu Y, Cui YH, Wang ZY, Xiong XL. [Emodin combined with rehabilitation training improves inflammation and nerve function damage in rats with cerebral infarction]. Linchuang He Shiyan Yixue Zazhi. 2022;21:337-341. [DOI] [Full Text] |
| 37. | Bo H, Zhao XP, Fan XX, Yu J, Zhang MJ, Sun W. [Discussion on the Mechanism of Rhubarb in the Treatment of Brain Edema Based on Network Pharmacology]. Zhongguo Zhongyi Jizheng. 2022;31:950-954. [DOI] [Full Text] |
| 38. | Tian QX, Zhang MX, Liu JL. [Effect of rhein on cerebral ischemia-reperfusion injury and its mechanism in rats]. Zhejiang Yixue. 2021;43:2316-2321. [DOI] [Full Text] |
| 39. | Li Z. [Effects of pretreatment with different extracts of Qianghuo on rats with liver ischaemia-reperfusion injury model]. M.Sc. Thesis, Qinghai University. 2022. Available from: http://cdmd.cnki.com.cn/Article/CDMD-10743-1022829134.htm. |
| 40. | Liu WW, Jiang XW, Zhang S, Zu Y X, Zhao Q C. [Chemical constituents of coumarins compounds from Notopterygium incisum and their anti-oxidant activity]. Zhongcaoyao. 2019;50:1310-1315. [DOI] [Full Text] |
| 41. | Wang YJ, Chang L, Yao TM. [Protective effect of qiangwuol on isoprenaline-induced H9 c2 cardiomyocyte injury]. Zhongguo Linchuang Yaolixue Zazhi. 2021;37:2015-2018. [DOI] [Full Text] |
| 42. | Li WJ, Kung ZH, Huang Y, Sun MJ. [Protective Effect of Sanhua Decoction on Blood-brain Barrier in Rats with Cerebral Ischemia-reperfusion Injury]. Zhongguo Zhongyi Jichu Yixue Zazhi. 2022;28:1072-1076. [DOI] [Full Text] |
| 43. | Gong ZH, Li WJ, Sun MJ, Huang Y. [Protective Effect and Mechanism of Sanhuatang and Its Modifications on Cerebral Ischemia-reperfusion Injury in Rats]. Zhongguo Shiyan Fangjixue Zazhi. 2022;28:11-18. [DOI] [Full Text] |