Published online Nov 26, 2024. doi: 10.12998/wjcc.v12.i33.6655
Revised: September 11, 2024
Accepted: September 19, 2024
Published online: November 26, 2024
Processing time: 127 Days and 17.8 Hours
By critically examining the work, we conducted a comprehensive bibliometric analysis on the role of nuclear factor erythroid 2-related factor 2 (NRF2) in ner
Core Tip: Based on the original paper, this study highlights two primary points: Bibliometric methodologies and future perspectives for nuclear factor erythroid 2-related factor 2 (NRF2) research. We emphasize the need for broader literature databases and diversified analysis methods in bibliometric research. Additionally, we highlight the importance of NRF2 modulation in regulating ferroptosis for treating nervous system diseases and discuss how nanoparticle drug delivery systems may overcome challenges associated with NRF2-related drugs.
- Citation: Huang YQ, Huang ZW, Zhang XJ. Targeting nuclear factor erythroid 2-related factor 2-regulated ferroptosis to treat nervous system diseases. World J Clin Cases 2024; 12(33): 6655-6659
- URL: https://www.wjgnet.com/2307-8960/full/v12/i33/6655.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i33.6655
We have thoroughly examined the high-quality bibliometric study by Chang et al[1], published in the high-impact journal, World Journal of Clinical Cases. In this study, a bibliometric analysis of the literature was conducted to provide a comprehensive overview of trends in evolutionary research trends on nuclear factor erythroid 2-related factor 2 (NRF2) in the nervous system disease, including focal points, frontiers, and potential future directions. The main content of the study is summarized in Table 1.
| Item | Details |
| Background | NRF2, a pivotal transcription factor in the antioxidant network, has been reported to play a crucial role in nervous system diseases. susceptible to oxidative damage. Consequently, the regulatory capacity of NRF2 and its potential clinical applications in these diseases have generated increasing interest in the scientific community |
| Methods | Data were gathered through a systematic review of the literature on NRF2 and nervous system diseases from the Web of Science Core Collection database and used the bibliometric tool CiteSpace for analysis |
| Results | Recent years have seen a rise in NRF2-related literature on nervous system diseases, with China holding a dominant position in terms of article publications, financial investments, and the number of authors with the highest publication records. NRF2 research hotspots have evolved from focusing solely on antioxidants to include anti-apoptosis and active ferroptosis |
| Conclusion | China should prioritize enhancing the quality and impact of research articles. Research on NRF2 in nervous system diseases has expanded to encompass various cell death mechanisms. Further clinical investigations of NRF2-related medications are necessary |
Herein, we critically analyze the paper by Chang et al[1] focusing on two aspects, i.e., bibliometric methodologies and future perspectives, to stimulate constructive discussion in clinical and basic research communities. It will be our great pleasure to receive a response from the author team soon.
The conceptualization and design of the bibliometric study were acceptable. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline-followed recruitment approach was illustrated. The analyzed results were relatively convincing and the discussion was appealing. The obtained findings can serve as a reference for future research on neurological diseases.
Chang et al[1] commented on the limitations of their study. Based on this, we suggest the following for future bibliometric studies.
First, it is suggested to expand the range of literature databases used. Although the Web of Science Core Collection is highly trusted, other major databases such as Scopus, PubMed, and Google Scholar are recommended for cross-comparison. Second, the diversity of the TS can be improved including core clinical topics from the published literature on nervous system disorders. Using these approaches will provide access to a wider range of sources and diverse literature, enhancing the comprehensiveness and credibility of bibliometric analyses.
Additional bibliometric tools and various bibliometric analytical methods can be used. Besides CiteSpace, other important software or online platforms like VOSviewer, SATI, HistCite, can be utilized for indicator analysis and visual representation. Additionally, statistical analysis and co-word analysis can be conducted alongside citation and cluster analyses to gain deeper insights.
The integration of strengths, weaknesses, opportunities, and threats (SWOT) analysis is an effective approach. SWOT analysis includes problem diagnosis and prescription. Through a comprehensive analysis of internal and external factors, SWOT analysis assists in identifying the research object's current capabilities and potential for further development[2]. This analysis has been comprehensively applied in medical and biomedical sciences[3,4]. The inclusion of SWOT analysis can offer more valuable information and implications to the status quo and future directions in these fields of research.
By incorporating a broader spectrum of literature, acquiring more bibliometric information, and enhancing the dimensions of the discussion on the results, bibliometric investigations will become more informative.
Importantly, as revealed by the study focus and frontiers in the original paper, the regulation of ferroptosis by NRF2 in nervous system cells has been a research hotspot in this field. NRF2 was first reported to participate in ferroptosis regulation by Sun et al[5] in 2016. In the study, the p62-Keap1-NRF2 antioxidative signaling pathway was identified as a crucial negative regulator of ferroptosis in hepatocellular carcinoma cells, exerting its effect through the transcriptional activation of genes involved in reactive oxygen species and iron metabolism. Up to now, ferroptosis has been shown to play an important role not only in the treatment of nervous system diseases but also in various cancer types. The regulatory pathways associated with NRF2 in diverse diseases have been widely examined and can be summarized as follows.
The NRF2 signaling pathway is intricately associated with multiple pathways involved in oxidoreductase synthesis, which plays an overlooked role in the process of ferroptosis. Specifically, the downstream targets of NRF2 encompass nicotinamide adenine dinucleotide phosphate [NAD(P)H] quinone oxidoreductase 1, heme oxygenase-1 (HO-1), solute carrier family 7 membrane 11, NAD(P)H quinone oxidoreductase 1, thioredoxin 1, phase II detoxifying enzymes, and various multidrug resistance-associated transporters[6,7].
Notably, NRF2 plays a crucial role in maintaining iron homeostasis, thereby influencing cell susceptibility to ferroptosis through its impact on free radical generation and lipid peroxidation[8]. For instance, NRF2 has been reported to control HERC2 (E3 ubiquitin ligase for nuclear receptor coactivator 4 and F-box/LRR-repeat protein 5), and vesicle-associated membrane proteins 8 (which mediates autophagosome-lysosome fusion) for the maintenance of iron homeostasis[9]. Additionally, various iron-related proteins such as ferritin, transferrin receptor, ferroportin, and HO-1 have been shown to be with NRF2[10,11]. In a nutshell, NRF2 is an inhibitor of ferroptosis.
The elucidation of the above-mentioned regulatory mechanisms contributes to a deeper understanding of NRF2’s role in nervous system diseases. It is well documented that multiple nervous system diseases, such as central nervous system injury (ischemic stroke, hemorrhage, and spinal cord injury)[12], neurodegenerative disease, such as Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis[13], and the brain cancers[14] are closely related to upregulated ferroptosis processes. For instance, the induction of ferroptosis has been shown to effectively suppress glioblastoma cells through the downregulation of the protein kinase B/mammalian target of rapamycin complex 1/glutathione peroxidase 4 signaling pathway in the study by Cai et al[15] in 2023. Suppressing the ferroptosis level in damaged cells is a reasonable and promising strategy to manage the above-mentioned nervous system diseases.
From this point of view, to upregulate NRF2 expression, by downregulating the expression of NRF2 competition factors like bric-a-brac and cap-n-collar homology 1[16], or activate the NRF2-involved signaling pathways, e.g. vitamin D receptor/NRF2/HO-1 pathway[17], Keap1-NRF2-ARE pathway[18], will become a ferroptosis-targeted therapy for nervous system diseases. Numerous NRF2 activators have entered clinical trials, highlighting their potential therapeutic applications[19]. Dimethyl fumarate (DMF), which has demonstrated the ability to induce NRF2 activation within the central nervous system, has been approved for psoriasis treatment[20]. Additionally, another NRF2 agonist, cyanoenone triterpenoid RTA-408 (omaveloxolone), was reported in a phase 2 randomized clinical trial to be well tolerated and to enhance neurological function when administered at a dosage of 160 mg/day over 12 weeks[21]. These findings underscore the significant clinical value of targeted NRF2 therapy and its growing prominence in research. To achieve the above targeted therapy, the utilization of various drugs, consistent with the findings of Chang et al[1], particularly, bioactive compounds from traditional Chinese medicine has been documented in relevant research. For instance, Salidroside[22], Forsythoside A[23], DMFs[24], Trehalose[25], Berberine[26], etc., and exerted promising activities.
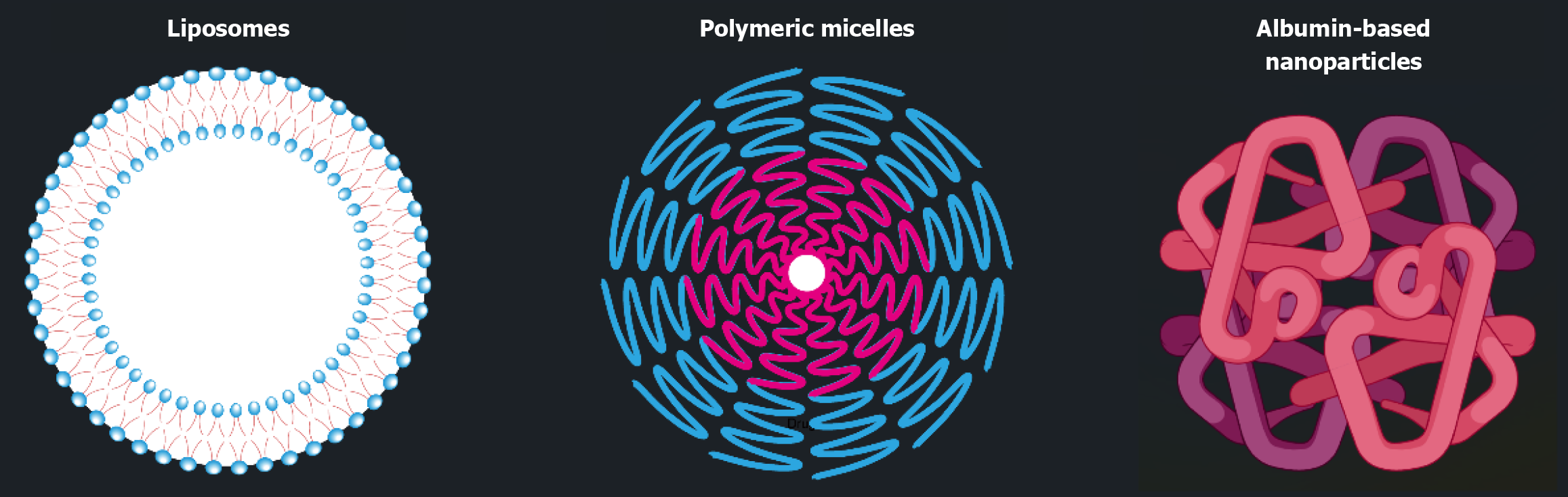
Furthermore, sophisticated nanocarriers can be designed to deliver the corresponding therapeutics to enhance the efficacy of ferroptosis-targeted therapy for nervous system diseases. Nanocarriers, e.g., lipid nanoparticles (e.g. liposomes), polymeric nanoparticles (e.g. polymeric micelles), and protein nanoparticles (e.g. albumin-based nanoparticles) (Figure 1), are versatile vesicles that are attracting attention from the clinic, industry, and laboratory. Appropriately designed nanocarriers can enhance the stability[27], bioavailability[28], and targetability[29] of encapsulated therapeutic agents, which are largely beneficial for treating nervous system diseases. For instance, Liu et al[30] designed a quercetin-modified ultrasmall Cu2-xSe antioxidative nanoparticles (CSPQ) to boost Parkinson's disease therapy by activating NRF2. Moreover, previous research on CSPQ by the same authors demonstrated the successful targeting of microglia by coating the nanoparticle with a dopaminergic neuron cell membrane[31]. Gai et al[32] prepared folate-modified liposome nanoparticles for the targeted co-delivery of erastin and putative metallothionein (to inhibit NRF2 expression) to enhance the bioavailability and efficiency of the drug/gene combination. These studies demonstrate the potential of nanoparticle drug delivery systems for precise and effective modulation of NRF2 signal pathways.
We predict that the NRF2 regulation can effectively modulate ferroptosis levels, which are closely associated with nervous system diseases, thereby facilitating the treatment of related disorders. Nanoparticle drug delivery systems are expected to enhance the bioavailability, targetability, and stability of NRF2-related drugs, thereby facilitating the clinical translation of relevant research. In our ongoing studies examining the involvement of NRF2 in a wide range of critical signaling pathways within the human body, we plan to explore the potential of the co-delivery of NRF2-related drugs in combination with other ferroptosis-regulating drugs. We hypothesize that this strategy will enhance cellular drug sensitivity, which exhibits limited efficacy across various tumor cell types. Additionally, NRF2 regulation has demonstrated potential therapeutic efficacy against neurological disorders; however, the treatment of these diseases inevitably encounters delivery challenges such as the blood brain barrier. We envision achieving precise and efficient regulation of NRF2 at specific sites by employing rational bioengineering design strategies for delivery systems, including the utilization of bionic "hitchhiking" mechanisms and charge-mediated penetration capabilities.
| 1. | Chang XQ, Xu L, Zuo YX, Liu YG, Li J, Chi HT. Emerging trends and hotspots of Nuclear factor erythroid 2-related factor 2 in nervous system diseases. World J Clin Cases. 2023;11:7833-7851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Teoli D, Sanvictores T, An J. SWOT Analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2023. [PubMed] |
| 3. | Dominguez JA, Pacheco LA, Moratalla E, Carugno JA, Carrera M, Perez-Milan F, Caballero M, Alcázar JL. Diagnosis and management of isthmocele (Cesarean scar defect): a SWOT analysis. Ultrasound Obstet Gynecol. 2023;62:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 4. | Stoller JK. A Perspective on the Educational "SWOT" of the Coronavirus Pandemic. Chest. 2021;159:743-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 1492] [Article Influence: 165.8] [Reference Citation Analysis (0)] |
| 6. | Furfaro AL, Traverso N, Domenicotti C, Piras S, Moretta L, Marinari UM, Pronzato MA, Nitti M. The Nrf2/HO-1 Axis in Cancer Cell Growth and Chemoresistance. Oxid Med Cell Longev. 2016;2016:1958174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 7. | Anandhan A, Dodson M, Schmidlin CJ, Liu P, Zhang DD. Breakdown of an Ironclad Defense System: The Critical Role of NRF2 in Mediating Ferroptosis. Cell Chem Biol. 2020;27:436-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 8. | Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35:830-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 1632] [Article Influence: 272.0] [Reference Citation Analysis (0)] |
| 9. | Anandhan A, Dodson M, Shakya A, Chen J, Liu P, Wei Y, Tan H, Wang Q, Jiang Z, Yang K, Garcia JG, Chambers SK, Chapman E, Ooi A, Yang-Hartwich Y, Stockwell BR, Zhang DD. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci Adv. 2023;9:eade9585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 250] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 10. | Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023-16029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1217] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 11. | Huang J, Tabbi-Anneni I, Gunda V, Wang L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1211-G1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Ou M, Jiang Y, Ji Y, Zhou Q, Du Z, Zhu H, Zhou Z. Role and mechanism of ferroptosis in neurological diseases. Mol Metab. 2022;61:101502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 13. | Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1629] [Article Influence: 543.0] [Reference Citation Analysis (0)] |
| 14. | Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Eyüpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med. 2008;14:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Cai J, Ye Z, Hu Y, Ye L, Gao L, Wang Y, Sun Q, Tong S, Zhang S, Wu L, Yang J, Chen Q. Fatostatin induces ferroptosis through inhibition of the AKT/mTORC1/GPX4 signaling pathway in glioblastoma. Cell Death Dis. 2023;14:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 16. | Nishizawa H, Yamanaka M, Igarashi K. Ferroptosis: regulation by competition between NRF2 and BACH1 and propagation of the death signal. FEBS J. 2023;290:1688-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 17. | Li J, Cao Y, Xu J, Li J, Lv C, Gao Q, Zhang C, Jin C, Wang R, Jiao R, Zhu H. Vitamin D Improves Cognitive Impairment and Alleviates Ferroptosis via the Nrf2 Signaling Pathway in Aging Mice. Int J Mol Sci. 2023;24:15315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 18. | Lu MC, Ji JA, Jiang ZY, You QD. The Keap1-Nrf2-ARE Pathway As a Potential Preventive and Therapeutic Target: An Update. Med Res Rev. 2016;36:924-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 595] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 19. | Dinkova-Kostova AT, Copple IM. Advances and challenges in therapeutic targeting of NRF2. Trends Pharmacol Sci. 2023;44:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 121] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 20. | Lastra D, Fernández-Ginés R, Manda G, Cuadrado A. Perspectives on the Clinical Development of NRF2-Targeting Drugs. Handb Exp Pharmacol. 2021;264:93-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Lynch DR, Farmer J, Hauser L, Blair IA, Wang QQ, Mesaros C, Snyder N, Boesch S, Chin M, Delatycki MB, Giunti P, Goldsberry A, Hoyle C, McBride MG, Nachbauer W, O'Grady M, Perlman S, Subramony SH, Wilmot GR, Zesiewicz T, Meyer C. Safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia. Ann Clin Transl Neurol. 2019;6:15-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Yang S, Xie Z, Pei T, Zeng Y, Xiong Q, Wei H, Wang Y, Cheng W. Salidroside attenuates neuronal ferroptosis by activating the Nrf2/HO1 signaling pathway in Aβ(1-42)-induced Alzheimer's disease mice and glutamate-injured HT22 cells. Chin Med. 2022;17:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 23. | Wang C, Chen S, Guo H, Jiang H, Liu H, Fu H, Wang D. Forsythoside A Mitigates Alzheimer's-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation. Int J Biol Sci. 2022;18:2075-2090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 188] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 24. | Qi D, Chen P, Bao H, Zhang L, Sun K, Song S, Li T. Dimethyl fumarate protects against hepatic ischemia-reperfusion injury by alleviating ferroptosis via the NRF2/SLC7A11/HO-1 axis. Cell Cycle. 2023;22:818-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 25. | Gong F, Ge T, Liu J, Xiao J, Wu X, Wang H, Zhu Y, Xia D, Hu B. Trehalose inhibits ferroptosis via NRF2/HO-1 pathway and promotes functional recovery in mice with spinal cord injury. Aging (Albany NY). 2022;14:3216-3232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 26. | Li X, Chen J, Feng W, Wang C, Chen M, Li Y, Chen J, Liu X, Liu Q, Tian J. Berberine ameliorates iron levels and ferroptosis in the brain of 3 × Tg-AD mice. Phytomedicine. 2023;118:154962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 27. | Huang Y, Chang Z, Gao Y, Ren C, Lin Y, Zhang X, Wu C, Pan X, Huang Z. Overcoming the Low-Stability Bottleneck in the Clinical Translation of Liposomal Pressurized Metered-Dose Inhalers: A Shell Stabilization Strategy Inspired by Biomineralization. Int J Mol Sci. 2024;25:3261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 28. | Li C, Zhang Y, Su T, Feng L, Long Y, Chen Z. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int J Nanomedicine. 2012;7:5995-6002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Wang S, Chen Y, Guo J, Huang Q. Liposomes for Tumor Targeted Therapy: A Review. Int J Mol Sci. 2023;24:2643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 107] [Reference Citation Analysis (0)] |
| 30. | Liu H, Zheng Q, Yuan J, Gao Y, Wang T, Zhang H, Li Z. Modulating SQSTM1/p62-dependent selective autophagy of neurons by activating Nrf2 with multifunctional nanoparticles to eliminate α-synuclein aggregates and boost therapy of Parkinson’s disease. Nano Today. 2023;49:101770. [DOI] [Full Text] |
| 31. | Liu H, Han Y, Wang T, Zhang H, Xu Q, Yuan J, Li Z. Targeting Microglia for Therapy of Parkinson's Disease by Using Biomimetic Ultrasmall Nanoparticles. J Am Chem Soc. 2020;142:21730-21742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 32. | Gai C, Liu C, Wu X, Yu M, Zheng J, Zhang W, Lv S, Li W. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death Dis. 2020;11:751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |