Published online Nov 26, 2024. doi: 10.12998/wjcc.v12.i33.6635
Revised: August 17, 2024
Accepted: September 2, 2024
Published online: November 26, 2024
Processing time: 88 Days and 11.6 Hours
Wound healing is a complicated process that can be heavily influenced by patient comorbidities, in some cases leading to a chronic non-healing wound. Evidence presented in the medical literature supporting the clinical use of autologous platelet-rich plasma (PRP) in treatment of such wounds is becoming increasingly compelling. Mechanisms involved include complex interactions between the patient’s thrombocytes, cytokines, and growth factors.
We present a case of a 72-year-old male patient with a long-standing chronic wound and multiple comorbidities. Over the course of more than 7 months, the patient was unsuccessfully treated with all routinely used measures, including different dressing approaches. Multiple antibiotic regimens were administered for wound infection, with repeated evaluation of microbiological swab results. Finally, after three PRP applications, the wound showed clinical improvement with complete restitution of the epithelial layer of the skin.
PRP treatment may be beneficial to reduce healing time in chronic wounds.
Core Tip: Chronic wounds present a huge burden on healthcare systems worldwide, and diminish the quality of life of affected patients tremendously. Chronic wounds are often a source of frustration for attending clinicians as well, since many different types of dressings and other forms of conservative therapy and surgical debridement are used. We believe that local platelet-rich plasma (PRP) application is an easily performed procedure that could be offered to patients with chronic wounds after the failure of standard dressing-type procedures. We also believe that other more invasive regenerative treatment options should be postponed until after PRP is attempted.
- Citation: Dimova A, Boroš M, Dimov S, Konjevod J, Svetec M. Platelet-rich plasma treatment for chronic wounds: A case report and literature review. World J Clin Cases 2024; 12(33): 6635-6643
- URL: https://www.wjgnet.com/2307-8960/full/v12/i33/6635.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i33.6635
A chronic wound is defined as a skin barrier defect that persists after 3 months of the wound being sustained. This represents a major therapeutic challenge faced in clinic and is common among older patients with obesity, diabetes mellitus, and vascular disorders. Tissue repair is a universal phenomenon across all multicellular organisms, and much of the insight about mechanisms of wound healing and the relative temporal progression of the healing phases has come from studies in mice[1,2]. Deviation from the normal physiological healing process may be due to various factors, such as poor blood supply, impaired immune function, or inadequate nutritional support. Chronic wounds can cause significant distress to patients and place a heavy burden on the medical system[3].
Platelet-rich plasma (PRP) is an autologous blood-derived product containing high thrombocyte concentrations and various platelet-related growth factors; the thrombocyte levels of such are often 2-5 times higher than in normal blood. The utilization of these plasma products was first described by hematologists in the 1960s and 1970s, and they have since been used in various surgical and non-surgical fields such as dermatology, sports medicine, and orthopedics[4,5]. Recent systematic reviews and meta-analyses of randomized controlled trials have demonstrated that autologous PRP therapy effectively enhances wound healing and is considered a viable biological adjuvant therapy option in patients with non-healing diabetic foot ulcers[6].
Alpha-granules present in thrombocytes contain an abundance of growth factors, such as transforming growth factor β1 (TGF-β1), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor 1, platelet-derived growth factor (PDGF), and fibroblast growth factor, all of which play valuable roles in the mechanisms of healing. Upon their release from the alpha-granules, polypeptides and their receptors are activated to promote wound closure, which is achieved through cell differentiation and proliferation, chemotaxis, collagen synthesis, and angiogenesis. The PRP itself promotes wound healing due to the increased concentrations of these factors[7,8].
Older adults are more prone to chronic wounds than their younger counterparts, although both suffer significant impact to their quality of life from such. The true prevalence and incidence are hard to distinguish due to a lack of standardization in data collection. A substantial number of chronic wounds are underreported or not accurately described, especially when studies focus on other endpoints, such as lower-extremity amputations. It is also difficult to predict and identify prognostic factors for wound healing because of the multifactorial pathogenesis and different wound etiologies[9].
Patients with diabetes mellitus have impaired healing capabilities and run the risk of prolonged wound healing. Between 1 in 3 to 1 in every 5 patients with diabetes mellitus will develop a chronic non-healing wound in their lifetime. Non-diabetic patients have better regulation of angiogenesis and local inflammatory response to wound healing, as well as a more effective immune response to colonizing bacteria, with lower levels of reactive oxygen species[10]. Even when chronic wounds in diabetic patients heal, there is a risk of recurrence. The risk of recurrence within 1 year is roughly 40%, although it can be as high as 65% within 5 years. Some risk factors for diabetic ulcer recurrence have been identified, such as those involving progression of neuropathy, wound infection, peripheral artery disease, pre-ulcerative lesions, glycated hemoglobin level above 7.5%, and social factors[11].
Herein, we present the case of a patient with a long-standing chronic wound and multiple comorbidities, who was unsuccessfully treated with all routinely used measures, including different dressing approaches. Multiple antibiotic regimens were administered for wound infection, with repeated evaluation of microbiological swab results. Finally, after three PRP applications, the wound showed clinical improvement with complete restitution of the epithelial layer of the skin.
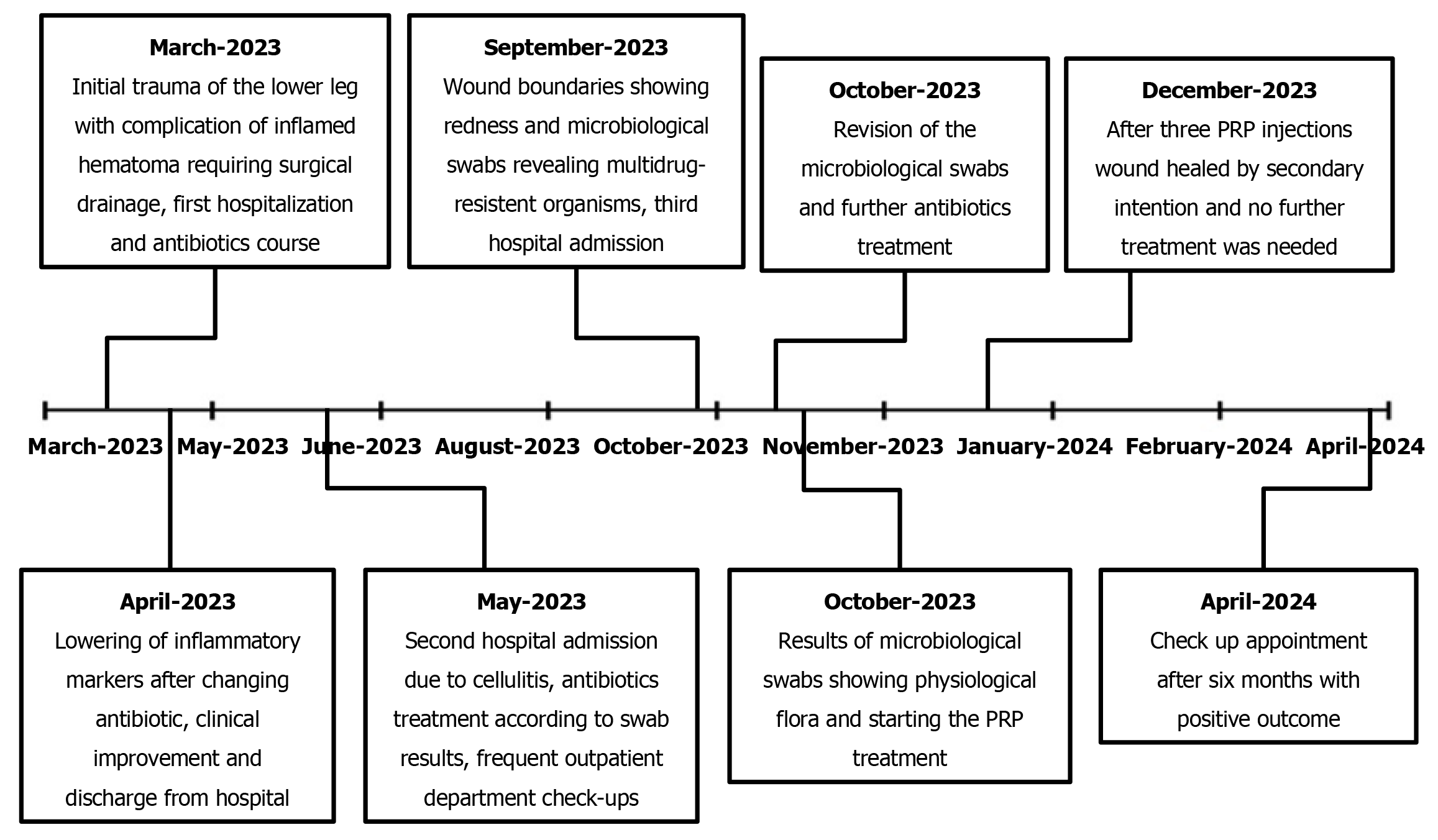
A 72-year-old male patient was admitted to our emergency department after suffering a contusion and superficial skin excoriation of the right lower leg from a wood log impact. His recovery was complicated by the development of a large hematoma that mandated incision and drainage. Eventually, a chronic wound developed at the incision site and persisted for 7.5 months.
Upon admission to our emergency department, the wound was cleaned and dressed. According to national guidelines and the patient’s antitetanic immunization status, combined active-passive immunization with tetanus toxoid and 50 U of human tetanus immunoglobulin was administered. Dual antibiotic first-line oral therapy was prescribed for 10 days in dosages of amoxicillin and clavulanic acid at 875 mg + 125 mg, twice daily, and clindamycin at 600 mg, three times daily.
The patient’s general health status was burdened with several comorbidities, including arterial hypertension, atrial fibrillation, type 2 diabetes mellitus, mitral regurgitation, and a cerebrovascular accident.
The patient’s personal and family history did not contain any significant events.
At presentation, the patient had localized swelling and a large area of superficial anterior lower leg skin excoriation, measuring 20 cm × 10 cm. Warfarin was temporarily discontinued from his longstanding therapy and replaced with low-molecular-weight heparin treatment in a dosage of 6000 IU daily, administered subcutaneously. However, 4 days after the traumatic incident, despite the exclusion of warfarin, the patient developed an inflamed hematoma measuring 10 cm × 7 cm.
After the patient developed the inflamed hematoma, laboratory tests revealed C-reactive protein (CRP) levels rising to 71.5 mg/L (normal range: 0.0–5.0 mg/L) and a leukocyte level of 9.1 × 109/L (normal range: 3.4–9.7 × 109/L), with a neutrophil relative count of 75.2% indicating a leftward shift. Incision and drainage of the hematoma was performed, with necrectomy of three separate marked areas of skin necrosis measuring 3 cm × 3 cm.
The patient was admitted to the hospital for intravenous antibiotic treatment of amoxicillin and clavulanic acid in dosages of 1 g + 200 mg, three times a day, and metronidazole at 500 mg, three times a day. Regular wound cleaning and dressing was performed daily, as well as addressing the possible signs of compartment syndrome. Due to lack of clinical response to the administered antibiotics (evidenced by persistent erythema and swelling with mild induration and CRP levels rising up to 161.0 mg/L, with leukocytes of 7.6 × 109/L and neutrophils making up 74.0% of white blood cells), on the 3rd day of hospitalization the amoxicillin was replaced with intravenous clindamycin in a dosage of 600 mg, three times daily, which led to clinical improvement.
Three days after the antibiotic replacement, a regression of local inflammation signs was observed and CRP levels dropped to 86.9 mg/L, with the leukocyte level lowering to 5.5 × 109/L and the relative neutrophil count to 68.1%.
Initial X-ray examinations revealed no fractures or other pathologies, apart from demineralization of the tibia and calcifications in the arteries of that region.
The patient was discharged on day 6 after hospitalization in good general condition, afebrile, and without signs of compartment syndrome. Satisfying local healing dynamics were seen, with an erythema regression observed in the proximal and distal part of the wound. Oral antibiotic therapy was continued for 7 days with clindamycin at 600 mg, three times daily, and metronidazole at 400 mg, three times daily. The patient’s right lower leg was cast-immobilized. The immobilization was removed at a surgical check-up appointment 2 weeks later.
After this first hospitalization, the wound healing progressed in an uneventful manner; the patient was asymptomatic over this 30-day period, recorded at three consequent follow-up appointments in the outpatient department. However, at 1 month after he had been discharged from the hospital, a markedly increased secretion was noted from the wound, along with erythema and induration of the lower leg. A microbiological swab was taken and an immobilization casting of the right lower leg was performed again. The patient was admitted to hospital for the second time because of the cellulitis that had developed. In addition, low-molecular-weight heparin treatment was again introduced to replace warfarin. Wound swabs after 3 days showed methicillin-resistant Streptococcus aureus (MRSA) and Klebsiella pneumoniae (extended-spectrum β-lactamases); sensitivity testing showed both to be responsive to sulfamethoxazole and trimethoprim treatment, and the combination antibacterial therapy was started immediately. The patient was discharged after 5 days, with continuation of the oral sulfamethoxazole and trimethoprim antibiotic treatment in dosages of 400 mg + 80 mg, twice daily, for 10 days.
During the next month, the wound healed by secondary intention, with several necrectomies required due to necrotic demarcation of the edges of the wound. Regular follow-up visits to the outpatient department with wound care took place every 7-14 days, with primary care visits every 2-3 days. During this treatment phase, the wound reduced in size, there was no induration or erythema, and wound secretion was minimal. Unfortunately, the reduction of wound size eventually stopped, leaving the patient with a chronic wound measuring 18 cm × 3 cm to 18 cm × 4 cm. Furthermore, 3 months after the incision and hematoma drainage, hypergranulation tissue was noted on the wound edges, and the patient started complaining of heavy pain in the wound area.
The patient complained of persistent pain 6 months after the initial trauma. At that point, the wound was 15 cm long with redness along its boundaries, but without secretion and fluctuation. Oral clindamycin antibiotic treatment was given three times daily in doses of 600 mg for 2 weeks.
In a follow-up appointment we noted no success and persistent erythema measuring 12 cm × 2 cm to 12 cm × 3 cm. Due to the lack of clinical response to the oral antibiotic regimen, the patient was readmitted to hospital for the third time. New swab results were reviewed by an infectologist to address the presence of multidrug-resistant organisms. Isolates of MRSA and Enterobacter spp had been found in the wound swabs, and the infectologist indicated application of oral sulfamethoxazole and trimethoprim in dosages of 800 mg + 160 mg, twice daily, and intravenous application of meropenem in a dosage of 1 g, daily. The wound infection subsided in response to this treatment regimen. The patient was discharged after 5 days with oral antibiotic sulfamethoxazole and trimethoprim treatment in a dosage of 800 mg + 160 mg, twice daily, for 7 more days.
The patient was ultimately diagnosed with a chronic wound of the right lower leg.
Seven and a half months after the initial trauma, microbiological swabs revealed physiological flora in a chronic wound measuring 12 cm × 3 cm with moderate-to-heavy persistent pain [visual analog scale (VAS), 6–8]. Hyperbaric oxygen treatment was considered; however, as the patient could not get an appointment within 3 months and due to the patient’s poor compliance to such a scenario, we opted for PRP treatment to promote wound healing and relieve his chronic pain.
A multidisciplinary consultation between the specialists involved in treatment concluded that PRP injection should not be administered if the swab results showed pathological microorganisms or anything except physiological flora. Also, the patient needed to be hemodynamically stable, without other acute illnesses and with adequate laboratory values of red blood cells and thrombocytes, which were within normal range for our patient. These requirements were met and we began the PRP treatment protocol.
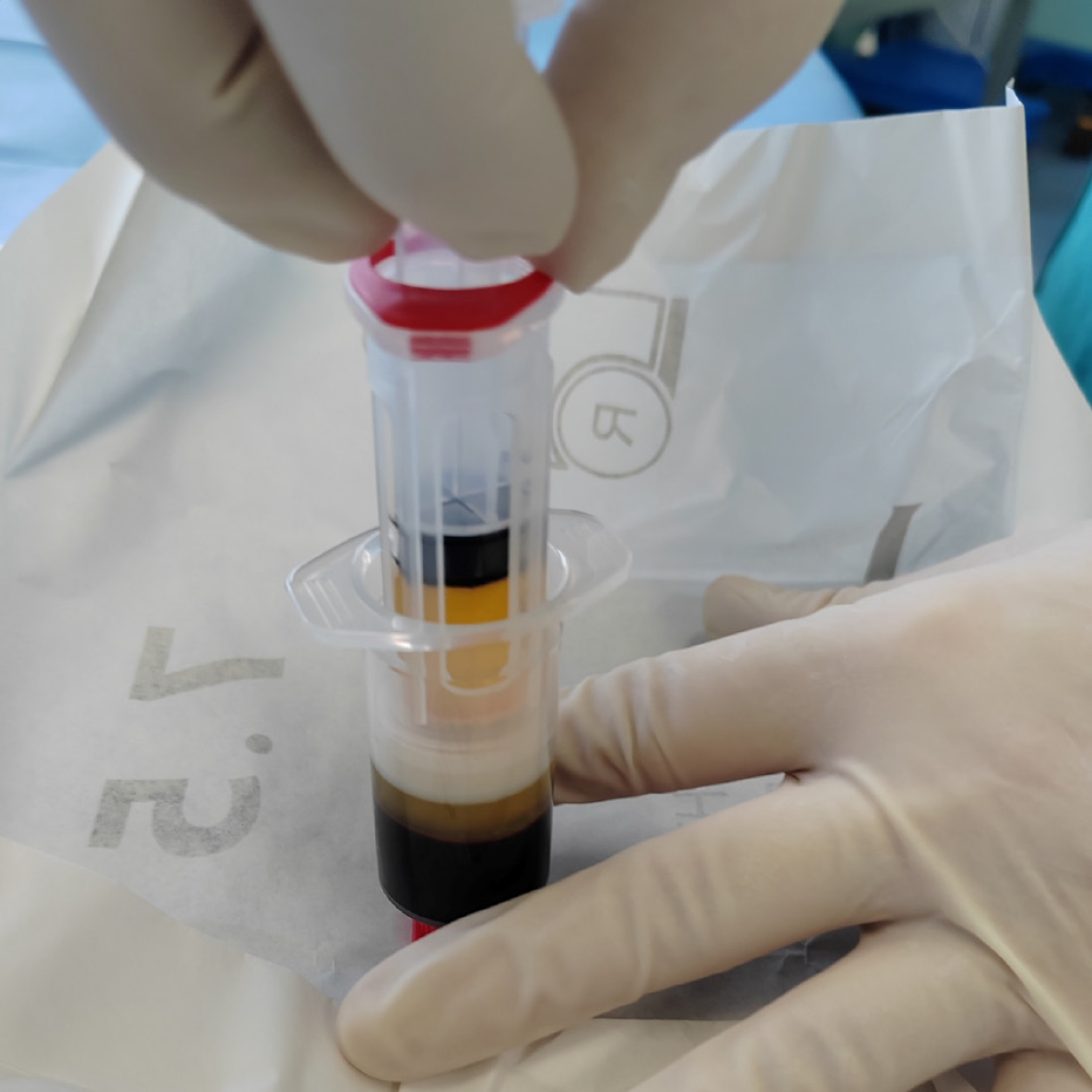
Autologous PRP was obtained by venipuncture and aspiration of 15 mL of the patient’s peripheral venous blood using the Arthrex ACP® Double-Syringe System (Naples, FL, United States). The test tube was then centrifuged at 1,500 rpm for 5 minutes (Horizon 24-AH centrifuge; Drucker Diagnostics, Port Matilda, PA, United States). From the separated plasma, we obtained 6 mL of PRP to be used for application via the Double-Syringe System (Figure 1). Autologous PRP (4 mL) was then administered around the wound edges, and the remaining PRP (2 mL) was freely sprayed onto the wound surface. Wound aftercare was performed every 2nd day with application of 0.9% NaCl and a sterile cotton mesh covering.
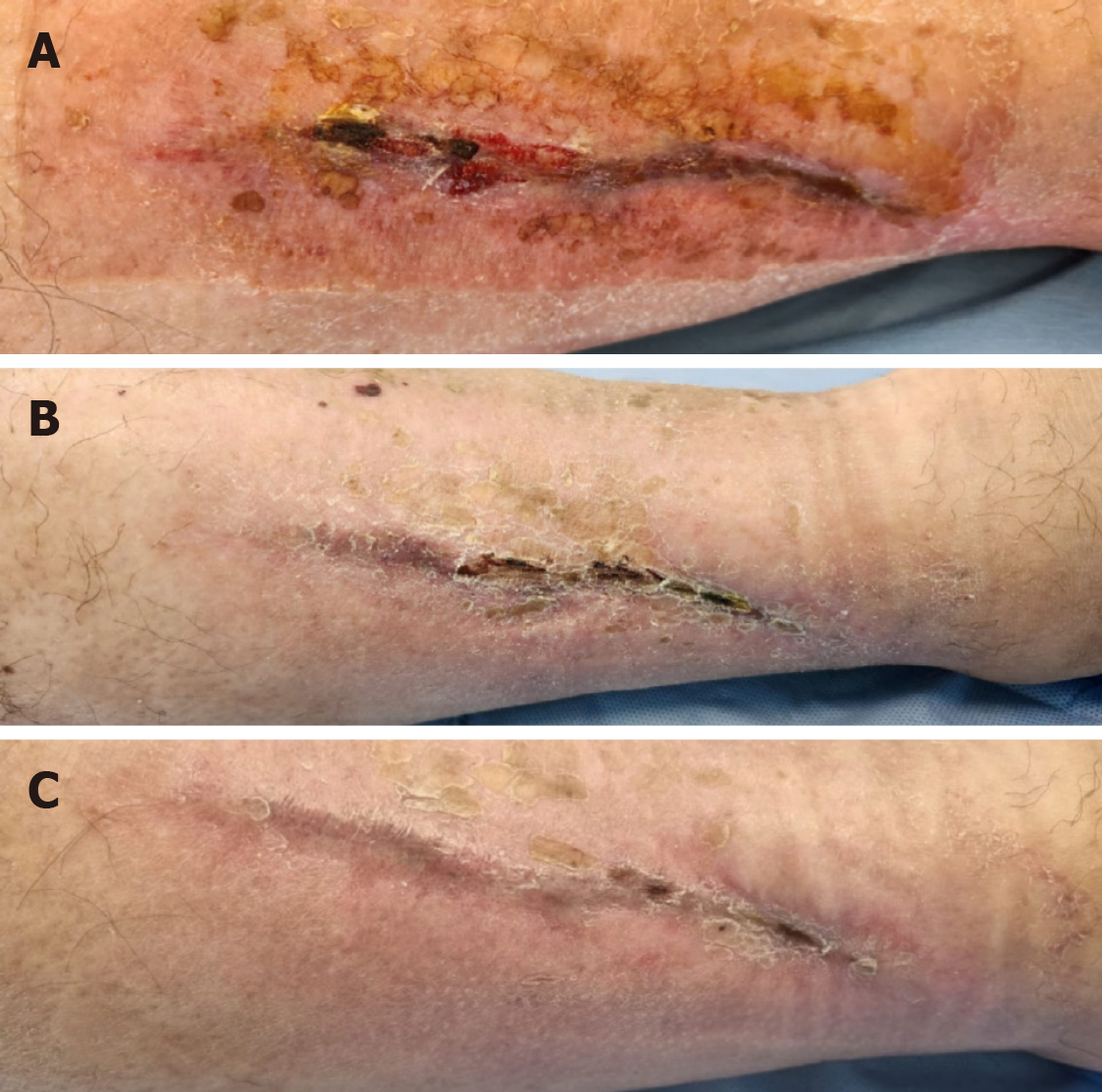
The patient showed markedly positive results with regard to VAS score diminution to 3–4 as early as 2 days post-procedurally. Two weeks after the first PRP application, improved healing was observed. The patient received a second and third PRP application in the same manner, at a day-hospital, with 2-week interval. Further improvement, both subjective and objective, was observed, and 4 weeks after the third PRP application we decided to cancel the further planned PRP applications due to the patient’s complete lack of pain and the observed complete restitution of skin integrity. Wound images taken immediately before the application of autologous PRP and after three PRP treatments show the tremendous improvement (Figure 2).
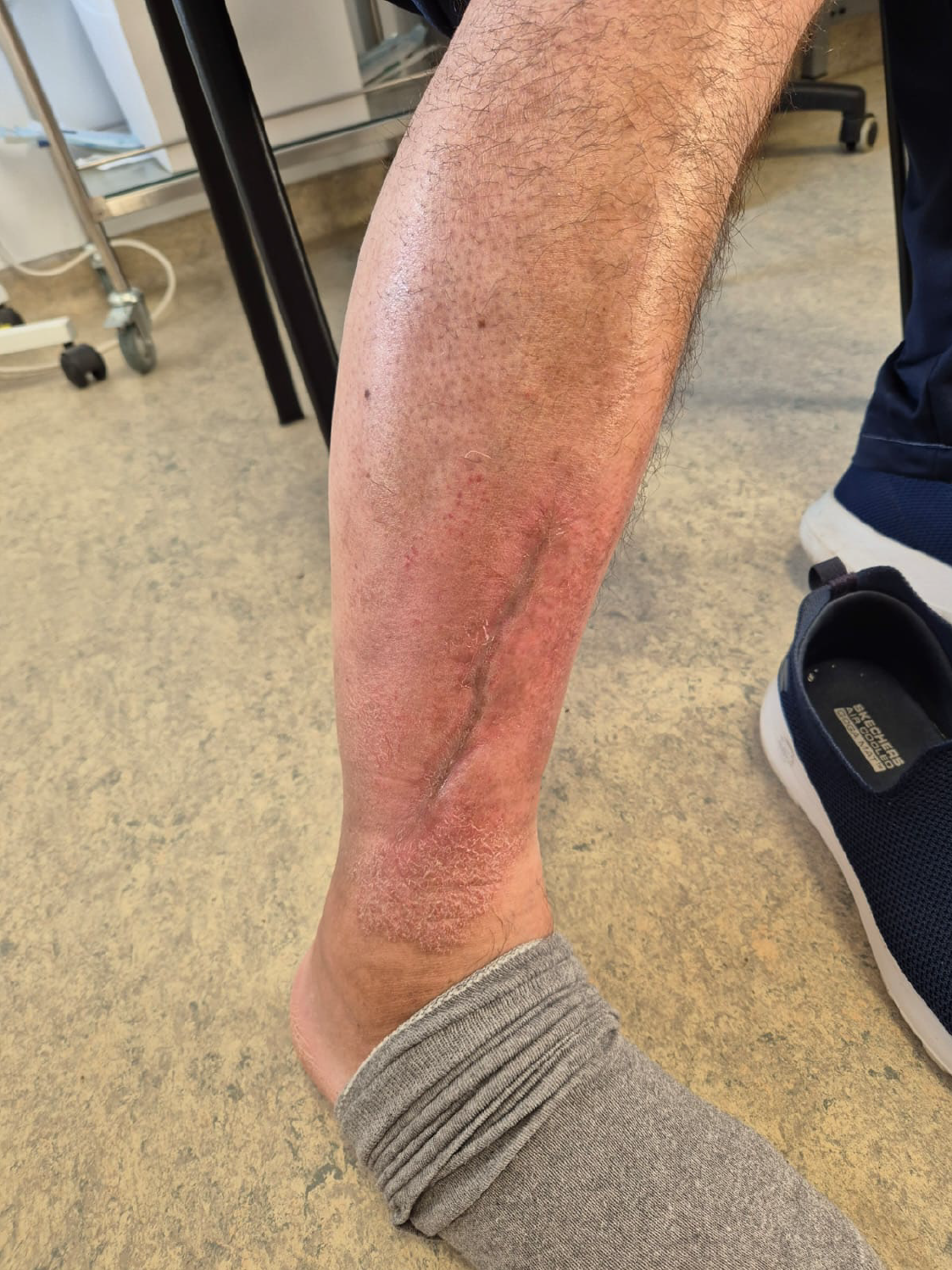
Six months after the third PRP application, the patient was still fully mobile, free of pain, and returned to his normal activities, with no residual skin defects (Figures 3 and 4). He reported no pain and complete quality of life and mobility restitution.
Chronic wounds with poor response to conventional wound dressings and treatments are a relatively common healthcare problem and can pose a real clinical challenge. Autologous PRP treatment could impact the costs in chronic wound management.
Regenerative medicine in wound healing is a constantly developing area with novel strategies and PRP treatment as an important adjuvant biological therapy. Efficacy and safety are key factors for PRP usage, which is generally considered safe in autologous form. However, the lack of standardization and high-quality randomized controlled trials have been a matter of dispute[12,13]. PRP is used to treat a wide array of wound etiologies including diabetic, pressure ulcer, venous ulcer, surgical, or traumatic wounds, and wounds of other etiologies. As there are currently many case reports and series showing promising results, further evidence-based controlled clinical studies on the effectiveness of PRP are awaited with great interest[14-18].
There are different types of autologous PRP systems available on the market. Each preparation technique has different properties and specifications. Researchers are encouraged to state exactly which system was used in a study, so that further scientific analyses can be made. We used a preparation technique which removed white blood cells. A study comparing five different PRP preparation techniques in a single-donor model, including the Arthrex ACP®, showed positive correlations between platelet dose and VEGF dose, PDGF dose, EGF dose, and TGF-β1 dose of growth factors. Significant biological variations from different PRP preparation systems were studied, which may explain the noted differences and variability in clinical benefits reported in the literature. Most products are prepared in an autologous manner, with only a few minor adverse effects reported after treatment protocols with PRP. Variability of PRP preparation systems makes it difficult to properly analyze adverse effects, but inflammatory reactions could be due to the residual living cells in products containing leukocytes and freshly used products. These types of products may exacerbate local inflammatory responses; however, PRP treatment is considered safe and has a low rate of adverse effects[19,20]. Leukocytes containing products are sometimes referred to as L-PRP in the literature. Some key features of PRP preparations are that we can distinguish between high-platelet dose systems (usually 5—9 times higher concentrations of platelets than in blood) and low-platelet dose systems (2.5—3 times higher concentration) like the Arthrex ACP® system. The Mini GPS III Platelet Concentration System (Zimmer Biomet, Warsaw, IN, United States) is referred to as a system with high-platelet dose; however, the Arthrex ACP®, Regen PRP (RegenLab, Brooklyn, NY, United States), and Selphyl System (Selphyl, Bethlehem, PA, United States) are low-platelet dose systems. The Mini GPS III and Regen PRP systems produce PRP with high leukocyte concentrations, as opposed to the Selphyl System and the Arthrex ACP® system[19,21]. We are interested in new systems that we can offer to our patients, and new bio formulations, especially those that offer autologous regenerative treatment covered by medical insurance or at a reasonable surcharge for the patient.
Our experience with PRP treatment in our institution is mostly related to orthopedic cases, such as intra-articular injections for degenerative knee disease; we have had positive experiences, high patient compliance, and good clinical feedback from these treatments. This patient is the first case of PRP treatment of a chronic wound in our institution, and while we were in the process of publishing this paper, we commenced treatment of other patients suffering from chronic wounds of different origins, for example, diabetic foot ulcers. We are currently exploring new options for our institution. We have been using the Arthrex ACP® system, which was the only system available in our hospital at the time. Such treatment was covered by social medical insurance at a state level for pain treatment, including pain from chronic wounds. We are open to exploring other PRP systems, if they can offer better quality product and a high clinical rate of success in treating our patients. In this case, we only used photograph documentation and VAS score measurement, but other factors like wound depth, localization, vascularization, and different comorbidities could be considered for further analysis.
Studies analyzed in a meta-analysis from 2022 showed no serious side effects of PRP treatment in chronic wound management. Most studies report no side effects. There were some reports of pain in the injection area, contact dermatitis, and maceration[22]. A case series from 2017 analyzed patients with chronic non-healing ulcers treated with autologous PRP. The treatment demonstrated safety and efficacy with a mean time of ulcer healing of 8.2 weeks. Of note, PRP treatment was found to have the potential to prevent lower-extremity amputations caused by non-healing wounds and their complications, but this needs to be assessed further[18]. Tsai et al[23] compared PRP and platelet-derived patches treatment vs traditional advanced wound dressings in patients with chronic wounds in their randomized controlled trial from 2019. A statistically significant clinical improvement was observed in the PRP group after only 2 weeks, and an improved overall treatment success was observed in patients with diabetes. Platelet-derived patches may be considered as an adjuvant to classic autologous PRP injections in the future[23]. PRP can also be combined with ablative lasers or microneedling for various types of atrophic scars, with evidence of safety and efficacy[24].
As an alternative to PRP treatment, our patient could have undergone hyperbaric oxygen therapy (HBOT). This type of treatment is beneficial for the treatment of hypoxic wounds and is available through many different healthcare providers. Absolute and relative contraindications, particularly those regarding restrictive and obstructive airway diseases, should also be considered when recommending HBOT treatment to patients[25]. HBOT is used in diabetic foot ulcer treatment, and enhances short term healing; however, the long term effects and reduction of risk for major amputations are still debatable[26,27].
Studies have presented interesting results for the application of PRP for chronic ulcers; however, the absence of precise clinical protocols and guidelines limit the extension of its use. There is a need for robust clinical trials to determine the most accurate indication and preferred method of obtaining PRP and application protocols. The international scientific community should be encouraged to review the present study and related articles to advance this area of research[12,28]. The literature offers some implications that delivering higher doses of growth factors may be effective[29], but there is little evidence of greater effectiveness in chronic wound management with higher PRP dose. Final bio formulations and biological properties of PRP are still not conclusive, and the full potential of PRP treatment remains to be determined. Scientific efforts to reveal explicit roles of thrombocytes, leukocytes, growth factors, mesenchymal stem cells, and cell-to-cell interactions should be continued. The analgesic effects of PRP treatment should also be further assessed[30]. We are looking forward to well-documented clinical studies to establish the full potential of PRP treatment in wound healing and regenerative medicine.
Delayed wound healing and stable chronic wound patterns prone to complications are a clinical reality, usually with prolonged healing, high costs, and low-to-modest success rates. This case shows success of PRP application in achieving a complete skin integrity restitution in a long-standing, repeatedly infected chronic wound that did not respond to other conservative treatment modalities. Autologous PRP treatment may be a valuable, cost-effective alternative and possible adjuvant treatment for chronic wounds with poor healing tendency in selected patients. However, further research and standardization of protocols are urgently needed.
| 1. | Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 640] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 2. | Grose R, Werner S. Wound-healing studies in transgenic and knockout mice. Mol Biotechnol. 2004;28:147-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Han G, Ceilley R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther. 2017;34:599-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 763] [Cited by in RCA: 1189] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 4. | Alves R, Grimalt R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018;4:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 378] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 5. | Shively JA, Sullivan MP, Chiu JS. Transfusion of platelet concentrates prepared from acidified platelet-rich plasma. Transfusion. 1966;6:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Deng J, Yang M, Zhang X, Zhang H. Efficacy and safety of autologous platelet-rich plasma for diabetic foot ulcer healing: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2023;18:370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Sánchez-González DJ, Méndez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: key for regeneration. Int J Pept. 2012;2012:532519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2305] [Cited by in RCA: 2405] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 9. | Gould L, Abadir P, Brem H, Carter M, Conner-Kerr T, Davidson J, DiPietro L, Falanga V, Fife C, Gardner S, Grice E, Harmon J, Hazzard WR, High KP, Houghton P, Jacobson N, Kirsner RS, Kovacs EJ, Margolis D, McFarland Horne F, Reed MJ, Sullivan DH, Thom S, Tomic-Canic M, Walston J, Whitney JA, Williams J, Zieman S, Schmader K. Chronic wound repair and healing in older adults: current status and future research. J Am Geriatr Soc. 2015;63:427-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 10. | Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic Wound-Healing Science. Medicina (Kaunas). 2021;57:1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 333] [Article Influence: 83.3] [Reference Citation Analysis (1)] |
| 11. | Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2320] [Article Influence: 290.0] [Reference Citation Analysis (2)] |
| 12. | Qu S, Hu Z, Zhang Y, Wang P, Li S, Huang S, Dong Y, Xu H, Rong Y, Zhu W, Tang B, Zhu J. Clinical Studies on Platelet-Rich Plasma Therapy for Chronic Cutaneous Ulcers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Wound Care (New Rochelle). 2022;11:56-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Li L, Chen D, Wang C, Yuan N, Wang Y, He L, Yang Y, Chen L, Liu G, Li X, Ran X. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen. 2015;23:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Carter MJ, Fylling CP, Parnell LK. Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty. 2011;11:e38. [PubMed] |
| 15. | Oliveira BGRB, Carvalho MR, Ribeiro APL. Cost and effectiveness of Platelet Rich Plasma in the healing of varicose ulcer: Meta-analysis. Rev Bras Enferm. 2020;73:e20180981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Gohar MM, Ali RF, Ismail KA, Ismail TA, Nosair NA. Assessment of the effect of platelet rich plasma on the healing of operated sacrococcygeal pilonidal sinus by lay-open technique: a randomized clinical trial. BMC Surg. 2020;20:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Trull-Ahuir C, Sala D, Chismol-Abad J, Vila-Caballer M, Lisón JF. Efficacy of platelet-rich plasma as an adjuvant to surgical carpal ligament release: a prospective, randomized controlled clinical trial. Sci Rep. 2020;10:2085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017;24:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 19. | Magalon J, Bausset O, Serratrice N, Giraudo L, Aboudou H, Veran J, Magalon G, Dignat-Georges F, Sabatier F. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy. 2014;30:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 20. | Acebes-Huerta A, Arias-Fernández T, Bernardo Á, Muñoz-Turrillas MC, Fernández-Fuertes J, Seghatchian J, Gutiérrez L. Platelet-derived bio-products: Classification update, applications, concerns and new perspectives. Transfus Apher Sci. 2020;59:102716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Pachito DV, Bagattini ÂM, de Almeida AM, Mendrone-Júnior A, Riera R. Technical Procedures for Preparation and Administration of Platelet-Rich Plasma and Related Products: A Scoping Review. Front Cell Dev Biol. 2020;8:598816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Meznerics FA, Fehérvári P, Dembrovszky F, Kovács KD, Kemény LV, Csupor D, Hegyi P, Bánvölgyi A. Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Clin Med. 2022;11:7532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Tsai HC, Lehman CW, Chen CM. Use of platelet-rich plasma and platelet-derived patches to treat chronic wounds. J Wound Care. 2019;28:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Ebrahimi Z, Alimohamadi Y, Janani M, Hejazi P, Kamali M, Goodarzi A. Platelet-rich plasma in the treatment of scars, to suggest or not to suggest? A systematic review and meta-analysis. J Tissue Eng Regen Med. 2022;16:875-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 25. | Oztürk F, Ermertcan AT, Inanir I. Hyperbaric oxygen therapy for the management of chronic wounds. Cutan Ocul Toxicol. 2013;32:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004;CD004123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;2015:CD004123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 28. | Gentile P, Garcovich S. Systematic Review-The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. Int J Mol Sci. 2020;21:5702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Tao X, Aw AAL, Leeu JJ, Bin Abd Razak HR. Three Doses of Platelet-Rich Plasma Therapy Are More Effective Than One Dose of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Arthroscopy. 2023;39:2568-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci. 2020;21:7794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 442] [Article Influence: 88.4] [Reference Citation Analysis (0)] |