Published online Nov 16, 2024. doi: 10.12998/wjcc.v12.i32.6526
Revised: August 8, 2024
Accepted: August 12, 2024
Published online: November 16, 2024
Processing time: 114 Days and 15.1 Hours
Myeloid sarcoma (MS) is a rare neoplasm characterized by the proliferation of immature myeloid precursor cells outside the bone marrow. The pathogenesis of MS is complex and not completely understood. Moreover, it develops in any extramedullary site of the body. In this editorial, we discuss the article published by Li et al, which presents a clinical case involving a 32-year-old man who exhibited gingival inflammation in the maxillary region. It was initially diagnosed as periodontal disease. However, clinical evaluation revealed a firm, grayish-white mass which underscored the need for comprehensive diagnostics to distinguish MS from other oral conditions. This article emphasizes the different clinical presentations of similar case studies in the literature, and highlights the difficulty in diagnosing oral MS due to its rarity and variability in clinical manifestation. The treatment of MS depends on the clinical presentation, tumor location, and the patient's response to conventional therapies. The various therapeutic options currently available are analyzed and discussed. Early inter
Core Tip: In this editorial, we highlight the analysis of oral myeloid sarcoma (MS) as discussed in the article by Li et al. We examine the diagnostic and therapeutic challenges inherent in MS, with focus on its diverse clinical presentations, particularly in the gingival tissue, and review the relevant literature. We emphasize the importance of understanding the pathogenesis of MS, the role of dental professionals in early detection of the disease, and the various treatment options available, such as chemotherapy, radiotherapy, stem cell transplantation, targeted therapies, and immunomodulatory therapies.
- Citation: Martínez Nieto M, González Gómez LA, Gómez Mireles JC, Lomelí Martínez SM. Diagnostic and therapeutic challenges of myeloid sarcoma in the oral cavity. World J Clin Cases 2024; 12(32): 6526-6533
- URL: https://www.wjgnet.com/2307-8960/full/v12/i32/6526.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i32.6526
Myeloid sarcoma (MS), also known as chloroma or granulocytic sarcoma, involves the proliferation and accumulation of immature myeloid precursor cells outside the bone marrow[1,2]. First described in 1811, MS is often referred to as granulocytic sarcoma, with the term “chloroma” attributed to its greenish color on exposure to air due to the presence of myeloperoxidase[3]. This neoplasm affects approximately 3%-8% of patients with acute myeloid leukemia (AML)[4,5]. MS can manifest in two primary forms: Primary MS, arising de novo without systemic involvement of leukemia, and secondary MS, which occurs concurrently with bone marrow involvement or as a relapse of leukemia[6]. This type of sarcoma can occur in a variety of extramedullary locations such as the skin, lymph nodes, soft tissues, gastrointestinal tract, bone, oral cavity, and periorbital tissues, among others[2].
Clinically, MS can manifest in diverse and non-specific ways[1,5]. The pathogenesis of MS is a complex process that involves genetic alterations, aberrant cell signaling, changes in the expression of adhesion molecules and chemokines, and the invasive capacity of malignant cells. These events allow the dissemination and proliferation of immature myeloid cells in various extramedullary sites, contributing to the heterogeneous clinical presentation of this disease[4,5]. The development of MS is associated with genetic and molecular alterations through mutations in genes such as NPM1, FLT3, and CEBPA, which are common in AML and may be present in MS, contributing to the extramedullary dissemination of malignant cells. These mutations lead to the activation of signaling pathways that promote cell proliferation, resistance to apoptosis, and abnormal migration of myeloid cells[5]. Abnormal expression of adhesion molecules and chemokines also plays a crucial role in the pathogenesis of MS. Malignant myeloid cells can express high levels of adhesion molecules such as CD44, which facilitate their adherence and migration to extramedullary sites[4,5]. A distinctive characteristic of MS is its ability to infiltrate various tissues. This invasive behavior is due to the ability of malignant myeloid cells to degrade the extracellular matrix and remodel the tissue microenvironment. Specifically, the overexpression of enzymes such as matrix metalloproteinases allows these cells to invade and colonize extramedullary tissues[4].
Histologically, MS is characterized by diffuse infiltration of immature blast cells with basophilic nuclei and increased nuclear-cytoplasmic ratios. It is common to observe rare mitoses and a positive reaction to myeloperoxidase (MPO) and CD117 in immunohistochemical studies, which helps in its diagnosis[7].
The present editorial emphasizes the analysis of a rare case of MS involving the gingival tissue, along with a review of the relevant literature. By reviewing the clinicopathological features, treatment regimens, prognosis, and future perspectives surrounding oral MS, we aim to provide clinicians with valuable insights into the recognition and management of this rare entity. Our goal is to increase awareness among dental professionals of the various clinical presentations of oral MS and its potential role in facilitating early diagnosis and appropriate referral for comprehensive evaluation and treatment. Ultimately, we aim to contribute to improving patient outcomes through early recognition, accurate diagnosis, and timely intervention in cases of oral MS.
MS occurs primarily in the bones and soft tissues but can affect several anatomical sites throughout the body, including the skin, lymph nodes, orbit and eye, oral cavity, bronchi, pericardium, peritoneum, gastrointestinal tract, kidneys, reproductive organs, breasts, and bladder[5,7]. Clinically, this rare form of myeloid neoplasm can present with pain, ulcerations, palpable masses, and systemic symptoms such as fever and weight loss. Although this pathology occurs predominantly in bony structures and soft tissues, its presentation in the oral cavity is exceptionally rare, with reported cases typically affecting single sites and rarely involving both the maxilla and the mandible. Oral MS exhibits diverse and non-specific clinical manifestations, with the gums and hard palatal mucosa being the most commonly involved sites (> 80%)[3,6]. The main clinical finding of this entity is often diffuse gingival inflammation, with other presentations including localized inflammation or mass, sometimes associated with unhealed extraction sockets and, rarely, ulcers[6]. Despite its rarity in oral tissues, MS may manifest during relapse in approximately 3%-5% of patients with AML, with a higher incidence observed in the skin, bone, or gastrointestinal tract[8].
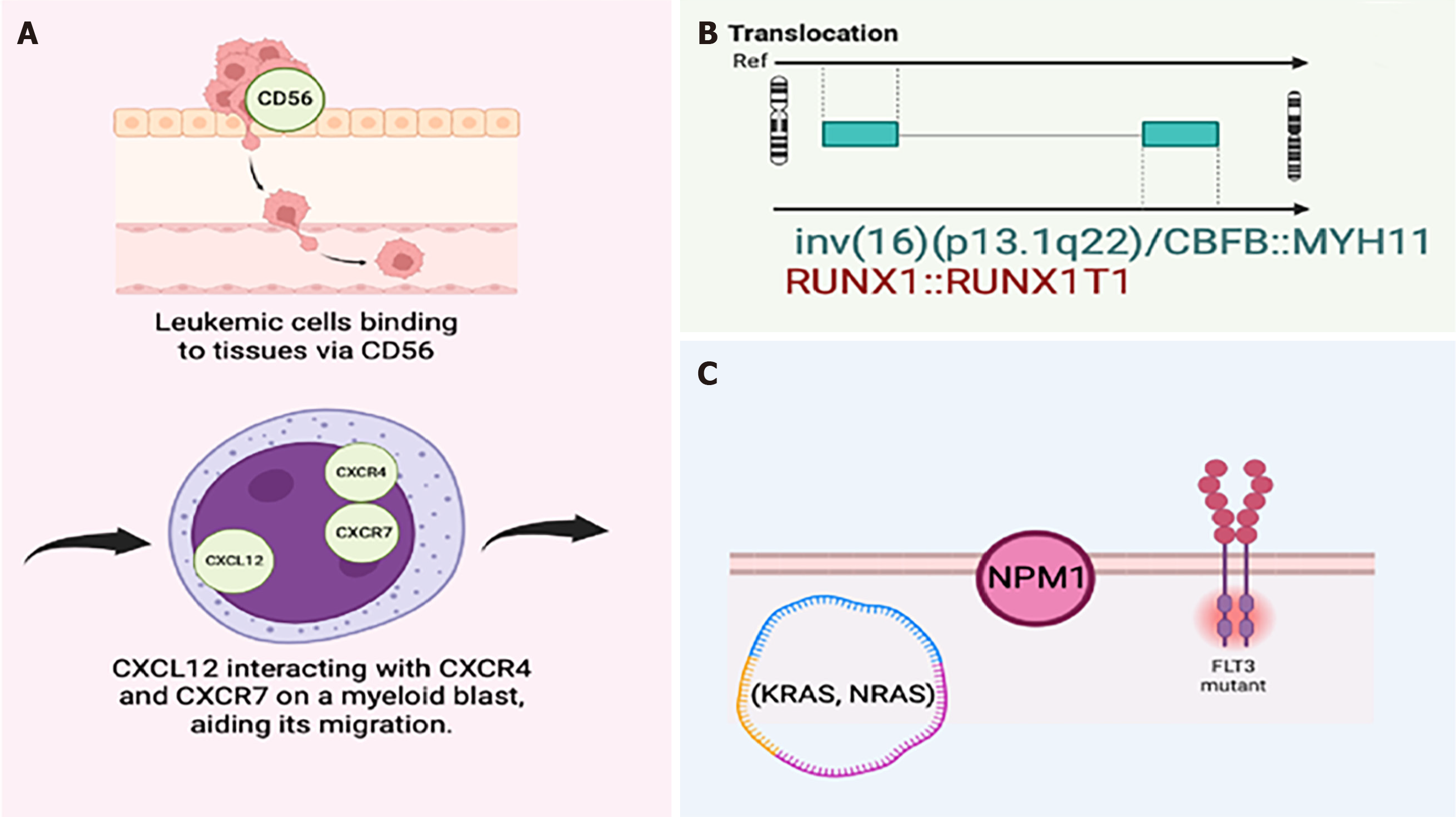
The molecular mechanisms driving the transformation of (MS) cells remain to be elucidated, but several pathways have been explored. Key areas of focus include cell-cell and cell-matrix interactions within the bone marrow microenvironment, involving adhesion molecules and chemokine receptor/ligand interactions. Differential expression of the adhesion molecule CD56 promotes the binding of leukemic blasts to tissues, particularly in common MS locations. The chemokine receptor CXCR4 and its ligand CXCL12, along with CXCR7, facilitate the migration of leukemia cells to peripheral tissues, contributing to MS progression (Figure 1)[1].
Chromosomal abnormalities are found in 54%-70% of MS cases, with initial studies suggesting a higher incidence (8%-21%) of the RUNX1:RUNX1T1 translocation. However, larger studies found this translocation to be rare, occurring in only 2%-3% of cases. The inv(16)(p13.1q22)/CBFB::MYH11 translocation is also noted in some MS cases. Other cytogenetic abnormalities include t(9;11), del(16q), and anomalies in chromosomes 4, 7, 8, and 11. FLT3-ITD mutations are found in up to 15% of MS cases, and NPM1 mutations occur in 20%-50% of cases. NPM1 is the most frequently mutated gene in MS, often associated with myelomonocytic, monocytic, or monoblastic differentiation. Mutations in the RAS pathway (KRAS, NRAS, BRAF, PTPN11, and CBL) are common in MS, with KRAS/NRAS mutations being the most frequent (70%). IDH1/2 mutations are less common, found in 26%-42% of MS cases[1]. The t(8;21) translocation is the most common cytogenetic abnormality in MS associated with medullary AML. Overall, findings suggest that bone marrow analysis is often sufficient for diagnosing MS due to the presence of these consistent abnormalities[1].
The location of MS in the oral cavity presents a diagnostic challenge due to its rarity and diverse clinical presentations. The literature on MS with maxillary gingival inflammation as the initial symptom provides valuable knowledge about its clinical presentation, diagnosis, and management. Therefore, it is important to understand the varied presentations of MS in the oral cavity for accurate diagnosis and timely management. Li et al[9] published an interesting paper, presenting an unusual MS in a 32-year-old man with gingival inflammation in the maxillary region, which resembled periodontal disease[10]. Intraoral examination revealed a firm, tender, grayish-white mass with regular surfaces and an inflamed mass in the gingival area of the left molar. The importance of considering MS as a possible diagnosis for gingival lesions is emphasized, particularly when the clinical features are unusual. The gray-white appearance of the lesion, in contrast to the typical colors of periodontal disease, underscored the need for comprehensive clinical and histopathological examinations to distinguish MS from other oral conditions. MS can mimic several oral diseases, requiring accurate diagnosis through histological and immunohistochemical analysis. A manifestation in the oral cavity as an initial symptom of MS coincides with the case studies published by Kurdoğlu et al[5], de Andrade et al[7], Shen et al[2], Wang et al[6], and Kole et al[10] (Table 1). The case presented by Kurdoğlu et al[5] highlights the challenges in the diagnosis and management of oral MS associated with AML. They reported the case of a 29-year-old woman who presented with a severely painful gray-brown sessile keratinized lesion on the anterior upper labial gingiva, accompanied by submandibular lymphadenopathy[5]. These signs and symptoms are similar to those reported by de Andrade et al[7]. In this latter study, they described the management of MS in a 24-year-old woman who, on physical examination, revealed cervical lymphadenopathy. The intraoral examination showed discrete areas of clotted blood within the gingival sulcus of some teeth and a painful 3 cm brown inflammation with a necrotic and bleeding surface located on the lower posterior gum[7]. Shen et al[2] reported the clinical case of a 41-year-old woman who, upon extraoral examination, showed facial symmetry and absence of lymphadenopathy. The intraoral examination revealed a gingival enlargement in the maxillary anterior region, extending from the first right premolar to the left lateral incisor, with sizes of 2 cm × 1.5 cm, 0.5 cm × 0.5 cm, and 0.5 cm × 1 cm. The hyperplasia involved the gingival papillae and free and attached gingiva, with the gingival papillae of the mandibular anterior teeth also enlarged. The affected gums were red, soft, edematous, and not painful, and dental plaque was observed. On general examination, no significant physical findings where noted, and the patient denied any pain in the oral cavity or throughout the body[2]. Similarly, Wang et al[6] reported a series of clinical cases, one of which highlighted an oral examination that revealed an asymptomatic, firm, and pink inflammation, measuring 4.0 cm × 1.5 cm, in the upper right gum from the third molar to the first premolar, with white papules on the surface. The lesion was neither painful nor ulcerated[6]. Likewise, Kole et al[10] described a rare case of granulocytic sarcoma in the jaw, where a hard bony swelling of approximately 3 cm × 2 cm was identified, extending from the distal surface of tooth 33 to the distal surface of tooth 37; paresthesia was present on the left side of the lower jaw and lip region[9]. The intraoral evaluations in the case studies of Kurdoğlu et al[5], de Andrade et al[7], Shen et al[2], Wang et al[6], and Kole et al[10] are similar to that reported by Li et al[9] regarding the site of MS manifestation, which is the gingival tissue, and the clinical presentation of the pathology. In contrast, Wang et al[6] published a series of clinical cases of MS in the oral cavity. In one case, oral evaluation revealed a lesion in the palate, and in another reported case, a lesion in the lower lip[6]. These two clinical cases had a diagnosis of MS in the oral cavity but differed from the case by Li et al[9], as they manifested in different locations[10]. The authors of all the previously mentioned oral MS case studies agree that given the frequency of reactive or neoplastic lesions in the oral cavity, particularly in gingival masses, a careful oral examination is essential for an accurate diagnosis. Additionally, they emphasize the need for greater awareness among dentists about the various presentations of MS and the importance of interdisciplinary collaboration between clinicians and pathologists for an accurate diagnosis.
| Ref. | Age (years) | Gender | Tumor location | Symptoms and signs | Imaging examinations | Results from histological examination | Immunohistochemical staining | Diagnosis | Treatment |
| Kurdoğlu et al[5], 2013 | 29 | Female | Gingiva | Painful buccal gingival swelling and bilateral submandibular lymphadenopathy, no gingival bleeding on probing | Radiological examinations did not show any bone pathology | Monomorphous diffuse infiltrate. Extramedullary myeloblastic malignancy | Positive reaction to CD117 and myeloperoxidase (MPO) | MS | Idarubicin and ARA-C chemotherapy regimen at first treatment phase. Second chemotherapy session 3-5-7 (daunorubicin, etoposide, and ARA-C) |
| de Andrade et al[7], 2017 | 24 | Female | Gingiva | Fast growing painful gingival swelling, fever, fatigue, and cervical lymphadenopathy. Bluish swelling on the right posterior lower gingiva exhibiting necrotic surface | Radiographic examination of the mandible showed no bone involvement | Undifferentiated tumor cells with granulocytic appearance | CD99, MPO, and Ki-67 (60%) | MS associated with AML | Chemotherapy, but the patient died of the disease one month later |
| Shen et al[2], 2018 | 41 | Female | Gingiva | Gingival swelling. No pain or pyorrhea, a blue-gray discoloration, and gingival masses | In the panoramic radiograph, the palatal bone was intact, and an ill-defined radiolucency was found in the left maxillary edentulous region. In addition, there was generalized mild horizontal alveolar bone loss, and a band-like radiolucent area was found in the alveolar bone crest from the left mandibular canine to the second premolar | Diffuse infiltration in the submucosa with small to medium sized blastic-like cells with increased nucleocytoplasmic ratio, round to oval, irregular nuclei, and fine chromatin. Rare mitotic figures were observed | Positive for myeloperoxidase | MS | Chemotherapy regimen based on anthracycline and cytarabine was instituted. The patient received 3 + 7 induction chemotherapy with doxorubicin and cytarabine, followed by 4 courses of consolidation therapy with cytarabine, L-asparaginase, and methotrexate |
| Wang et al[6], 2021 | 82 | Female | Gingiva | Painless swelling on the right maxillary posterior gingiva, tender lump of the right neck, firm mass without purulence | Computed tomography imaging revealed increased fluorodeoxyglucose uptake via the maxillary sinuses bilaterally and the right submandibular lymph node | NA | NA | MS | Radiation therapy (25 Gy over 12 fractions) and subsequent chemotherapy (decitabine) |
| 65 | Male | Palate | 2.0-0.8 cm craterform ulcer with diffuse erythema, induration, and swelling on the left soft palate, extending into the oropharynx | NA | NA | Bone marrow biopsy confirmed relapsed AML | MS associated with AML | Localized radiation therapy (24 Gy over 14 fractions) and immunotherapy (ipilimumab) | |
| 58 | Female | Lip | 0.3-0.3 cm indurated, nontender ulcer with surrounding erythema, scaling, and crusting on the midvermillion border of the lower lip | NA | NA | Bone marrow biopsy confirmed relapsed AML | MS associated with AML | Investigational chemotherapeutic drugs, including mitoxantrone, etoposide, and cytarabine and lenalidomide | |
| Kole et al[10], 2023 | 29 | Male | Gingiva | Swelling, paresthesia | CECT showed an osteolytic lesion extending from teeth 35 to 37 | Fibro-collagenous tissue infiltrated by nests and trabeculae of intermediate size mononuclear cells having dispersed nuclear chromatin and irregular nuclear margin | Positive for MPO and CD15 | MS | Segmental mandibulectomy |
| Li et al[9], 2024 | 32 | Male | Gingiva | Gingival swelling | No extensive bone loss | Diffuse infiltration of abnormal cells | Neoplastic cells were MPO positive, and the Ki-67 staining rate was 80% | MS | Idarubicin (IDA) regimen (IDA hydrochloride 10 mg/1 d-3 d, Cytarabine 100 mg/1 d-7 d) treatment, supplemented with gastric protection and antiemetic therapy |
The diagnosis of MS is based on the combination of clinical findings, imaging studies, and histopathological confirmation[1,7]. The choice of imaging techniques depends on the anatomical site. Magnetic resonance imaging is more sensitive for assessing central nervous system, spinal, and musculoskeletal injuries. On the other hand, a computed tomography (CT) scan is best suited for soft tissue assessments. 18-Fluorodeoxy-glucose positron emission tomography/CT (18-FDG-PET/CT) can be performed to search for multiple site involvement. These tools are useful for both radiotherapy treatment planning and response assessment. Biopsy and immunohistochemistry are essential to confirm the diagnosis and distinguish this tumor from other malignancies such as lymphomas and soft tissue sarcomas[1]. Immunohistochemistry is an essential tool for the diagnosis of MS. This technique is particularly used to identify specific myeloid lineage markers in tumor cells, such as MPO, CD13, and CD33, while T-cell markers like CD3, CD5, and CD7 are not present. To confirm the diagnosis, the expression of these markers must be present in at least 20% of the tumor cells[2,5]. Histopathological analysis is fundamental for diagnosis; MS cells typically show a diffuse infiltration of immature blast cells with a homogeneous appearance, large round or ovoid nuclei, and scant cytoplasm[1,5]. These characteristics allow for the diagnosis by ruling out other extramedullary malignant neoplasms, such as lymphomas or poorly differentiated carcinomas. Genetic and molecular tests are becoming increasingly important in the diagnosis and management of MS. These include Polymerase Chain Reaction (PCR), Fluorescence In Situ Hybridization (FISH), and Next-Generation Sequencing (NGS). PCR is used to detect specific genetic translocations and mutations. FISH is implemented to identify chromosomal alterations that may be characteristic of certain MS subtypes. NGS allows for a detailed analysis of the tumor's mutational profile, identifying specific mutations that can be therapeutic targets, directly impacting prognosis and treatment response. For example, mutations in KIT and TET2 are relevant to the pathogenesis of MS and can be identified using this method[1].
The treatment of MS varies depending on the clinical presentation, the location of the entity, and the patient's response to conventional therapies. Currently, several treatment alternatives are available, including chemotherapy, radiotherapy, hematopoietic stem cell transplantation (allo-HSCT), targeted therapies, and immunomodulatory therapies[1].
Chemotherapy is the mainstay of treatment for MS, especially when it occurs together with AML[1,11]. In the clinical case of MS presented by Li et al[9], chemotherapy with an idarubicin (IDA) regimen (IDA hydrochloride 10 mg/d from days 1 to 3, cytarabine 100 mg/d from days 1 to 7) was implemented. After a course of chemotherapy, the patient was switched to a mitoxantrone (MA) regimen (MA 8 mg/d from days 1 to 3, cytarabine 100 mg/d from days 1 to 7). At 12 mo of follow-up, the patient presented a stable condition with no progression[10]. Similarly, Shen et al[2], Kurdoğlu et al[5], and de Andrade et al[7] managed MS with gingival swelling as the initial symptom using chemotherapy[2,5,7] (Table 1). Shen et al[2] established an anthracycline and cytarabine-based regimen. The patient received 3+7 induction chemotherapy with doxorubicin and cytarabine, followed by four courses of consolidation therapy with cytarabine, L-asparaginase, and methotrexate. Subsequently, the patient received the next two cycles of chemotherapy with doxo
Radiotherapy is another treatment alternative, particularly viable for the palliative relief of symptoms in MS refractory to chemotherapy. For local control of gingival MS, studies have shown that low doses of radiotherapy (between 20-24 Gy in 10–12 fractions) can be effective[4]. However, this treatment can be associated with significant toxicities, such as severe mucositis, requiring a careful risk-benefit assessment for each case[4].
For patients with MS who have achieved remission with chemotherapy, allo-HSCT is a consolidative treatment option. This regimen not only provides a new hematopoietic system but also introduces a graft-versus-leukemia effect that may help prevent relapses[1,11]. However, extramedullary relapses remain a significant challenge, and the relapse rate after transplantation can reach 50%[11].
In patients with specific genetic mutations, targeted therapies such as tyrosine kinase inhibitors (TKIs) show promise. In particular, treatment with FLT3 inhibitors such as sorafenib has been shown to improve remission rates in patients with FLT3-ITD mutations. These treatment regimens can be implemented in combination with chemotherapy and transplantation to maximize their effectiveness[11].
Immunomodulatory therapies using hypomethylating agents such as decitabine (5-aza-2′-deoxycytidine) and azacytidine (5-azacytidine) play an important role in the prevention of post-transplant relapses[1,11]. These agents may improve immune surveillance and antileukemic response by increasing the expression of tumor antigens and major histocompatibility complex molecules. Maintenance therapy with decitabine following allo-HSCT has been shown to prolong disease-free survival in patients with MS[11].
The prognosis of MS remains poor, with a median survival of only 4 mo without aggressive treatment. Studies suggest that patients receiving allo-HSCT show a significant improvement in survival, although relapse remains a major concern. Early identification and aggressive intervention are crucial to improve outcomes in these patients[11].
The survival prognosis for patients with MS is generally unfavorable, particularly when associated with AML[1,5,11]. The survival rate is only 30.8%, with higher survival in cases of primary MS not associated with preexisting hematological malignancies (50%) compared to those associated with malignancy (22.2%)[5]. The median survival time varies according to several factors: Early diagnosis, extent and location of the tumor, response to treatment, and comorbidities. The complexity in achieving an early diagnosis due to the atypical presentation of MS can negatively impact prognosis. Additionally, cases without preexisting hematological findings are particularly challenging to diagnose[5]. The type and response to treatment play a crucial role in the outcomes for patients with MS. Some clinicians implement standard treatment with chemotherapy regimens such as idarubicin and cytarabine (ARA-C), followed by bone marrow transplantation; on the other hand, studies suggest that patients receiving allo-HSCT show a significant improvement in survival, although relapse remains a major concern[5,11]. The response to treatment varies, and the presence of additional metastases during treatment can complicate management and decrease survival; the persistence or recurrence of the tumor in other organs is a critical factor in reducing survival. Comorbidities present in patients with MS are a determining factor in survival outcomes; the presence of AML or other myeloproliferative disorders can negatively impact prognosis, while patients with bone marrow involvement have a higher relapse rate and lower survival.
MS is a rare and aggressive entity with a generally poor prognosis. Early detection and aggressive treatment can improve outcomes, although the presence of comorbidities and tumor extent remain critical factors. The management of MS requires a multidisciplinary and personalized approach. Continued research and clinical trials are essential to optimize these therapeutic strategies and improve outcomes for patients with MS.
The findings presented in the study by Li et al[9] on MS with maxillary gingival swelling as the initial symptom open the door to several important directions for future research regarding the diagnosis, treatment, and understanding of MS.
Although relevant diagnostic, clinical, and therapeutic aspects of MS are known, there is still uncertainty around the exact pathogenesis and the molecular mechanisms that lead to its development[1]. Future research could focus on genomic sequencing and the signaling pathways involved in MS, especially in unusual presentations such as intraoral cases. Given that cases of MS in the oral cavity are atypical, additional studies could focus on better understanding the epidemiology of this presentation, helping to identify possible risk factors or associated demographic characteristics.
The diagnosis of MS remains challenging due to its clinical and pathological features that overlap with other hematological and neoplastic diseases[1,11]. It would be beneficial to integrate research projects aimed at developing more precise diagnostic tools, such as advanced immunohistochemical panels or specific biomarkers detectable in less invasive analyses.
Chemotherapy is the main treatment for MS due to its ability to effectively treat the disease at both systemic and local levels, its effectiveness in treating related diseases such as AML, and the lack of equally effective alternatives in many cases[1,12]. Although the patient in the clinical case presented by Li et al[9] responded adequately to chemotherapy, the relationship between different treatment modalities and survival rates remains uncertain. Further research on high-dose chemotherapy and radiotherapy could provide new insights into the most effective treatment strategies. Additionally, since MS can progress to AML, it is crucial to develop therapeutic approaches that can prevent this progression or treat it more effectively. Targeted therapies that address specific genetic mutations in MS could be a promising avenue. It would be beneficial to propose longitudinal studies with long-term follow-up of patients to better understand the evolution of the disease and the effectiveness of current treatments, and to explore prognostic factors related to longevity and quality of life post-treatment.
In summary, it is essential to prioritize these future lines of research to not only deepen the understanding of MS, especially in unusual presentations such as in the gingiva, but also significantly improve diagnostic and therapeutic approaches for affected patients.
The identification and management of MS require a multidisciplinary approach due to the complexity and variability in its clinical presentations. Given that MS can present with nonspecific symptoms and in atypical locations such as the oral cavity, it is crucial that dental professionals are fully aware of the various clinical presentations of this rare neoplasm. Ultimately, increased awareness and education among dental professionals, along with effective interdisciplinary collaboration, can lead to earlier and more accurate diagnosis and timely treatment of MS. This would not only improve survival but could also reduce the morbidity associated with this disease, offering patients a better quality of life. Early detection and treatment, supported by ongoing research and advanced therapeutic approaches, are critical to meeting the challenges presented by MS.
| 1. | Loscocco GG, Vannucchi AM. Myeloid sarcoma: more and less than a distinct entity. Ann Hematol. 2023;102:1973-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 2. | Shen Y, Zhao L, Wu Y, Huang P. Multifocal occurrence of intraoral isolated MS in a patient without leukemic presentation: A case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e42-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Hu YG, Deng XH, Lei W, Li XL. Clinical characteristics and management of primary granulocytic sarcoma of the oral cavity: A case report and literature review. Medicine (Baltimore). 2020;99:e22820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 4. | Lee DY, Baron J, Wright CM, Plastaras JP, Perl AE, Paydar I. Radiation Therapy for Chemotherapy Refractory Gingival Myeloid Sarcoma. Front Oncol. 2021;11:671514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Kurdoğlu B, Oztemel A, Barış E, Sengüven B. Primary oral myeloid sarcoma: Report of a case. J Oral Maxillofac Pathol. 2013;17:413-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Wang D, He K, Sroussi H, Treister N, Luskin M, Villa A, Woo SB, Shazib MA. Oral myeloid sarcoma as an uncommon manifestation of acute myeloid leukemia: A case series and review of the literature. J Am Dent Assoc. 2021;152:393-400.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | de Andrade BA, Farneze RB, Agostini M, Cortezzi EB, Abrahão AC, Cabral MG, Rumayor A, Romañach MJ. Myeloid sarcoma of the oral cavity: A case report and review of 89 cases from the literature. J Clin Exp Dent. 2017;9:e1167-e1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Zisis V, Zisis S, Anagnostou E, Dabarakis N, Poulopoulos A, Andreadis D. Gingival Enlargement Can Constitute the Only Diagnostic Sign of Leukemia: Report of an Unusual Case. Cureus. 2023;15:e47959. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Li SH, Yang CX, Xing XM, Gao XR, Lu ZY, Ji QX. Myeloid sarcoma with maxillary gingival swelling as the initial symptom: A case report and review of literature. World J Clin Cases. 2024;12:3985-3994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Kole L, Ray A, Bandyopadhyay R, Paul S. Granulocytic Sarcoma of Mandible: A Case Report. JDMS. 2023;5:12-17. |
| 11. | Zhao H, Dong Z, Wan D, Cao W, Xing H, Liu Z, Fan J, Wang H, Lu R, Zhang Y, Cheng Q, Jiang Z, He F, Xie X, Guo R. Clinical characteristics, treatment, and prognosis of 118 cases of myeloid sarcoma. Sci Rep. 2022;12:6752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Shah K, Panchal H, Patel A. Spine Myeloid Sarcoma: A Case Series with Review of Literature. South Asian J Cancer. 2021;10:251-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |