Published online Nov 6, 2024. doi: 10.12998/wjcc.v12.i31.6493
Revised: August 22, 2024
Accepted: August 28, 2024
Published online: November 6, 2024
Processing time: 124 Days and 18.7 Hours
High-dose steroid administration is a common initial therapeutic approach for Vogt–Koyanagi–Harada disease (VKH). Nonetheless, administering substantial doses of steroids to pregnant women necessitates meticulous consideration due to the potential impacts on the mother and fetus. We present a case wherein steroid pulse therapy was administered to a patient who developed VKH during the late stages of pregnancy.
The patient was a 26-year-old nulliparous woman. At 33 weeks and 1 day of her pregnancy, she experienced a decline in visual acuity and noticed metamor
VKH management in pregnancy requires multidisciplinary coordination, emphasizing collaboration with ophthalmologists and specialists in internal medicine and neonatology.
Core Tip: Generally, high-dose steroid administration is the initial treatment for Vogt–Koyanagi–Harada disease (VKH). However, administering large doses of steroids to pregnant women requires careful treatment, considering their effects on the mother and fetus. We report a case in which steroid pulse therapy was administered to a patient who developed VKH during late pregnancy, and multidisciplinary management led to normal delivery. When treating VKH that develops during pregnancy, multidisciplinary management is considered necessary, with close collaboration not only with ophthalmologists, but also internal medicine and neonatologists.
- Citation: Ueyama K, Kakinuma T, Mori K, Hayashi A, Kakinuma K, Okamoto R, Kaneko A, Yanagida K, Takeshima N, Ohwada M. Managing Vogt–Koyanagi–Harada disease during pregnancy with steroid pulse therapy: A case report. World J Clin Cases 2024; 12(31): 6493-6499
- URL: https://www.wjgnet.com/2307-8960/full/v12/i31/6493.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i31.6493
Vogt–Koyanagi–Harada disease (VKH) is a systemic autoimmune disease that targets melanocytes or melanin pigments, and although environmental factors are thought to be involved, the exact pathogenesis has not been elucidated[1,2].
The maternal immune system undergoes suppression during pregnancy, attributed to heightened levels of immunosuppressive factors, including endogenous steroids, and a reduction in cellular immunity[3,4]. While the potential impact of pregnancy on the pathophysiology of VKH has been postulated, reports of its association with pregnancy exist, yet a consensus regarding pregnancy's influence on VKH remains elusive[5-10]. Notably, in cases of VKH coinciding with pregnancy, initial treatment typically entails high-dose steroid administration[2]. However, caution must be exercised regarding the potential effects of these therapeutic agents on pregnancy and fetal health.
Herein, we present the case of a pregnant woman who developed VKH during late pregnancy and underwent steroid pulse therapy. Through multidisciplinary management, the patient experienced a normal delivery and a favorable clinical outcome.
The patient was 26 years old, not pregnant, and nulliparous. She was referred to our facility.
The patient's pregnancy had been managed without complications until the 33rd week and 1st day, when she noted diminished visual acuity and metamorphopsia in her left eye. In the 33rd week and 3rd day of pregnancy, she sought consultation at a previous ophthalmology clinic. Referral to our facility was made at 33 weeks and 4 days of gestation for further evaluation and treatment.
Nothing noteworthy.
Nothing noteworthy.
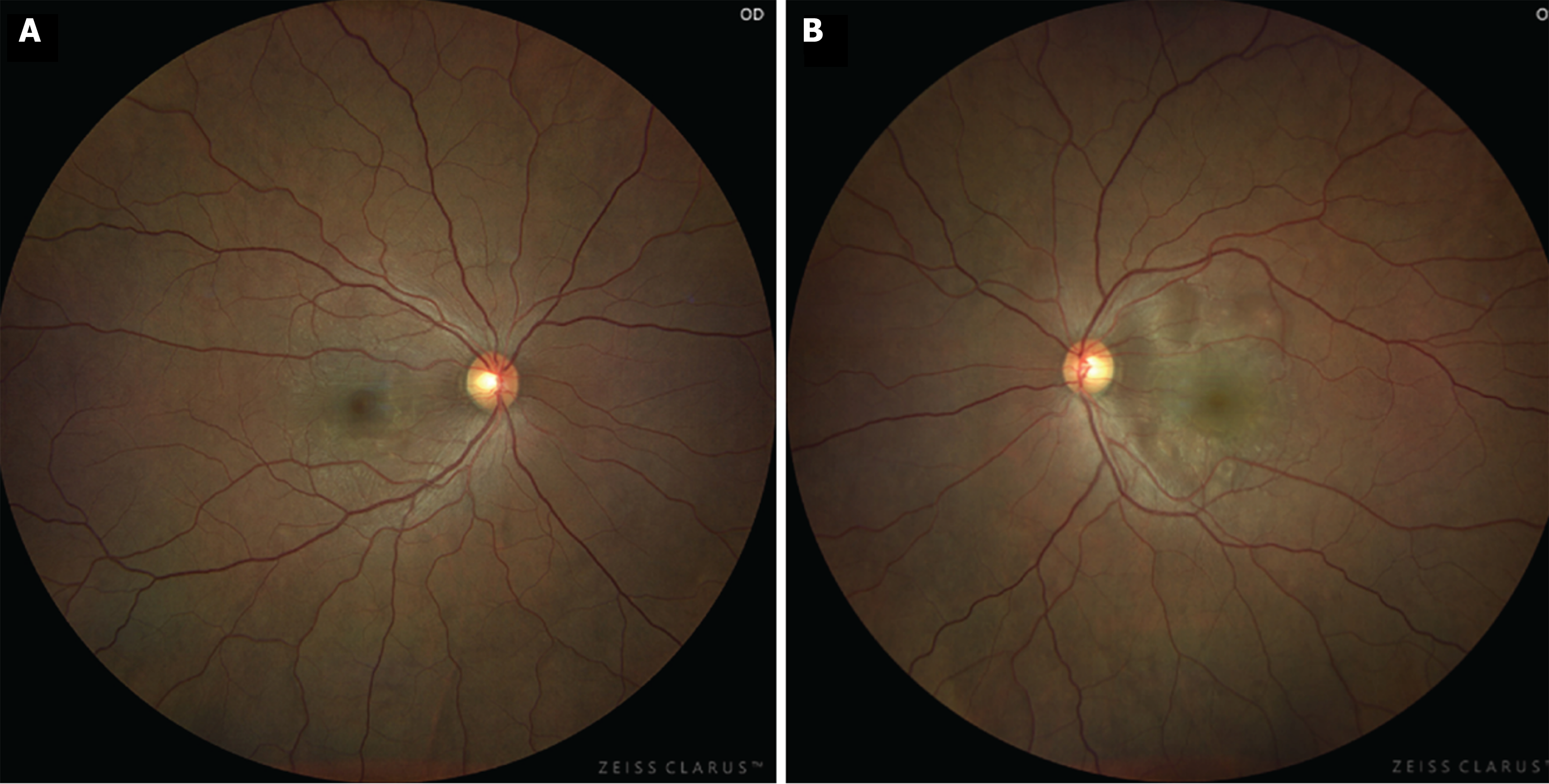
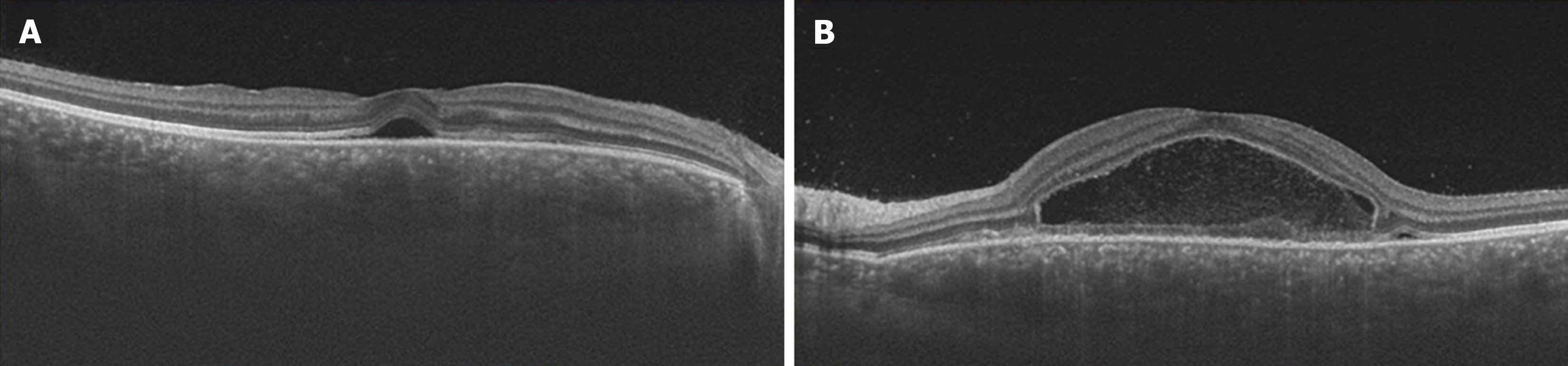
Ocular examination findings at initial assessment: The corrected visual acuity was measured at 1.2 in the right eye and 0.2 in the left eye, with intraocular pressure recorded as 17 mmHg bilaterally. Fundus examination revealed the presence of multiple serous retinal detachments (SRDs) in the posterior pole of both eyes (Figure 1). Additionally, optical coherence tomography (OCT) imaging demonstrated SRD with fibrin septa formation in both eyes, accompanied by central choroidal thickness, which was markedly thickened in both eyes (Figure 2).
Initial laboratory findings: Initial blood and urine analyses revealed a white blood cell count of 10.6 × 106/μL and a red blood cell count of 4.01 × 106/μL. Hemoglobin level measured at 12.9 g/dL, with a hematocrit value of 38.3%. Platelet count was 27.2 × 103/μL, blood glucose level was 78 mg/dL, HbA1C was 5.4%, and serum creatinine was 0.42 mg/dL. The uric acid level was 2.0 mg/dL, the estimated glomerular filtration rate was 200.5 mL/min, and urinalysis showed negative results for both sugar and protein.
Ultrasound tomography findings at initial assessment: The biparietal diameter was measured at 84.3 mm, the abdominal circumference at 280.1 mm, and the fetal length at 60.1 mm, with an estimated fetal weight of 2056 g. Fetal development appeared normal, and there were no discernible abnormalities noted in the placenta or umbilical cord, nor were there any evident fetal malformations. The resistance index of the umbilical artery was recorded at 0.74, while the resistance index of the middle cerebral artery was within normal limits at 0.84. The amniotic fluid index was 10.6 cm. Re-assuring fetal heart rate monitoring was observed during the non-stress test, yielding a Biophysical Profile Score of 10 points.
Due to the patient's pregnancy status, invasive diagnostic procedures, such as fluorescence fundus photography and cerebrospinal fluid testing, were not conducted.
Following the diagnostic evaluation, the patient received a diagnosis of VKH.
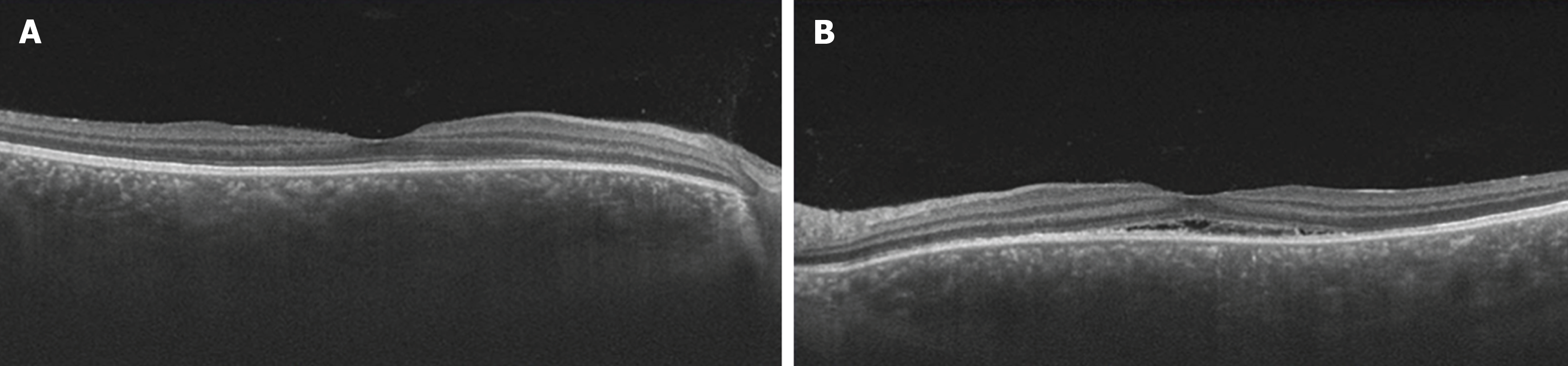
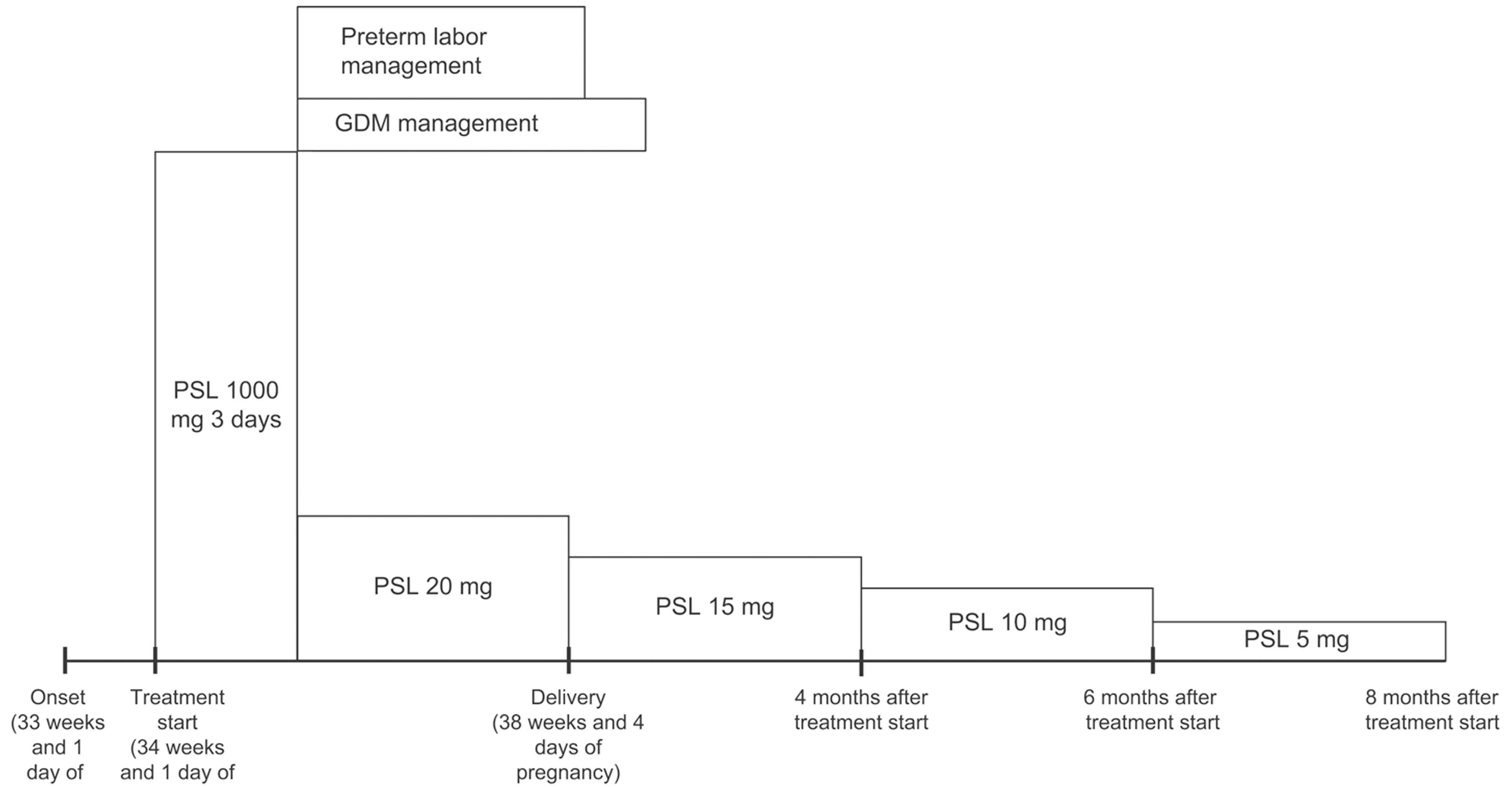
With the patient's informed consent, hospitalization was initiated at 34 weeks and 1 day of pregnancy, and she underwent steroid pulse therapy. This involved the intravenous infusion of 1000 mg of methylprednisolone over a 3-day period, with continuous monitoring of maternal and fetal well-being using cardiotocography. Subsequent improvements in SRDs and papilledema led to the transition to oral prednisolone at a dose of 20 mg/day as maintenance therapy. On the fourth day of treatment (34 weeks and 4 days of pregnancy), the onset of gestational diabetes mellitus (GDM) was detected, necessitating the initiation of insulin therapy. Furthermore, signs indicative of impending preterm labor were noted on the fifth day of treatment (34 weeks and 5 days of gestation), prompting the initiation of oral ritodrine hydrochloride therapy and advising bed rest. At 10 days after treatment initiation, OCT imaging revealed a significant reduction in subretinal fluid in both eyes (Figure 3). Furthermore, 1 month post-treatment initiation, the patient's corrected visual acuity improved to 1.2 in both eyes. Regarding GDM, HbAlc remained around 5% with insulin therapy, and no perinatal complications, such as heavy for dates or polyhydramnios, or diabetic complications, such as nephropathy, were observed.
Labor pains began on the 32nd day after the initiation of steroid pulse therapy (38 weeks and 4 days of pregnancy), and the baby was delivered vaginally on the same day. The infant's weight was 2593 g, Apgar score was 8 points at 1 min and 9 points at 5 min, and cord blood pH was 7.28. No obvious abnormalities were observed in the child. The puerperal course was uneventful, and both mother and baby were discharged from the hospital on the 5th day postpartum, 37 days after the initiation of steroid pulse therapy. After delivery, the dose of prednisolone was gradually reduced, but no recurrence of VKH was observed, and prednisolone was discontinued 8 months after starting steroid pulse therapy. The course of treatment is shown in Figure 4.
At 15 months after steroid pulse therapy initiation, there was no recurrence of VKH. No chronic lesions were observed, and corrected visual acuity was 1.2 in both eyes. Furthermore, no obvious abnormalities were observed in the child's growth.
VKH is an autoimmune disorder characterized by the targeting of melanin-producing cells, known as melanocytes, across various bodily tissues. A comprehensive understanding of its pathology remains elusive. However, individuals with a genetic predisposition, particularly those exhibiting human leukocyte antigen -DR4 positivity, may experience activation of cell-mediated immunity in response to innate immune stimuli, such as incidental infections. This immune response is believed to involve T helper 1 (Th1) cells, Th17 cells, and other immune cells with specificity for melanocyte antigens, thereby triggering inflammation against melanocyte antigens throughout the body. The acute phase typically presents with bilateral panuveitis, primarily driven by choroidal inflammation. Clinical manifestations may extend to inner ear disturbances and aseptic meningitis. In the chronic or late stages, features, such as granulomatous uveitis, sunset glow fundus appearance, skin depigmentation, and premature graying of hair, may manifest[1,2,11,12]. Pregnant individuals experience immune tolerance suppression, characterized by heightened levels of immunosuppressive factors, such as endogenous steroids and diminished cellular immunity[3,4]. Nonetheless, the exact impact of pregnancy on VKH remains uncertain.
In VKH cases, robust suppression of inflammation during the early stages of onset is deemed crucial for averting subsequent recurrence and disease prolongation. Typically, high-dose steroid pulse therapy is favored, wherein intravenous steroid infusion is initially administered, followed by a transition to oral therapy with gradual dose reduction while monitoring clinical improvement[3,13,14]. Failure to administer adequate steroid doses during the early stages of VKH may result in recurrent inflammation and progression to a prolonged disease state with an unfavorable prognosis. This progression can lead to irreversible visual impairment due to chorioretinal degeneration and secondary glaucoma[15]. Notably, Kitaichi et al[16] emphasized the necessity of initiating systemic steroid therapy within 14 days of symptom onset to mitigate the risk of disease progression.
Two systemic administration methods of steroids for VKH include high-dose steroid therapy and steroid pulse therapy. High-dose steroid therapy entails the intravenous administration of a long-acting steroid, such as betame
Betamethasone and dexamethasone, both synthetic glucocorticoids, exhibit minimal metabolism by 11β-hydroxysteroid dehydrogenase (11β-HSO), resulting in significant placental penetration[17,18]. Conversely, prednisolone undergoes inactivation by 11β-HSO, resulting in lower placental penetration, estimated at approximately 10%. Consequently, prednisolone is considered to have a lesser impact on the fetus compared to dexamethasone and betamethasone[19]. Given these considerations, steroid pulse therapy may be preferred when administering systemic steroids to pregnant women. Additionally, there is evidence suggesting a slight increase in the risk of fetal cleft palate with the administration of high doses of steroids during early pregnancy[20]. In the context of VKH developing in late pregnancy and considering the relatively safer placental permeability profile of prednisolone, steroid pulse therapy was selected for this case.
Conversely, systemic steroid administration to pregnant women is associated with heightened risks of preterm birth, preeclampsia, GDM, and fetal growth restriction[21-23]. In this particular case, hospitalization and treatment with steroid pulse therapy were implemented. Following steroid pulse therapy, the patient experienced instances of threatened preterm labor and gestational diabetes. To address threatened preterm labor, ritodrine hydrochloride tablets, a tocolytic medication, were administered alongside instructions for bed rest to prevent premature labor.
Moreover, pregnant women diagnosed with GDM may encounter complications, such as macrosomia (heavy for date baby) or polyhydramnios, necessitating treatment for threatened premature delivery due to symptoms, such as abdominal distension, uterine contractions, and cervical shortening associated with polyhydramnios[24]. In the present case, GDM manifested following the initiation of steroid pulse therapy. However, effective blood sugar management was achieved through insulin therapy in collaboration with the attending physician. Consequently, we successfully managed the condition without encountering perinatal complications, such as polyhydramnios or macrosomia, and the patient did not develop diabetic complications, such as new ocular lesions or nephropathy.
Steroid pulse therapy was administered for VKH, which manifested during late pregnancy, resulting in favorable outcomes for both the mother and baby. Nevertheless, the efficacy of this treatment approach remains subject to debate. The existing literature on VKH cases occurring during pregnancy is sparse, and clear treatment guidelines are lacking.
Moreover, in recent years, there has been growing recognition that the intrauterine environment not only influences fetal growth and development but also has enduring effects that extend into adulthood, giving rise to the concept of Developmental Origins of Health and Disease. This concept posits that prenatal exposures can impact long-term health outcomes and increase the risk of developing lifestyle-related diseases[25]. Excessive maternal corticosteroid administration during pregnancy can alter fetal hypothalamic-pituitary-adrenal axis regulation, leading to a prolonged stress response state. Consequently, long-term effects have been reported on neurodevelopmental and behavioral patterns, cardiovascular health, and glucose metabolism (resulting in insulin resistance)[26].
Henceforth, we aim to gather additional cases of VKH occurring during pregnancy to deepen our understanding of optimal treatment strategies. Moreover, we advocate for the necessity of conducting long-term follow-up studies to assess the impact of steroid therapy on infants.
When managing VKH that arises during pregnancy, it is imperative to adopt a multidisciplinary approach, necessitating close collaboration with ophthalmologists and specialists in internal medicine and neonatology.
| 1. | Diallo K, Revuz S, Clavel-Refregiers G, Sené T, Titah C, Gerfaud-Valentin M, Seve P, Jaussaud R. Vogt-Koyanagi-Harada disease: a retrospective and multicentric study of 41 patients. BMC Ophthalmol. 2020;20:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | O'Keefe GA, Rao NA. Vogt-Koyanagi-Harada disease. Surv Ophthalmol. 2017;62:1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 3. | Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 415] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 4. | PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, Mor G, Schulz L, Soares M, Spencer T, Strominger J, Way SS, Yoshinaga K. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 460] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 5. | Steahly LP. Vogt-Koyanagi-Harada syndrome and pregnancy. Ann Ophthalmol. 1990;22:59-62. [PubMed] |
| 6. | Friedman Z, Granat M, Neumann E. The syndrome of Vogt-Koyanagi-Harada and pregnancy. Metab Pediatr Ophthalmol. 1980;4:147-149. [PubMed] |
| 7. | Miyata N, Sugita M, Nakamura S, Isobe K, Matoba H, Tsuda K, Tanaka K, Ohno S. Treatment of Vogt-Koyanagi- Harada's disease during pregnancy. Jpn J Ophthalmol. 2001;45:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Sanchez-Vicente JL, Moruno-Rodríguez A, De Las Morenas-Iglesias J, Gonzalez-Jauregui B, Franco-Ruedas C, Lechon-Caballero B, Talego-Sancha A, Rueda-Rueda T, Del Estad-Cabello A, López-Herrero F. Clinical Manifestations and Treatment of Vogt-Koyanagi-Harada Disease during Pregnancy and after Birth: A Case Report. Ocul Immunol Inflamm. 2023;31:830-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Alromaih AZ, Almater AI, Albloushi AF, Alkheraiji NF, Abu El-Asrar AM. Outcomes of initial-onset acute uveitis associated with Vogt-Koyanagi-Harada disease occurred during pregnancy. Int Ophthalmol. 2023;43:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Chan CC. Gender differences in vogt-koyanagi-harada disease and sympathetic ophthalmia. J Ophthalmol. 2014;2014:157803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Urzua CA, Herbort CP Jr, Takeuchi M, Schlaen A, Concha-Del-Rio LE, Usui Y, Cuitino L, Papasavvas I. Vogt-Koyanagi-Harada disease: the step-by-step approach to a better understanding of clinicopathology, immunopathology, diagnosis, and management: a brief review. J Ophthalmic Inflamm Infect. 2022;12:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, Pivetti-Pezzi P, Tessler HH, Usui M. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 815] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 13. | Iwahashi C, Okuno K, Hashida N, Nakai K, Ohguro N, Nishida K. Incidence and clinical features of recurrent Vogt-Koyanagi-Harada disease in Japanese individuals. Jpn J Ophthalmol. 2015;59:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Lai TY, Chan RP, Chan CK, Lam DS. Effects of the duration of initial oral corticosteroid treatment on the recurrence of inflammation in Vogt-Koyanagi-Harada disease. Eye (Lond). 2009;23:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Read RW, Rechodouni A, Butani N, Johnston R, LaBree LD, Smith RE, Rao NA. Complications and prognostic factors in Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2001;131:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Kitaichi N, Horie Y, Ohno S. Prompt therapy reduces the duration of systemic corticosteroids in Vogt-Koyanagi-Harada disease. Graefes Arch Clin Exp Ophthalmol. 2008;246:1641-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Murphy VE, Fittock RJ, Zarzycki PK, Delahunty MM, Smith R, Clifton VL. Metabolism of synthetic steroids by the human placenta. Placenta. 2007;28:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Asztalos E. Antenatal corticosteroids: a risk factor for the development of chronic disease. J Nutr Metab. 2012;2012:930591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 319] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Xiao WL, Liu XY, Liu YS, Zhang DZ, Xue LF. The relationship between maternal corticosteroid use and orofacial clefts-a meta-analysis. Reprod Toxicol. 2017;69:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Martel MJ, Rey E, Beauchesne MF, Perreault S, Lefebvre G, Forget A, Blais L. Use of inhaled corticosteroids during pregnancy and risk of pregnancy induced hypertension: nested case-control study. BMJ. 2005;330:230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Schatz M, Dombrowski MP, Wise R, Momirova V, Landon M, Mabie W, Newman RB, Hauth JC, Lindheimer M, Caritis SN, Leveno KJ, Meis P, Miodovnik M, Wapner RJ, Paul RH, Varner MW, O'Sullivan MJ, Thurnau GR, Conway DL; Maternal-Fetal Medicine Units Network, The National Institute of Child Health and Development; National Heart, Lung and Blood Institute. The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol. 2004;113:1040-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Perlow JH, Montgomery D, Morgan MA, Towers CV, Porto M. Severity of asthma and perinatal outcome. Am J Obstet Gynecol. 1992;167:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 143] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Zhang X, Liao Q, Wang F, Li D. Association of gestational diabetes mellitus and abnormal vaginal flora with adverse pregnancy outcomes. Medicine (Baltimore). 2018;97:e11891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1243] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 26. | Braithwaite EC, Pickles A, Sharp H, Glover V, O'Donnell KJ, Tibu F, Hill J. Maternal prenatal cortisol predicts infant negative emotionality in a sex-dependent manner. Physiol Behav. 2017;175:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |