Published online Nov 6, 2024. doi: 10.12998/wjcc.v12.i31.6451
Revised: July 22, 2024
Accepted: August 16, 2024
Published online: November 6, 2024
Processing time: 181 Days and 3.4 Hours
Allergic rhinitis (AR) poses a significant global health burden, with the potential to progress to asthma, thereby impacting patients’ quality of life. Immunotherapy has demonstrated effectiveness in mitigating clinical symptoms by altering the underlying disease mechanisms of AR. This article provides a thorough review of the current state of immunotherapy for AR, encompassing various facets of immunotherapeutic strategies, elucidating their mechanisms and clinical implications. By presenting a nuanced understanding of the present landscape of immunotherapy for AR, this review aims to serve as a valuable reference for informing clinical treatment strategies. The subsequent analysis of diverse immunotherapeutic pathways offers a comprehensive understanding of their mechanisms and clinical implications. A meticulous examination is conducted on subcutaneous immunotherapy, sublingual immunotherapy, oral immunotherapy, intralymphatic immunotherapy, and innovative intravenous gold-induced autologous serum injection therapy. Each pathway is systematically elucidated, with its distinctive features and potential contributions to managing AR empha
Core Tip: This comprehensive review thoroughly examines various immunotherapeutic pathways. Each pathway is systematically elucidated, with its distinctive features, mechanisms, and clinical implications highlighted. The review critically assesses the epidemiological landscape of allergic rhinitis, shedding light on its diverse regional characteristics and influencing factors. Furthermore, combining immunotherapy with antibody therapy signifies a promising frontier in severe allergic rhinitis treatment. The review outlines relevant indicators for predicting immunotherapy efficacy, emphasizing the importance of identifying immune cells, cytokines, metabolites, and metabolic pathways associated with treatment outcomes. Despite the proven benefits of immunotherapy, the review candidly addresses its limitations.
- Citation: Fu Y, Song YL, Liu ZG. Recent developments in immunotherapy approaches for allergic rhinitis. World J Clin Cases 2024; 12(31): 6451-6461
- URL: https://www.wjgnet.com/2307-8960/full/v12/i31/6451.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i31.6451
Allergic rhinitis (AR) profoundly impacts individuals’ occupational and daily lives. AR, characterized as a non-infectious chronic inflammatory condition affecting the nasal mucosa, primarily involves immunoglobulin E (IgE)-mediated responses in atopic individuals upon exposure to allergens. The treatment paradigm for AR adheres to the principle of a combination of prevention and treatment, encompassing environmental control, pharmaceutical interventions, immunotherapy, and health education. While traditional drugs like nasal glucocorticoids and antihistamines provide temporary symptomatic relief, immunotherapy stands out for its capacity to confer enduring benefits to patients. The global prevalence of AR is noteworthy, with 15.2% observed in adolescents (13-14 years old) across 25 countries and 11.1% in children (6-7 years old) in 16 countries[1]. The allergen immunotherapy (AIT) process involves administering the allergen extract to the patient according to an effective dose plan within a specified time[2]. It has been widely used in food allergy, AR, insect venom allergy, atopic dermatitis, and even some cancers[3-6]. Notably, it can prevent AR from evolving into allergic asthma and control asthma symptoms[7]. Choosing the appropriate allergen is the key to achieving the therapeutic effect of AIT. Allergens can be determined if the patient has a positive result in an allergy skin prick test or serum IgE measurement and exhibits allergic symptoms when exposed to allergens[8]. Immunotherapy has been used for more than 110 years since 1911[9]. Researchers focus on understanding factors leading to patient discontinuation at different stages, emphasizing the need for enhanced patient education, bolstered confidence in treatment, and improved compliance[10]. Ongoing advancements in AIT, including allergen types, doses, treatment duration, and administration methods, underscore the dynamic nature of this therapeutic approach. The allergen types, doses, treatment times, and ways of immunotherapy are constantly updated. However, despite the promising benefits, it is imperative to acknowledge the inherent limitations embedded within immunotherapy. This comprehensive review critically delves into the current immunotherapy landscape for AR, meticulously scrutinizing its various facets to offer a nuanced and discerning perspective. Immunotherapy, while presenting a formidable approach to managing AR, is a challenge. Treatment duration, patient adherence, and potential adverse effects necessitate thorough consideration. This review takes a critical stance, dissecting these limitations to provide an in-depth understanding of the constraints that may impact the efficacy of immunotherapeutic interventions. Furthermore, the evolving nature of AIT, encompassing changes in allergen formulations and administration protocols, contributes to the complexity of its application. This article delves into these intricacies, shedding light on the dynamic nature of immunotherapy and its ongoing adaptations. By critically examining the merits and limitations of immunotherapy, this review provides a comprehensive guide for clinicians and researchers navigating the intricate landscape of immunotherapy for AR. It aims to foster a well-rounded understanding beyond the optimistic aspects, acknowledging the challenges of pursuing effective AR treatment. Currently, several allergens are contributing to AR in patients[11,12].
AR is a pervasive chronic nasal disease, representing a significant global health challenge. Its prevalence exhibits considerable variation across different countries, reflecting the complex interplay of environmental and regional factors. In Konya, the influence of urbanization has manifested in a noteworthy increase in the prevalence of AR among Turkish students in recent years[13]. The prevalence of current AR, cumulative AR, diagnosed eczema, and diagnosed food allergy in a 6-12-year-old population in Budapest has continued to display an increasing trend[14]. Conversely, in a specific subgroup of children in Bavaria, Germany, parent-reported childhood atopic diseases and airway-related symptoms have exhibited stagnation between 2004 and 2017. Notably, the prevalence of “wheeze” and “rhinitis” has demonstrated a decreasing trend over this period[15]. The Southeast Asian region reveals notable disparities and gaps in the prevalence of AR and local AR[16]. China, particularly the steppe area of the Xilingol League in Inner Mongolia, experiences a high seasonal pollen exposure rate, contributing to an exceptionally elevated prevalence of AR[17]. Studies conducted in China underscore the urban-rural divide in allergic disease prevalence. Urban areas exhibit a higher self-reported AR prevalence among children than their rural counterparts. Additionally, the sensitization rate to food and inhalant allergens in urban children is lower than that in rural areas[18]. The incidence of AR varies among patients of different ages and genders. A multicenter study demonstrated that the prevalence of AR in males was significantly higher than that in females[19]. In the southern edge of the plateau grassland region of northern China, patients ≥ 40 years of age were 1.79-fold and 2.16-fold more likely to have asthma and AR combined with asthma, respectively, than patients < 40 years of age, indicating that age > 40 years is an independent risk factor for AR combined with asthma[20]. An investigation by Lee et al[21] revealed a noteworthy surge in the prevalence of wheezing and AR, with a pivotal association identified between this increase and the heightened prevalence of parental atopic history. Beyond genetic predispositions, the development of allergic diseases and allergen sensitization is intricately linked to early, specific environmental exposures in urban and rural settings. In urban children, frequent changes in residence and early antibiotic usage emerge as significant risk factors for sensitization. Furthermore, sensitization itself, along with a family history of allergy, constitutes notable risk factors for the manifestation of allergic diseases. In contrast, rural children exhibit a protective effect against allergen sensitization and allergic diseases through early exposure to the rural environment[18]. Children who manifested long-lasting disease before the appearance of allergy had a 6.3-fold and 5.5-fold risk of AR in the two examined years, and the respiratory tract may be considered a unique morphofunctional entity because of infections in this site[14]. Recent findings underscore a novel dimension in the complex etiology of AR, revealing an association between overweight/obesity and an increased risk of atopic allergic diseases[22]. This implies a multifaceted etiological framework where genetic and environmental factors influence the susceptibility and manifestation of AR. Scholars have identified up to 76 potential ecological risk factors, environmental protective factors, and biomarkers for AR[23]. There is a connection between social, behavioral, and biological factors and AR; lifestyle and environmental risk factors play an essential role in AR[19]. The epidemiological data will be beneficial in guiding the formulation of healthcare policies and developing plans for the appropriate use of healthcare resources, especially the modifiable factors related to individuals and families[20,24] (Table 1). Recent investigations affirm the distinct regional characteristics of AR, marked by substantial variations in allergen types across different geographical areas. In Thailand, pollen extracts from rice, corn, sorghum, and para grass have been implicated in pollen allergies, highlighting significant regional differences in allergenic sources[25]. Meanwhile, fungal sensitization is a prominent factor influencing AR manifestations among the Southeast Asian Chinese population[26]. In tropical regions, the perennial presence of Aedes aegypti raises the prospect of its whole-body extract being linked to respiratory allergies, underscoring the diverse allergenic landscape in such climates[27]. China exhibits localized variations in allergen prevalence, with Xinjiang primarily characterized by herbaceous allergens, Inner Mongolia dominated by Artemisia pollen, and Ningxia featuring mugwort as the primary allergen[17,28,29]. Shenzhen, within the Chinese context, presents a distinctive airborne allergen profile. Notable allergens in the region include Brucella tropicalis, house dust mites, Dermatophagoides farinae, cockroaches, and ragweed, emphasizing the need for localized considerations in understanding AR triggers[30]. The capital city of Beijing stands out for the sensitivity of its population to ragweed and juniper pollen, both strongly associated with AR and/or allergic asthma. Juniper pollen is associated with a higher prevalence of AR-related allergic diseases[31,32] (Table 2).
| No. | Risk/protective factor(s) | Details |
| 1 | Environmental factors | Many potential environmental risk factors, protective factors, and biomarkers of AR have been published. Tic disorders (class I), early-life antibiotic use, exposure to indoor dampness, acetaminophen exposure, childhood acid suppressant use, and exposure to indoor mold were environmental risk factors (class II), and coronavirus disease 2019 and prolonged breastfeeding were environmental protective factors (class II). The biomarkers graded as suggestive evidence were nasal nitric oxide in AR patients (class II) and interleukin-13 rs20541 polymorphism in AR patients (class III)[23] |
| 2 | Age (> 40 years old) | Age > 40 is an independent risk factor for AR combined with asthma[20] |
| 3 | Demographic factors | Smoking, drinking habits, and pet adoption are demographic factors affecting the presentation of AR[24] |
| 4 | Male | Being male is a risk factor for AR[19] |
| 5 | Family history | A family history of asthma or allergy is an independent risk factor for AR[19,20] |
| 6 | Allergic reactions | Adverse food reactions and mold allergies are independent risk factors for AR[20] |
| 7 | Air purifier use | The use of air purifiers is associated with AR risk[19] |
| 8 | Environmental exposure | Exposure to dust is a risk factor for AR[19] |
| 9 | Living location | Living in towns or urban areas is associated with AR risk[19] |
| 10 | Trends in prevalence | Trends in the prevalence of current AR and factors affecting symptoms have been documented. The prevalence of cumulative AR and current AR symptoms (AR in the past 12 mo) in 6-12-year-old children increased significantly. Longlasting disease before the appearance of the allergy significantly increases the risk of the development of cumulative AR[14] |
| Region | Allergens |
| Thailand | Rice, corn, sorghum, and para grass |
| Southeast Asian Chinese population | Fungus |
| Tropical regions | Aedes aegypti |
| China | |
| Xinjiang | Herbaceous allergens |
| Inner Mongolia | Artemisia pollen |
| Ningxia | Mugwort |
| Shenzhen | Brucella tropicalis, house dust mite, Dermatophagoides farinae, cockroach, and ragweed |
| Beijing | Ragweed and juniper pollen |
Subcutaneous immunotherapy (SCIT) is the cornerstone of treating allergic respiratory diseases. It provides an opportunity to achieve long-term clinical remission through its disease-modifying properties. It can also prevent AR from developing into asthma, prevent new allergen sensitivity, and improve patients’ quality of life[33]. SCIT can be divided into conventional (CIT) and accelerated immunotherapy. The latter can be further divided into cluster immunotherapy and rush immunotherapy (RIT); both CIT and RIT benefit AR, with similar clinical efficacy, safety, and cost-effectiveness ratios. RIT is more effective early, and patients’ compliance is higher. Therefore, RIT is worthy of clinical promotion and exploration[34]. CIT and cluster immunotherapy have similar efficacy, which does not change with age; both schemes are safe and reliable[35]. SCIT is effective against house dust mite allergy in AR patients and can produce a robust immune response[36]. A study confirmed that the initial SCIT treatment of mite extract products Novo Helisen Depot, Strengths 1 to 3 in Chinese children and adolescents with AR and allergic asthma was safe and well-tolerated[37]. Unlike traditional SCIT, Rondon et al[38] reported a unique, cost-effective alternative to immunotherapy, intradermal low-dose house dust mite immunotherapy. This unique immunotherapy is an allergen mixture consisting of 50 ng house dust mite/Dermatophagoides farinae and 120 ng tropical Blumea. It is used in poor allergic communities in the tropics and needs further study. In addition, Tversky et al[39] showed that the novel, universal, low-dose, and species-rich SCIT was well-tolerated and could significantly improve the symptoms of patients with moderate to severe AR. Although the dosage and allergen types of immunotherapy are being updated, further research on the effectiveness and safety of these new ways of immunotherapy is still needed.
Sublingual immunotherapy (SLIT) tablets have more significant benefits than montelukast, antihistamines, and nasal corticosteroids, especially for patients with perennial AR. Compared with SCIT, SLIT tablets are superior to SCIT in safety but slightly inferior to SCIT in efficacy. The safety of SLIT tablets has been fully demonstrated[40]. SCIT mainly induces sIgG4, while SLIT induces sIgA[41]. SLIT can significantly reduce the symptoms of AR patients in the second year of treatment. It can also improve AR patients’ quality of life and mood, reduce the use of symptom relief drugs, and improve their overall health[42]. The clinical efficacy of SLIT tablets is affected by the dose of allergen and the duration of treatment. Some studies have shown that the different doses of mite SLIT tablets have no significant difference in their ability to improve the quality of life of children with AR. The longer the duration of treatment, the better the effect[43]. In addition, Suárez-Fueyo et al[44] showed that patients treated with SLIT tablets had significantly higher total serum IgE levels than those treated with placebo. The patients’ peripheral blood IgE Fc receptor CD23+ cells correlated positively with the therapeutic effect. Patients treated with SLIT tablets also showed a significant decrease in the ability of CD23+ cells to capture and transport specific IgE. At present, SLIT tablets are not recommended for asthma patients. Still, some studies have shown that the combination of SLIT tablets and omalizumab can improve the symptoms of asthma patients with AR. Thus, SLIT tablets can provide a new treatment for asthma patients with AR[45]. In addition, some scholars have proposed the use of novel allergens and food allergens. SLIT is a novel treatment for respiratory allergic diseases, but more extensive clinical trials are needed[46].
Oral immunotherapy (OIT) is a more aggressive treatment involving escalating doses of allergens to induce desensitization and tolerance. It is a promising strategy for managing food allergies and has shown efficacy in treating AR and asthma. OIT’s success is attributed to its ability to modify the immune response, leading to sustained unresponsiveness to allergens and reducing the risk of severe allergic reactions upon accidental exposure[47]. A study suggested that OIT could be a potential therapeutic option for patients with respiratory allergies to tree pollen, house dust mites, and animal dander[48]. OIT has shown promise in treating respiratory allergies, including AR, by inducing immune tolerance and modifying the immune response to allergens[49,50]. OIT has successfully improved AR symptoms, with potential benefits extending to asthma control[51]. Moreover, OIT has been explored as a possible treatment for asthma, especially in individuals with overlapping AR, showcasing its potential as a comprehensive therapeutic approach[52]. OIT is considered an evolving therapeutic avenue with considerable potential for enhancing the quality of life for individuals with AR and other respiratory allergies[53]. However, the complexities associated with determining optimal dosing regimens, managing adverse reactions, and ensuring long-term efficacy underscore further research to refine OIT protocols and establish its role in the broader landscape of allergic disease management.
Intralymphatic immunotherapy (ILIT) is an emerging approach that involves injecting allergens into lymph nodes to induce a more targeted and potent immune response. Increased sIgG4 levels have been associated with the effectiveness of ILIT[54]. ILIT holds promise as a rapid and effective treatment for AR and has shown encouraging results in clinical studies[55]. ILIT has effectively reduced AR symptoms and improved quality of life[56]. The targeted nature of ILIT allows for lower allergen doses, minimizing the risk of systemic side effects while maximizing therapeutic benefits[57]. A study comparing ILIT with SCIT found that ILIT induced a faster and more significant decrease in nasal symptoms and medication use in patients with AR[58]. ILIT represents a novel and innovative immunotherapeutic approach with the potential to revolutionize the treatment landscape for allergic diseases. While further research is needed to establish its long-term efficacy and safety, ILIT holds promise as a valuable addition to the armamentarium of allergy treatments.
Gold-induced autologous serum injection therapy (GIASIT) is an emerging immunotherapeutic approach that involves the injection of autologous serum treated with gold salts to induce desensitization and alleviate symptoms of AR[59]. GIASIT has shown promise in reducing nasal symptoms, improving quality of life, and decreasing medication use in patients with AR[60]. The underlying mechanisms of GIASIT involve the modulation of immune responses and the induction of tolerance to allergens[61]. GIASIT represents a unique and innovative immunotherapeutic approach for AR, offering an alternative for individuals who may not be suitable candidates for traditional immunotherapy approaches. While more research is needed to elucidate its long-term efficacy and safety, GIASIT holds potential as a complementary option in the spectrum of allergy treatments.
The integration of immunotherapy with antibody therapy has emerged as a viable and effective treatment option for individuals grappling with severe AR. While immunotherapy is an efficacious approach for treating AR, its applicability and benefits are only sometimes experienced among all patients. A significant breakthrough in this realm is evident in the confirmed substantial efficacy of omalizumab in addressing allergic asthma in patients concurrently affected by AR[60]. This innovative therapeutic combination represents a promising avenue for improving treatment outcomes, particularly for individuals facing the challenges of severe AR. Many scholars found that when the ratio of the total IgE level to baseline at week 16 was ≥ 2.0, this ratio in patients with moderate to severe AR was significantly correlated with the clinical response to omalizumab. Omalizumab effectively treats patients with moderate to severe AR and improves their quality of life[61]. For some patients with severe local AR, SLIT combined with omalizumab can improve the treatment effect for these refractory patients[62]. In addition, a study showed that the addition of dupilumab to mite SLIT tablets could also effectively treat the symptoms of asthma patients with AR[63]. Corren et al[64] reported on a study combining SCIT and human monoclonal anti-thymic stromal lymphopoietin antibodies in treating AR patients. It was found that the inhibition of thymic stromal lymphopoietin enhanced the efficacy of SCIT during treatment and may promote tolerance after one year of treatment (Table 3).
| Method | Delivery route | Mechanism | Safety |
| SCIT | Subcutaneous (systemic) injection | IgG4 antibody induction[41] | Higher rates of systemic reactions[40] |
| SLIT | Sublingual (local) administration | IgA antibody induction[41] | Fewer systemic reactions than SCIT[40] |
| OIT | Oral cavity/gastrointestinal tract | Suppression of allergen-specific T-cell proliferation[50] | Oral pruritus[53] |
| ILIT | Lymph nodes | IgG4 antibody induction[54] | Safer than SCIT[58] |
| GIASIT | Intravenous infusion | Increased plasma gelsolin levels[59] | Mild side effects[59] |
| Combination of AIT and monoclonal antibody therapy | Subcutaneous monoclonal antibody and AIT route | Omalizumab (anti-IgE). Dupilumab (anti-IL4Rα). Tezepelumab (anti-TSLP)[62-64] | Mild or moderate application-site reactions[63] |
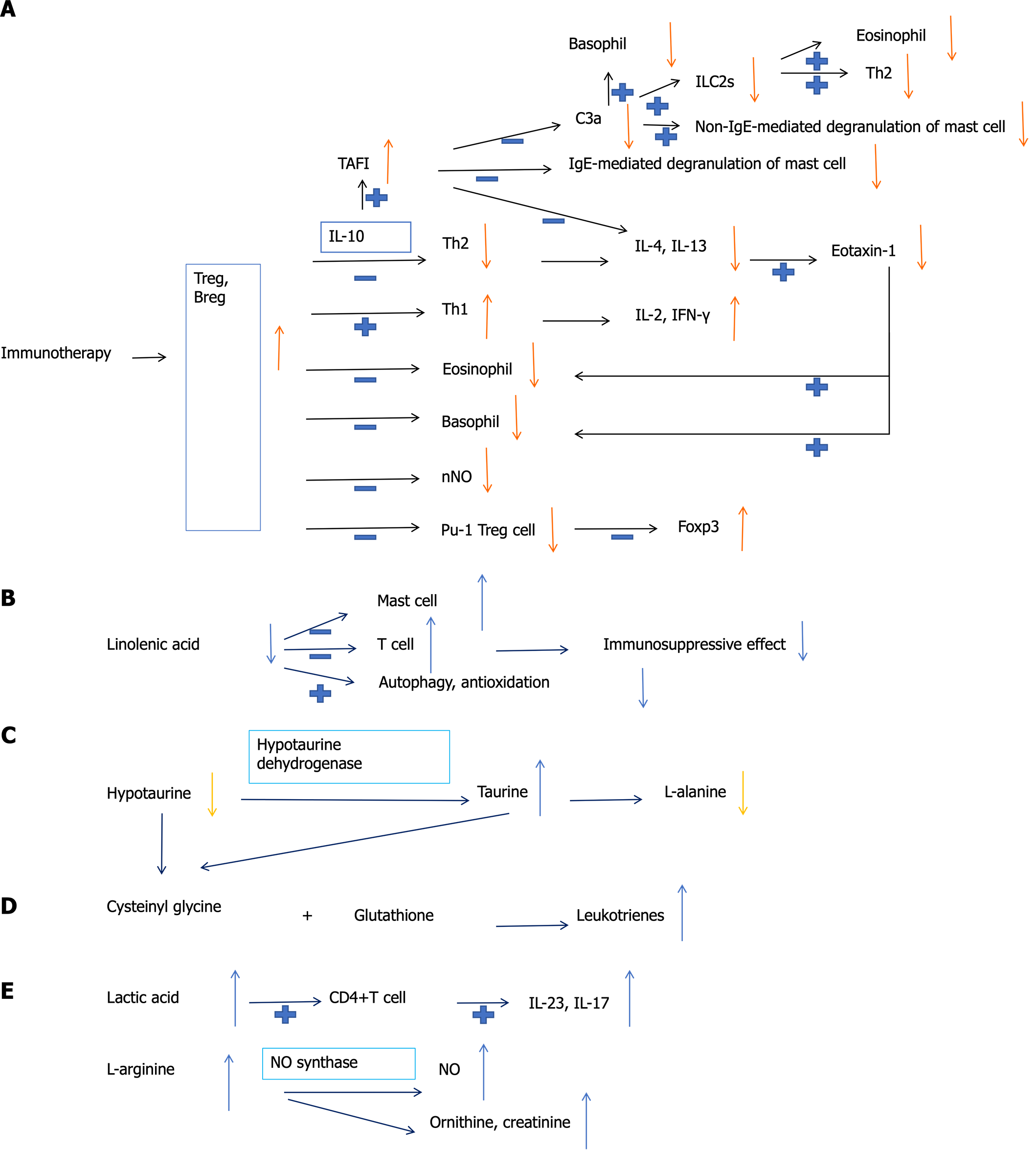
Studies have shown that many indicators can predict the efficacy of immunotherapy. Immunotherapy can reduce the proportion of PU-1+ regulatory T cell (Treg) subtype in AR patients. The number of PU-1+ Treg cells may be used as an indicator to monitor the therapeutic effect of immunotherapy on AR[65]. In addition, some metabolites, such as hypotaurine, taurine, and L-alanine, can potentially become predictive biomarkers of effective SCIT[66]. Interleukin-4, eotaxin, and interferon-γ can also serve as robust biomarkers for the early prediction of SCIT response[67]. It has been reported that the decreased basophil sensitivity after 3 wk of SCIT can predict the clinical outcome of this treatment[68]. A study showed that the onset of action and clinical response to SCIT in the second year can be expected as early as the fourth month[69]. Parisi et al[70] found that nasal nitric oxide and nasal cytology can also be used as predictors of the short-term efficacy of SLIT treatment. It is reported in the literature that six metabolites, including lactate, ornithine, linolenic acid, creatinine, arachidonic acid, and sphingosine, perform well in predicting the efficacy of SLIT. The changes in these metabolites mainly involve glycolysis and pyruvate metabolism, arginine and proline metabolism, and fatty acid metabolism pathways[71]. In addition, SLIT may induce high serum thrombin activatable fibrinolysis inhibitor levels. Thrombin activatable fibrinolysis inhibitor may play a key protective role in the pathogenesis of AR by inactivating C3a and inhibiting mast cell degranulation and chemokine expression in fibroblasts[72]. A recent study reported that the regulatory effect on type II innate lymphoid cells is also part of the therapeutic mechanism of SLIT[73]. Identifying the immune cells, cytokines, metabolites, and metabolic pathways related to immunotherapy’s efficacy will help better understand the mechanism of immunotherapy in AR patients and screen out the people who benefit from immunotherapy (Figure 1).
Immunotherapy is the only causal treatment confirmed by research that can improve the symptoms of AR patients. Many scholars have found that immunotherapy is related to significantly reduced drug treatment in children[10,74-79]. It showed sustained clinical efficacy during the treatment period, and the disease remission effect lasted at least two years after stopping the treatment[74]. This is the hallmark of clinical control[75]. In addition, immunotherapy is a treatment accompanied by high patient expectations for efficacy and satisfaction. Long-term continuation is possible in case of patients’ correct understanding of treatment methods and compliance with immunotherapy. Immunotherapy is expected to be increasingly popular, from treating specialists to treating general clinicians[76]. Although the efficacy of immunotherapy has been confirmed, immunotherapy also has shortcomings. Patients with allergic diseases have poor compliance with immunotherapy, which is related to gender and the number of diseases. The main reasons for withdrawal are self-conscious inconvenience and unsatisfactory treatment effect[77]. Children’s compliance is better than that of adults. A one-year large-scale reality study in Germany confirmed that standardized house dust mite SLIT was effective. The adverse drug reactions were consistent with the data of controlled clinical trials. Most adverse reactions resolved within the first 2 wk, highlighting the importance of patient education to optimize compliance[78]. In addition, a recent study has shown that the current SLIT guidelines are of average quality. The formulation methods and reporting standards of these guidelines must be formulated to regulate the treatment of SLIT correctly[79] (Table 4).
| Limitation | Details | Resolution |
| Poor compliance with immunotherapy, which is related to gender and the number of diseases | Self-conscious inconvenience and unsatisfactory treatment effect, the compliance of children is better than that of adults | Patient education |
| Current SLIT guidelines are of average quality | The formulation methods and reporting standards of these guidelines must be formulated |
AR is a prevalent and burdensome global health issue with far-reaching implications for affected individuals. Immunotherapy has emerged as a pivotal and evolving therapeutic approach for AR, offering the prospect of sustained clinical remission and the prevention of disease progression. This comprehensive review provides a thorough examination of various immunotherapeutic pathways, encompassing SCIT, SLIT, OIT, ILIT, and GIASIT. Each pathway is systematically elucidated, with its distinctive features, mechanisms, and clinical implications highlighted. The review critically assesses the epidemiological landscape of AR, shedding light on its diverse regional characteristics and influencing factors. Furthermore, combining immunotherapy with antibody therapy signifies a promising frontier in severe AR treatment. The review outlines relevant indicators for predicting immunotherapy efficacy, emphasizing the importance of identifying immune cells, cytokines, metabolites, and metabolic pathways associated with treatment outcomes. Despite the proven benefits of immunotherapy, the review candidly addresses its limitations, including poor patient compliance and the need for enhanced education and guideline quality. The evolving nature of AIT, coupled with ongoing advancements in treatment protocols, reinforces this therapeutic modality’s dynamic and adaptable nature. In conclusion, this review can serve as a comprehensive guide for clinicians, researchers, and policymakers, offering a nuanced understanding of the current state of immunotherapy for AR and its potential implications for the future of allergic disease management.
| 1. | García-Marcos L, Asher MI, Pearce N, Ellwood E, Bissell K, Chiang CY, El Sony A, Ellwood P, Marks GB, Mortimer K, Martínez-Torres AE, Morales E, Perez-Fernandez V, Robertson S, Rutter CE, Silverwood RJ, Strachan DP; Global Asthma Network Phase I Study Group. The burden of asthma, hay fever and eczema in children in 25 countries: GAN Phase I study. Eur Respir J. 2022;60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 2. | Wise SK, Damask C, Roland LT, Ebert C, Levy JM, Lin S, Luong A, Rodriguez K, Sedaghat AR, Toskala E, Villwock J, Abdullah B, Akdis C, Alt JA, Ansotegui IJ, Azar A, Baroody F, Benninger MS, Bernstein J, Brook C, Campbell R, Casale T, Chaaban MR, Chew FT, Chambliss J, Cianferoni A, Custovic A, Davis EM, DelGaudio JM, Ellis AK, Flanagan C, Fokkens WJ, Franzese C, Greenhawt M, Gill A, Halderman A, Hohlfeld JM, Incorvaia C, Joe SA, Joshi S, Kuruvilla ME, Kim J, Klein AM, Krouse HJ, Kuan EC, Lang D, Larenas-Linnemann D, Laury AM, Lechner M, Lee SE, Lee VS, Loftus P, Marcus S, Marzouk H, Mattos J, McCoul E, Melen E, Mims JW, Mullol J, Nayak JV, Oppenheimer J, Orlandi RR, Phillips K, Platt M, Ramanathan M Jr, Raymond M, Rhee CS, Reitsma S, Ryan M, Sastre J, Schlosser RJ, Schuman TA, Shaker MS, Sheikh A, Smith KA, Soyka MB, Takashima M, Tang M, Tantilipikorn P, Taw MB, Tversky J, Tyler MA, Veling MC, Wallace D, Wang Y, White A, Zhang L. International consensus statement on allergy and rhinology: Allergic rhinitis - 2023. Int Forum Allergy Rhinol. 2023;13:293-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 193] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 3. | Kim EH, Bird JA, Keet CA, Virkud YV, Herlihy L, Ye P, Smeekens JM, Guo R, Yue X, Penumarti A, Qaqish B, Li Q, Kulis MD, Burks AW. Desensitization and remission after peanut sublingual immunotherapy in 1- to 4-year-old peanut-allergic children: A randomized, placebo-controlled trial. J Allergy Clin Immunol. 2024;153:173-181.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 4. | Kappen JH, Agache I, Jutel M, Pillai P, Corrigan CJ. Allergen Immunotherapy for Asthma. J Allergy Clin Immunol Pract. 2024;12:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Pfützner W. Allergen immunotherapy of insect venom allergy: Almost 100 years old, but steadily updated. Allergol Select. 2023;7:211-218. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Li Y, Li H, Huang W, Yu Q, Wang K, Xiong Y, Wang Q, Qin Y, Kuang X, Tang J. Single-cell RNA sequencing reveals the landscape of biomarker in allergic rhinitis patient undergoing intracervical lymphatic immunotherapy and related pan-cancer analysis. Environ Toxicol. 2024;39:2817-2829. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Lommatzsch M, Brusselle GG, Levy ML, Canonica GW, Pavord ID, Schatz M, Virchow JC. A(2)BCD: a concise guide for asthma management. Lancet Respir Med. 2023;11:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 8. | Lee HY, Lee SM, Kang SY, Kim K, Kim JH, Ryu G, Min JY, Park KH, Park SY, Sung M, Lee Y, Yang EA, Jee HM, Ha EK, Shin YS, Chung EH, Choi SH, Koh YI, Kim ST, Nahm DH, Park JW, Shim JY, An YM, Han DH, Han MY, Lee YW, Choi JH; Korean Academy of Asthma Allergy and Clinical Immunology (KAAACI) Allergen Immunotherapy and Allergen Working Group. KAAACI Guidelines for Allergen Immunotherapy. Allergy Asthma Immunol Res. 2023;15:725-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Noon L. Prophylactic inoculation against hay fever. Int Arch Allergy Appl Immunol. 1953;4:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Xia C, Yan R, Wang Q. [Compliance and withdraw reason of sublingual immunotherapy in 245 patients with allergic rhinitis]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2023;37:277-281. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Sio YY, Victoria Nanong GA, Lim JA, Matta SA, Say YH, Teh KF, Wong YR, Rawanan Shah SM, Reginald K, Chew FT. Sensitization to oil palm pollen associates with risks and severity of allergic diseases. World Allergy Organ J. 2024;17:100853. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Jeong KY, Sang M, Lee YS, Gadermaier G, Ferreira F, Park JW. Characterization of Hum j 6, a Major Allergen From Humulus japonicus Pollen, the Primary Cause of Weed Pollinosis in East Asia. Allergy Asthma Immunol Res. 2023;15:767-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Yazar B, Meydanlioglu A. The prevalence and associated factors of asthma, allergic rhinitis, and eczema in Turkish children and adolescents. Pediatr Pulmonol. 2022;57:2491-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Sultész M, Balogh I, Katona G, Mezei G, Hirschberg A, Gálffy G. Trends in prevalence and risk factors of allergic rhinitis symptoms in primary schoolchildren six years apart in Budapest. Allergol Immunopathol (Madr). 2017;45:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Pirner C, Korbely C, Heinze S, Huß J, Summer B, Oppel E, Nowak D, Herr C, Kutzora S; HMU Study Group. Atopic diseases and airway-related symptoms in Bavarian children before starting primary school: Time trend analyses. Respir Med. 2022;191:106707. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Pham DL, Trinh THK, Le KM, Pawankar R. Characteristics of allergen profile, sensitization patterns and Allergic Rhinitis in SouthEast Asia. Curr Opin Allergy Clin Immunol. 2022;22:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Zhuang Y, Ma T, Kang Z, Siqin B, Yan W, Bai Y, Shan G, Wang X. [Epidemiological survey of allergic rhinitis in steppe area of Xilingol League, Inner Mongolian of China]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;35:1004-1008. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Fu W, Zheng Z, Zhao J, Feng M, Xian M, Wei N, Qin R, Xing Y, Yang Z, Wong GWK, Li J. Allergic disease and sensitization disparity in urban and rural China: A EuroPrevall-INCO study. Pediatr Allergy Immunol. 2022;33:e13903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Tong X, Tong H, Gao L, Deng Y, Xiang R, Cen R, Zhao Y, Wang P, Li G, Shen J, Xu B, He B, Kong Y, Tao Z, Xu Y; Hubei Medical Quality Control Center for Allergic Disease. A Multicenter Study of Prevalence and Risk Factors for Allergic Rhinitis in Primary School Children in 5 Cities of Hubei Province, China. Int Arch Allergy Immunol. 2022;183:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Ma T, Chen Y, Pang Y, Wang X, Dai D, Zhuang Y, Shi H, Zheng M, Zhang R, Jin W, Yang X, Wang Y, Shan G, Yan Y, Wang D, Wang X, Wei Q, Yin J, Wang X, Zhang L. Prevalence and risk factors of allergic rhinitis and asthma in the southern edge of the plateau grassland region of northern China: A cross-sectional study. World Allergy Organ J. 2021;14:100537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Lee SL, Lau YL, Wong WH, Tian LW. Childhood Wheeze, Allergic Rhinitis, and Eczema in Hong Kong ISAAC Study from 1995 to 2015. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Vehapoglu A, Cakın ZE, Kahraman FU, Nursoy MA, Toprak A. Is overweight/obesity a risk factor for atopic allergic disease in prepubertal children? A case-control study. J Pediatr Endocrinol Metab. 2021;34:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Xu X, Liu X, Li J, Deng X, Dai T, Ji Q, Xiong D, Xie H. Environmental Risk Factors, Protective Factors, and Biomarkers for Allergic Rhinitis: A Systematic Umbrella Review of the Evidence. Clin Rev Allergy Immunol. 2023;65:188-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 24. | Chong SN, Chew FT. Epidemiology of allergic rhinitis and associated risk factors in Asia. World Allergy Organ J. 2018;11:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Jarupund P, Jantrakulroj P, Suwanphakdee C, Sinthuvanich C. A Pilot Study to Identify Grass Species That Mediate Pollen Allergy in Thailand. Int Arch Allergy Immunol. 2023;184:875-881. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Sio YY, Pang SL, Say YH, Teh KF, Wong YR, Shah SMR, Reginald K, Chew FT. Sensitization to Airborne Fungal Allergens Associates with Asthma and Allergic Rhinitis Presentation and Severity in the Singaporean/Malaysian Population. Mycopathologia. 2021;186:583-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Sánchez J, Toro Y, Cantillo JF, Martínez D, Fernández-Caldas E, Cardona R, Puerta L. Nasal Provocation Challenges with Aedes aegypti Whole-Body Extract Induces Allergic Rhinitis. Int Arch Allergy Immunol. 2023;184:366-369. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Xiao LM, Yan HS, Yang YP, Wang LL, Zhang H. [Analysis of inhaled allergen spectrum characteristics of allergic rhinitis in 5 019 cases in Xinjiang area]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022;57:474-478. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Zhang Y, Yan X, Shen X, Liu M, Zhou Y, He J, Zhang N, Chen B, Yang F, Ma R. [Distribution characteristics and results of allergens in patients with allergic rhinitis in Ningxia area]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2023;37:562-569. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Zhang N, Wu Y, Zhang Q, Wei Z, Liu Y. [Analysis of inhalation allergen of patients with allergic rhinitis in Shenzhen]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022;36:467-472. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Ma TT, He N, Wang HT, Chen YL, Zhuang Y, Shi HY, Lan TF, Guo MY, Yu RL, Wang Y, Wang XY. [Sensitization characteristics of Juniperus chinensis pollen in Beijing area]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022;57:479-484. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Suo S, Ma T, Wang H, Wang Y, Wang X. [Sensitization characteristics of ragweed pollen in Beijing area]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2023;37:380-386. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Creticos PS. Subcutaneous allergen immunotherapy in the treatment of allergic respiratory disease. Allergy Asthma Proc. 2022;43:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Shen Y, Ke X, Yang YC, Huang JJ, Liu J, Zhang M, Chen ZQ, Hong SL. [Clinical observation and preliminary economic study of rush immunotherapy in patients with allergic rhinitis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022;57:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Yu J, Zhong N, Luo Q, Liu Y, Yi H, Ye J, Zhang J. Early Efficacy Analysis of Cluster and Conventional Immunotherapy in Patients With Allergic Rhinitis. Ear Nose Throat J. 2021;100:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Valero A, Ibáñez-Echevarría E, Vidal C, Raducan I, Castelló Carrascosa JV, Sánchez-López J. Efficacy of subcutaneous house dust mite immunotherapy in patients with moderate to severe allergic rhinitis. Immunotherapy. 2022;14:683-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Xiang L, Liu F, Zhi L, Jiang W, Liu C, Xie H, Zhou X, Sun Y, Zheng Y, Zhu R, Tao Z, Xia W, Lai H, Wei Q, Cheng L, Tang Y, Xu R, Huang H, Zhou Q, Chang P. Safety of semi-depot house dust mite allergen extract in children and adolescents with allergic rhinitis and asthma. Immunotherapy. 2021;13:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Rondon C, Sánchez-Borges M, Cupello ER, Fabiano F, Capriles-Hulett A. Aqueous intradermal low-dose house dust mite immunotherapy in tropical settings: a valid cost-effective approach for developing nations? Allergol Immunopathol (Madr). 2021;49:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Tversky J, Patel P, Sowho M, Natarajan R, Chung T, Whelton A, Azar A. Randomized double-blind pilot study of universal, species abundant, multiallergen subcutaneous immunotherapy for moderate-severe allergic rhinitis. Ann Allergy Asthma Immunol. 2023;131:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | DuBuske L. Efficacy and safety of sublingual allergen immunotherapy. Allergy Asthma Proc. 2022;43:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Shamji MH, Larson D, Eifan A, Scadding GW, Qin T, Lawson K, Sever ML, Macfarlane E, Layhadi JA, Würtzen PA, Parkin RV, Sanda S, Harris KM, Nepom GT, Togias A, Durham SR. Differential induction of allergen-specific IgA responses following timothy grass subcutaneous and sublingual immunotherapy. J Allergy Clin Immunol. 2021;148:1061-1071.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 42. | Horn A, Bernstein DI, Okubo K, Dalgaard T, Hels O, Sørensen HF, Henriksen M, Azuma R, Mikler J, Nolte H. House dust mite sublingual immunotherapy tablet safety in adolescents with allergic rhinoconjunctivitis: Worldwide clinical trial results. Ann Allergy Asthma Immunol. 2023;130:797-804.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 43. | Kajiume T. Sublingual immunotherapy for pediatric patients with mite allergies. Medicine (Baltimore). 2022;101:e28690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 44. | Suárez-Fueyo A, Ramos T, Galán A, Jimeno L, Wurtzen PA, Marin A, de Frutos C, Blanco C, Carrera AC, Barber D, Varona R. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J Allergy Clin Immunol. 2014;133:130-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Woehlk C, Ramu S, Sverrild A, Nieto-Fontarigo JJ, Vázquez-Mera S, Cerps S, Pulga A, Andreasson LM, Eriksen LL, Dyhre-Petersen N, Menzel M, Klein DK, Hansen S, Uller L, Porsbjerg C. Allergen Immunotherapy Enhances Airway Epithelial Antiviral Immunity in Patients with Allergic Asthma (VITAL Study): A Double-Blind Randomized Controlled Trial. Am J Respir Crit Care Med. 2023;207:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 46. | Soyyiğit S, Aydın Ö, Seçil D, Doğan C, Gökmen D, Sin BA, Mısırlıgil Z, Mungan VD. Pre-seasonal immunotherapy is effective in both monosensitized and polysensitized patients with allergic rhinitis. Eur Ann Allergy Clin Immunol. 2023;55:122-130. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 47. | Yang J, Shen Z, Liu L, Kang W, Shao Y, Zhang P, Quan F. Clinical Efficacy and Safety of Artesimia annua-Sublingual Immunotherapy in Seasonal Allergic Rhinitis Patients Based on Different Intervention Time. Int Arch Allergy Immunol. 2022;183:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 48. | Yonekura S, Gotoh M, Okano M, Kurokawa T, Maekawa Y, Okubo K, Okamoto Y. Japanese cedar pollen sublingual immunotherapy is effective in treating seasonal allergic rhinitis during the pollen dispersal period for Japanese cedar and Japanese cypress. Allergol Int. 2022;71:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Würtzen PA, Grønager PM, Lund G, Gupta S, Andersen PS, Biedermann T, Ipsen H. Simplified AIT for allergy to several tree pollens-Arguments from the immune outcome analyses following treatment with SQ tree SLIT-tablet. Clin Exp Allergy. 2021;51:284-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Endo T, Asaka D, Nakayama T, Saito S, Kodama H, Mitsuyoshi R, Takaishi S, Sugimoto N, Omae S, Takagi H, Wakasa Y, Ozawa K, Takano M, Takaiwa F, Kojima H, Saito S. Immunological and Symptomatic Effects of Oral Intake of Transgenic Rice Containing 7 Linked Major T-Cell Epitopes from Japanese Cedar Pollen Allergens. Int Arch Allergy Immunol. 2021;182:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Yuta A, Ogawa Y, Murao T, Kozaki H, Shimizu T. [Enhanced clinical effects of Cedarcure(®) tablets of sublingual immunotherapy over the years of treatment and the impact of outcome by combined use of mite sublingual immunotherapy (dual SLIT) for Japanese Cedar pollinosis]. Arerugi. 2022;71:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Contoli M, Porsbjerg C, Buchs S, Larsen JR, Freemantle N, Fritzsching B. Real-world, long-term effectiveness of allergy immunotherapy in allergic rhinitis: Subgroup analyses of the REACT study. J Allergy Clin Immunol. 2023;152:445-452.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 53. | González-Bravo L, García JL, Privitera M, Rosado A. Off-label Use of Oral Immunotherapy for Rhinoconjunctivitis and Asthma due to Grass Pollen: A Safe and Effective Alternative in Patients over 65 Years Old: A Series of Case Reports. Curr Drug Saf. 2023;18:599-602. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Shamji MH, Kappen JH, Akdis M, Jensen-Jarolim E, Knol EF, Kleine-Tebbe J, Bohle B, Chaker AM, Till SJ, Valenta R, Poulsen LK, Calderon MA, Demoly P, Pfaar O, Jacobsen L, Durham SR, Schmidt-Weber CB. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72:1156-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 258] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 55. | Skaarup SH, Schmid JM, Skjold T, Graumann O, Hoffmann HJ. Intralymphatic immunotherapy improves grass pollen allergic rhinoconjunctivitis: A 3-year randomized placebo-controlled trial. J Allergy Clin Immunol. 2021;147:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 56. | Ahlbeck L, Ahlberg E, Björkander J, Aldén C, Papapavlou G, Palmberg L, Nyström U, Retsas P, Nordenfelt P, Togö T, Johansen P, Rolander B, Duchén K, Jenmalm MC. Intralymphatic immunotherapy with one or two allergens renders similar clinical response in patients with allergic rhinitis due to birch and grass pollen. Clin Exp Allergy. 2022;52:747-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Hellkvist L, Hjalmarsson E, Weinfeld D, Dahl Å, Karlsson A, Westman M, Lundkvist K, Winqvist O, Georén SK, Westin U, Cardell LO. High-dose pollen intralymphatic immunotherapy: Two RDBPC trials question the benefit of dose increase. Allergy. 2022;77:883-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Wang W, Wang X, Wang H, Wang X. Evaluation of Safety, Efficacy, and Compliance of Intralymphatic Immunotherapy for Allergic Rhinoconjunctivitis: A Systematic Review and Meta-Analysis. Int Arch Allergy Immunol. 2023;184:754-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 59. | Schneider U, Hollands P. Intravenous gold-induced autologous serum injection therapy (Go ACT®) as a new treatment for seasonal pollen-based allergies. Eur Rev Med Pharmacol Sci. 2021;25:4121-4127. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Qian XJ, Hu XT, Jiang P. Exploration of the efficacy of anti-immunoglobulin E monoclonal antibodies in the treatment of allergic asthma. Immunology. 2023;169:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Zhao Z, Deng Y, Xiang L, Chen J, Wan J, Sun J, Kong Y, Hua Q. The ratio of total IgE level at week 16 to baseline significantly correlated with the clinical response to omalizumab in moderate to severe allergic rhinitis patients. Int Immunopharmacol. 2023;122:110623. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 62. | Bozek A, Kozłowska R, Misiołek M, Ścierski W, Gawlik R. Omalizumab added to allergen immunotherapy increased the effect of therapy in patients with severe local allergic rhinitis. Hum Vaccin Immunother. 2022;18:2097818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Hoshino M, Akitsu K, Kubota K, Ohtawa J. Efficacy of a house dust mite sublingual immunotherapy tablet as add-on dupilumab in asthma with rhinitis. Allergol Int. 2022;71:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 64. | Corren J, Larson D, Altman MC, Segnitz RM, Avila PC, Greenberger PA, Baroody F, Moss MH, Nelson H, Burbank AJ, Hernandez ML, Peden D, Saini S, Tilles S, Hussain I, Whitehouse D, Qin T, Villarreal M, Sever M, Wheatley LM, Nepom GT, Sanda S; Immune Tolerance Network ITN057AD CATNIP Study Team. Effects of combination treatment with tezepelumab and allergen immunotherapy on nasal responses to allergen: A randomized controlled trial. J Allergy Clin Immunol. 2023;151:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 65. | Qiao YL, Jiao WE, Xu S, Kong YG, Deng YQ, Yang R, Hua QQ, Chen SM. Allergen immunotherapy enhances the immunosuppressive effects of Treg cells to alleviate allergic rhinitis by decreasing PU-1+ Treg cell numbers. Int Immunopharmacol. 2022;112:109187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 66. | Yu RL, Pan C, Ma TT, Wang XY, Shi HY, Zhuang Y, Yan WJ, Liu JG, Cao MD, Sun JL, Wang DY, Yin JS, Wei JF, Wang XY. Prediction of clinical efficacy of subcutaneous immunotherapy for Artemisia sieversiana pollen allergic rhinitis by serum metabolomics. J Formos Med Assoc. 2022;121:2465-2480. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 67. | Xie S, Fan R, Tang Q, Cai X, Zhang H, Wang F, Xie S, Gao K, Zhang J, Xie Z, Jiang W. Identification of Robust Biomarkers for Early Predicting Efficacy of Subcutaneous Immunotherapy in Children With House Dust Mite-Induced Allergic Rhinitis by Multiple Cytokine Profiling. Front Immunol. 2021;12:805404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Schmid JM, Würtzen PA, Siddhuraj P, Jogdand P, Petersen CG, Dahl R, Erjefält JS, Hoffmann HJ. Basophil sensitivity reflects long-term clinical outcome of subcutaneous immunotherapy in grass pollen-allergic patients. Allergy. 2021;76:1528-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Lin Y, Liu J, He J, Wu L, Li S, Cheng B, Shao Y, Zhang Y, Wang Y, Tang L, Chen Z. Effects of subcutaneous immunotherapy in allergic rhinitis children sensitive to dust mites. Allergol Immunopathol (Madr). 2023;51:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 70. | Parisi GF, Manti S, Papale M, Amato M, Licari A, Marseglia GL, Leonardi S. Nasal Nitric Oxide and Nasal Cytology as Predictive Markers of Short-Term Sublingual Allergen-Specific Immunotherapy Efficacy in Children with Allergic Rhinitis. Am J Rhinol Allergy. 2022;36:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Xie S, Jiang S, Zhang H, Wang F, Liu Y, She Y, Jing Q, Gao K, Fan R, Xie S, Xie Z, Jiang W. Prediction of sublingual immunotherapy efficacy in allergic rhinitis by serum metabolomics analysis. Int Immunopharmacol. 2021;90:107211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 72. | Yoshida K, Takabayashi T, Imoto Y, Sakashita M, Kato Y, Narita N, Fujieda S. Increased Thrombin-Activatable Fibrinolysis Inhibitor in Response to Sublingual Immunotherapy for Allergic Rhinitis. Laryngoscope. 2021;131:2413-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 73. | Wang X, Shen Y, Hong S, Kang H, Ke X. Changes in type 2 innate lymphoid cells and serum cytokines in sublingual immunotherapy in pediatric patients with allergic rhinitis. BMC Pediatr. 2023;23:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 74. | Yonekura S, Gotoh M, Kaneko S, Maekawa Y, Okubo K, Okamoto Y. Disease-Modifying Effect of Japanese Cedar Pollen Sublingual Immunotherapy Tablets. J Allergy Clin Immunol Pract. 2021;9:4103-4116.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Sánchez J, Alvarez L, García E. Real-world study: drug reduction in children with allergic rhinitis and asthma receiving immunotherapy. Immunotherapy. 2023;15:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 76. | Kawashima K. [Sublingual immunotherapy]. Arerugi. 2022;71:92-101. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 77. | Huang RR, Zhu XH, Wang JL. [Demographic features and compliance of children with allergic diseases receiving sublingual immunotherapy]. Zhongguo Dang Dai Er Ke Za Zhi. 2022;24:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 78. | Reiber R, Wolf H, Futschik T, Schwab JA, Hölscher U, Schnitker J, Wüstenberg E. Safety and tolerability of the standardized quality house dust mite sublingual immunotherapy tablet in real life: A noninterventional, open-label study. J Allergy Clin Immunol Pract. 2021;9:3221-3223.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Wang Q, Zhu R, Ning Y, Feng Y, Feng Y, Han S. Evaluation of the quality of guidelines for sublingual immunotherapy of allergic rhinitis. Eur Arch Otorhinolaryngol. 2023;280:4319-4325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |