Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.616
Peer-review started: October 24, 2023
First decision: November 28, 2023
Revised: December 16, 2023
Accepted: January 2, 2024
Article in press: January 2, 2024
Published online: January 26, 2024
Processing time: 85 Days and 17.7 Hours
Brain abscess is a serious and potentially fatal disease caused primarily by microbial infection. Although progress has been made in the diagnosis and treatment of brain abscesses, the diagnostic timeliness of pathogens needs to be improved.
We report the case of a 54-year-old male with a brain abscess caused by oral bacteria. The patient recovered well after receiving a combination of metagenomic next-generation sequencing (mNGS)-assisted guided medication and surgery.
Therefore, mNGS may be widely applied to identify the pathogenic microorganisms of brain abscesses and guide precision medicine.
Core Tip: Brain abscesses are mixed infections, and there is difficulty in detecting all pathogens on routine bacterial cultures. We report the case of a 54-year-old male with a brain abscess caused by oral bacteria. The patient recovered well after receiving a combination of metagenomic next-generation sequencing (mNGS)-assisted guided medication and surgery. mNGS may be used for the rapid diagnosis of pathogenic microorganisms and may play an important role in precision medicine.
- Citation: Zhu XM, Dong CX, Xie L, Liu HX, Hu HQ. Brain abscess from oral microbiota approached by metagenomic next-generation sequencing: A case report and review of literature. World J Clin Cases 2024; 12(3): 616-622
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/616.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.616
Brain abscess is a serious and potentially fatal disease caused primarily by microbial infection[1,2]. Brain abscesses can result from the spread of bacteria from distant sites of primary infection or from direct sequential invasion from adjacent sites[3,4]. Pus evolves from a localized area of encephalitis to an enveloped membrane in the brain parenchyma[5].
Patients with brain abscesses have had high morbidity, disability, and mortality rates in recent decades[6,7]. In recent years, the morbidity and mortality of brain abscesses have only declined with significant advances in imaging techniques, neurosurgical techniques, microbiological detection techniques, and antibiotic therapy[8]. However, the predisposing factors of bacterial brain abscesses and the prevalence of associated bacterial pathogens have different effects on mortality.
Brouwer et al[8] found that the common pathogens in blood and abscess fluid cultures from patients with brain abscesses were Streptococci, Diplococci, and Gram-negative bacteria. Notably, most cases were mixed infections with multiple microorganisms, including rare ones. In recent years, the application of new molecular biotechnologies has expanded the range of microbial pathogens isolated in brain abscesses[9].
Detection and identification of the pathogen are essential to confirm the diagnosis and select the best antibiotic regimen. Microbiological cultures of abscesses remained negative in approximately 20% of patients with bacterial brain abscesses. In recent years, metagenomic next-generation sequencing (mNGS) has been used for its ability to detect all potential pathogens - bacteria, viruses, fungi, and parasites in a sample and to detect host responses simultaneously[10]. Thus, mNGS has great potential utility in the diagnosis of infectious diseases.
Herein, we present a case of a brain abscess due to periodontitis pathogens in a 54-year-old man. The patient recovered well after receiving a combination of mNGS-assisted guided medication and surgery.
A 54-year-old man presented to the neurology department with a headache and fever for 5 d.
The symptoms started 3 mo before the presentation and included headaches while bowing his head, which started 19 d before the presentation with double vision while viewing distant objects, and fevers during the headaches started 5 d before presentation.
Seven years prior, the patient underwent surgery for appendicitis in a local hospital. Moreover, he was diagnosed with chronic severe periodontitis for 5 years, type 2 diabetes for 2 years, and an allergic reaction to aminotriol keto chromate injection. Ten days prior, the patient was admitted to a local hospital complaining of headaches for three months, and brain magnetic resonance imaging (MRI) revealed multiple signal abnormalities in the left cerebellum and abnormal signal foci in the cavernous sinus region. Laboratory examinations showed that the C-reactive protein (CRP) was 44.91 mg/L (normal 0-10), and the erythrocyte sedimentation rate (ESR) was 45 mm/h (normal 0-15). cyfra21-1 (CF21-1) was 3.5 ng/mL (normal 0-3), neuron-specific enolase (NSE) was 22.8 ng/mL (normal 0.6-5.4), and the levels of immune markers were normal. No abnormalities were found in the electrolyte test, kidney function test, liver renal function test, or routine blood and urine analyses. Therefore, the local hospital’s staff considered that the patient could have metastases or inflammatory lesions. Five days prior, the treatment showed no avail, and the patient presented with an aggravated headache and pyrexia. The patient was admitted to our hospital for further diagnosis and treatment.
The patient denied any family history of genetic disorders.
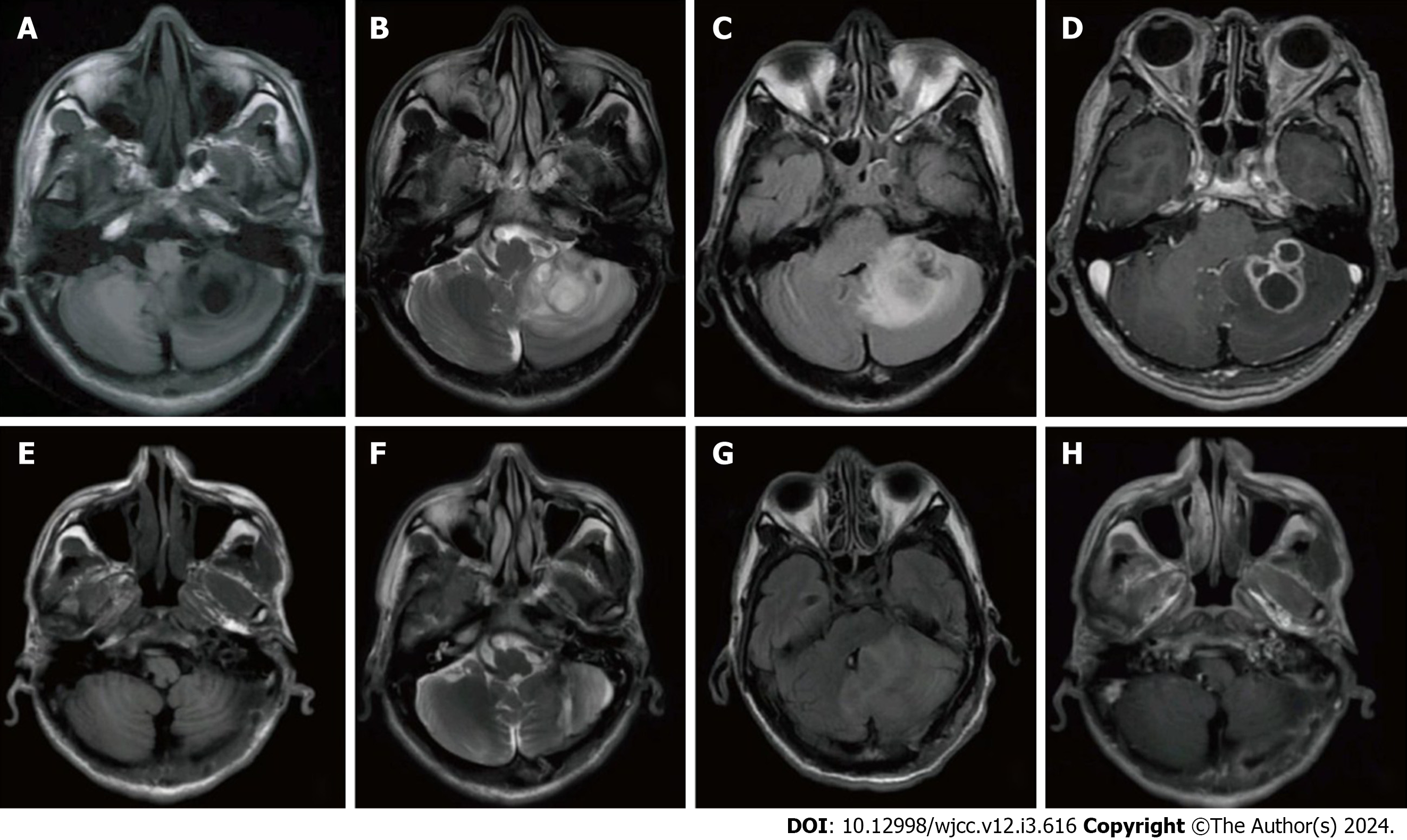
Thyroid and abdominal ultrasound, chest computed tomography (CT), and abdominal CT did not reveal any tumor lesions, and lymph node biopsy showed no significant abnormalities. Brain CT showed abnormal signal foci in the cavernous sinus region. Brain MRI showed irregular patchy long T1 and long T2 signal shadows in the left cerebellar hemisphere, with blurred borders, and patchy low signal shadows within them. Enhancement scanning of the lesion area showed a multiventricular ring-like enhancement (Figure 1A-D).
The serum sodium was 133.6 mmol/L (normal 135-145), the serum chlorine was 92.6 mmol/L (normal 95-100), the blood glucose was 8.68 mmol/L (normal 3.9-6.1), the glycated hemoglobin was 11.3% (normal 4%-6%), the CRP was 11.00 mg/L (normal 0-10), and the ESR was 23 mm/h (normal 0-15). The cytomegalovirus IgG was 92.2 U/mL (normal 0-4), and the rubella virus IgG was 24.5 IU/mL (normal 0 < 10). The antinuclear extract antibody profile (Anti-Sm antibody, anti-Scl-70 antibody, anti-RNP antibody, anti-Jo-1 antibody, anti-SSA antibody and anti-SSB antibody), tumor anti-nervous system antibody profile (anti-hu, anti-Ri, anti-Yo, anti-Amphiphysin), and tumor marker (CA125, CA199, CA72-4, CY21-1, CEA, NSE, SCC, AFP, TPSA, FPSA and ProGRP), all of which were normal. No abnormalities were found in the eight tests for infectious diseases, tuberculosis T-cell test, G test, GM test, kidney function, liver renal function test, or routine blood and urine analyses.
The vital signs were as follows: body temperature, 36.5 °C; blood pressure, 105/70 mmHg; heart rate, 70 beats per min; and respiratory rate, 16 breaths per min. Furthermore, the patient had the following: clear mind, clear language, normal emotions; orientation, understanding, memory, computing power were all normal; stable vitals; gums with residual crowns and roots; reduced tendon reflexes in the extremities; no obvious abnormality in cardiopulmonary and abdominal physical examination; and no edema in the lower limbs. Moreover, nonpersistent horizontal nystagmus was observed in both eyeballs, especially in left eye fixation. The tongue was centered, the right pharyngeal reflex was slightly diminished, the left finger nose test and heel knee tibial test were slightly less accurate, and other physical examinations showed no obvious abnormalities.
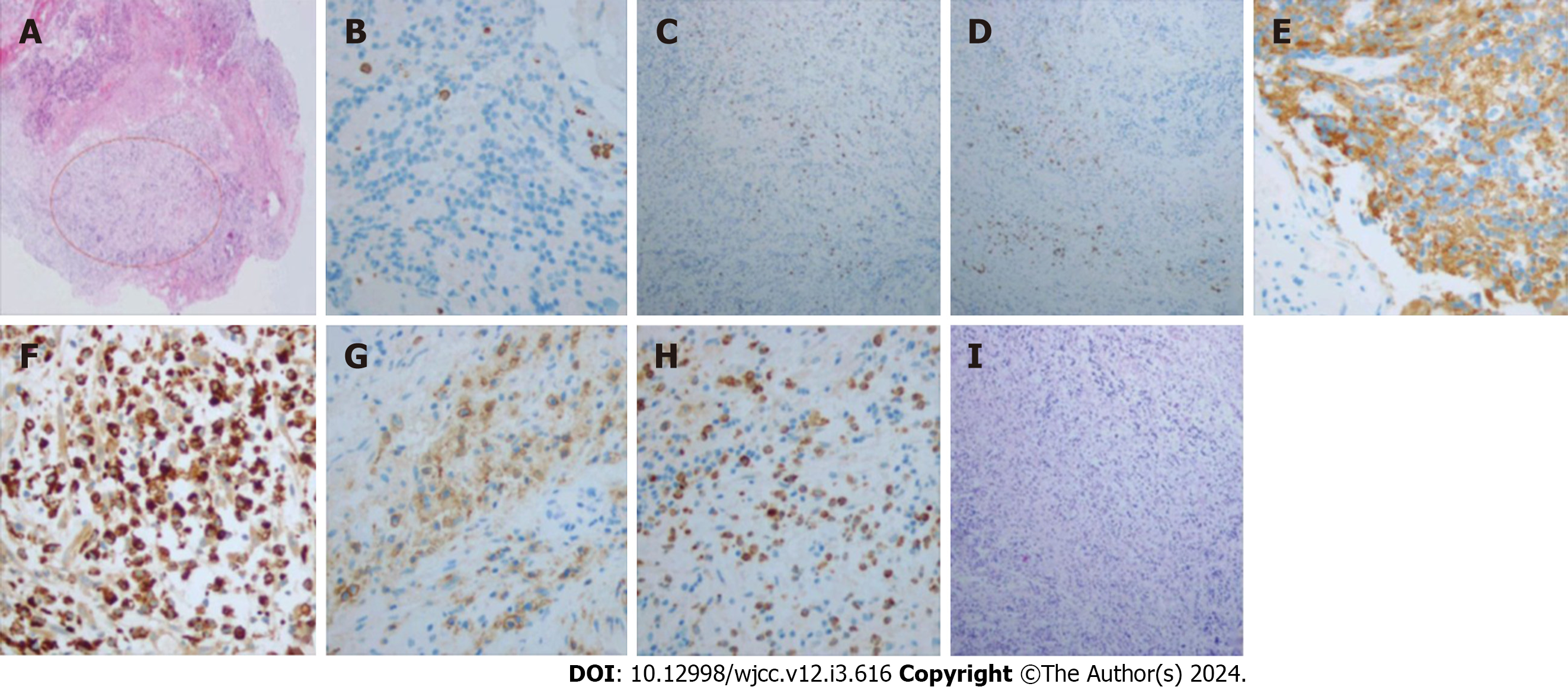
On day 7 of admission, mNGS was used to detect pathogenic microorganisms in the CSF, and the results showed that the pathogens were two oral bacteria, Tannerella forsythensis and Campylobacter rectus (Table 1). Moreover, the intracranial lesions were preliminarily considered inflammatory tissue, which may have been secondary to oral bacteria. On day 10 of admission, these lesion tissues were extracted. Intraoperatively, three flexible, light yellow lesion tissues with a normal blood supply were found in the left cerebellum. We used a syringe to puncture the abscess and aspirate it and found yellow, sticky, foul-smelling pus. Histopathological examination of the resected specimen (size, 1 cm × 0.6 cm × 0.3 cm) confirmed that the abscess originated from a bacterial infection (Figure 2A). Immunohistochemical analysis revealed that inflammation was positive for CD3, CD4, CD8, CD20, CD56, CD68, CD138, and MPO and negative for PAS (Figure 2B-I).
| Sample type | Type | Genus name | Species name |
| Cerebrospinal fluid | G- | Tannerella | Tannerella forsythensis |
| G- | Campylobacter | Campylobacter rectus |
The final diagnosis was brain abscess caused by oral bacteria.
Postoperatively, the antibiotic treatment was changed to linezolid (600 mg, IV, q12h). Meanwhile, the patient received treatment to regulate blood sugar, relieve pain, and protect the gastric mucosa. On day 13 of admission, the patient's body temperature returned to normal, the headaches were gradually relieved, and the mental state gradually improved. On day 25 of admission, the patient recovered well and was discharged.
MRI results showed that the intracranial condition had basically recovered well after 2 wk (Figure 1E-H). The patient was in good condition after 6 months postoperatively.
Brain abscesses are usually caused by serious infections such as bacteria, fungi, or parasites and can develop from encephalitis, which can be very disturbing to the quality of life of patients[11]. In most patients, susceptibility factors for brain abscess include immune suppression, chronic systemic disease, disruption of the natural protective barrier around the brain, and systemic infection sources[12]. Diabetic patients have a higher prevalence of microvascular disease, periapical inflammation, and brain abscess than nondiabetic patients[13].
MRI is the radiological method of choice for diagnosis and identification[14]. Clinically, CT must be complemented with MRI. MRI is helpful in differentiating brain abscesses from primary and cystic abscesses and in excluding tumor lesions[15]. CT scanning with contrast enhancement can be used to rapidly detect the size, number, and location of abscesses. The availability of these diagnostic techniques has led to a decrease in brain abscess mortality over the years[16]. The etiology, clinical manifestations, and complications of brain abscesses vary significantly in different populations. Fever, meningitis, elevated ESR, and reduced cyclic enhancement on delayed scans favor the diagnosis of abscesses. However, these are not sufficient to differentiate the pathogens.
Pathogenic bacteria can enter brain tissue through hematogenous spread, closed diffusion, head trauma, and traumatic infection from neurosurgery, which can lead to brain abscess[8]. Brain abscesses are often caused by multiple microorganisms, including staphylococci and streptococci or gram-negative bacilli[17]. Maraki et al[18] found that the mortality rate of brain abscesses caused by odontogenic infections was approximately 14%. Lateralized intraoral and intracranial infections help to identify possible routes of transmission. When both sides are consistent, direct venous dissemination should be considered. Otherwise, systemic hematogenous dissemination is considered[19]. The most closely related and abundant pathogen in the oral cavity associated with brain abscesses is anaerobic bacteria. Anaerobic bacteria are present in almost all dental infections, including periodontal and endodontic diseases. Aerobic bacteria are the initiators of infection, while anaerobic bacteria contribute to severe infection[20]. Infections in maxillary teeth can spread to the maxillary sinus, sphenoid sinus, septal sinus, and orbital cavity. Some blood vessels may spread oral infections to the cavernous sinus. Therefore, maxillary teeth are more likely to cause brain abscesses than mandibular teeth[21]. Most odontogenic infections penetrate the bony and fascial spaces and form infected ducts of brain abscesses through the bloodstream, lymphatic vessels, or direct venous drainage[22]. If the infectious process is not treated early, the clinical situation can deteriorate to the point where it becomes life-threatening for patients. The patient was definitively diagnosed with periodontitis, and the bacteria detected in the cerebrospinal fluid were all common in periodontal disease. The skull base CT showed no signs of trauma, such as a fracture. Combined with the abnormal signal in his cavernous sinus area, the brain abscess was thought to be caused by the venous return route or direct diffusion.
Microorganism testing in the patient's mouth, blood, and brain abscesses can be a great diagnostic aid in establishing the relationship between the mouth and the brain[21]. Performing microbiological testing to determine pathogens is key to making an accurate diagnosis[23]. The detection of pathogenic microorganisms in intracranial infections is limited, with low sensitivity and specificity. In approximately 25% of patients, the causative pathogens are identified in blood and cerebrospinal fluid cultures[8]. The researchers identified 80 different bacterial taxa, 44 of which have not been previously described in brain abscesses, and 37 of which have not been reported in culture[17]. Classical identification techniques include bacterial culture combined with Sanger sequencing of 16S rDNA. Bacterial culture usually does not accommodate both anaerobic and aerobic bacteria, so anaerobic cultures are generally negative. In the cases with positive bacterial culture, the results of bacterial culture could not be returned until 3-4 d after the examination. Sanger sequencing is only applicable to single microbial samples and is not applicable to multiple microbial infections[24]. Polymerase chain reaction (PCR) is a tool with good specificity and sensitivity for detecting Mycobacterium tuberculosis that needs to be detected quickly[25]. However, PCR results can sometimes have false-positives and false-negatives. The application of 16S rDNA-based mNGS enables the detection of as many microorganisms as possible in the sample compared to these technologies[26]. mNGS is performed for unbiased sequencing and untargeted diagnostics of microorganisms in a sample (viruses, fungi, bacteria) to obtain the entire sequence of the microorganism. mNGS can quickly and accurately identify the species and source of pathogenic bacteria in brain abscess[27], which guide the clinical use of antibiotics more targeted. In this situation, mNGS avoid the abuse of antibiotics, and reduce the cost of antibiotics. It is an effective method to detect the pathogenic bacteria of brain abscess. The patient's CSF smear, as well as culture, were negative; mNGS detected Tannerella forsythensis and Campylobacter rectus and postoperative pus culture-confirmed bacterial infections, both of which are oral resident bacteria, especially in the mouth of patients with periodontal disease, but not necessarily with acute periodontal infections. Both bacteria are opportunistic pathogens and parthenogenic anaerobes, which are more difficult to detect and culture clinically. Brain abscesses caused by odontogenic pathogenic bacteria are mostly reported as case reports and are mostly mixed bacterial infections[28].
The cure rate for patients with bacterial brain abscesses treated with a combination of surgical approaches and antimicrobial drugs exceeds 90%. The success of bacterial brain abscess treatment depends on the identification of the pathogen and the targeting of the treatment. Broad-spectrum antibiotics are used preferentially in initial therapy until the results of pathogenic microbial testing are available[29]. Once the pathogen is clearly diagnosed, the antimicrobial agents can be modified to achieve the most effective treatment. In addition, these drugs should have the ability to cross the blood-brain barrier. Drug therapy is preferred for deep infections, small abscesses, or multiple abscesses. When the abscess is larger than 2.5 cm in diameter, surgical excisional treatment can be used to completely remove the purulent material to shorten the hospital stay and the need for reoperation[30]. The patient was treated with anti-infection therapy in the early stage, the symptoms were mild and severe, there was no obvious acute severe inflammatory process clinically, and pathologically, there was also both acute inflammatory reaction and chronic granuloma formation, which was related to the type, virulence, site, and organism immunity of the pathogen. After admission to our hospital, the disease progressed faster, the envelope formed quickly, and the edema increased. Finally, good results were achieved through timely surgical treatment. However, we did not perform mNGS on the surgically removed abscesses.
Hence, we need to consider the possibility of odontogenic infection in common brain abscesses when the patient has poor oral hygiene. Then, the aerobic and anaerobic nature of the potentially pathogenic bacteria must be taken into account when sending samples for laboratory tests. mNGS performed as soon as possible in brain abscesses has a certain value for the determination of clinical medicines. Finally, appropriate surgical treatment can improve the prognosis of patients.
Brain abscesses are mixed infections, and there is difficulty in detecting all pathogens on routine bacterial cultures. mNGS may be used for the rapid diagnosis of pathogenic microorganisms and may play an important role in precision medicine.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soriano-Ursúa MA, Mexico S-Editor: Zhang H L-Editor: A P-Editor: Zhang YL
| 1. | Lajolo C, Favia G, Limongelli L, Tempesta A, Zuppa A, Cordaro M, Vanella I, Giuliani M. Brain abscess of odontogenic origin in children: a systematic review of the literature with emphasis on therapeutic aspects and a new case presentation. Acta Otorhinolaryngol Ital. 2019;39:67-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Costa Mendes L, Vaysse F, Maret D. Brain Abscess Secondary to a Dental Infection. Case Rep Emerg Med. 2020;2020:3248174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Moazzam AA, Rajagopal SM, Sedghizadeh PP, Zada G, Habibian M. Intracranial bacterial infections of oral origin. J Clin Neurosci. 2015;22:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Bodilsen J, Dalager-Pedersen M, van de Beek D, Brouwer MC, Nielsen H. Risk Factors for Brain Abscess: A Nationwide, Population-Based, Nested Case-Control Study. Clin Infect Dis. 2020;71:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Lu CH, Chang WN, Lui CC. Strategies for the management of bacterial brain abscess. J Clin Neurosci. 2006;13:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Helweg-Larsen J, Astradsson A, Richhall H, Erdal J, Laursen A, Brennum J. Pyogenic brain abscess, a 15 year survey. BMC Infect Dis. 2012;12:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Bodilsen J, Dalager-Pedersen M, van de Beek D, Brouwer MC, Nielsen H. Incidence and mortality of brain abscess in Denmark: a nationwide population-based study. Clin Microbiol Infect. 2020;26:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology. 2014;82:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 311] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 9. | Patel R. MALDI-TOF MS for the diagnosis of infectious diseases. Clin Chem. 2015;61:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20:341-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 901] [Article Influence: 150.2] [Reference Citation Analysis (0)] |
| 11. | Brouwer MC, Tunkel AR, McKhann GM 2nd, van de Beek D. Brain abscess. N Engl J Med. 2014;371:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 292] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 12. | Tan IL, Smith BR, von Geldern G, Mateen FJ, McArthur JC. HIV-associated opportunistic infections of the CNS. Lancet Neurol. 2012;11:605-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Marotta PS, Fontes TV, Armada L, Lima KC, Rôças IN, Siqueira JF Jr. Type 2 diabetes mellitus and the prevalence of apical periodontitis and endodontic treatment in an adult Brazilian population. J Endod. 2012;38:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Berndt M, Lange N, Ryang YM, Meyer B, Zimmer C, Hapfelmeier A, Wantia N, Gempt J, Lummel N. Value of Diffusion-Weighted Imaging in the Diagnosis of Postoperative Intracranial Infections. World Neurosurg. 2018;118:e245-e253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Zhou W, Shao X, Jiang X. A Clinical Report of Two Cases of Cryptogenic Brain Abscess and a Relevant Literature Review. Front Neurosci. 2018;12:1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Viviano M, Cocca S. Multiple brain abscesses after professional tooth cleaning: Case report and literature review. J Stomatol Oral Maxillofac Surg. 2018;119:432-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Al Masalma M, Lonjon M, Richet H, Dufour H, Roche PH, Drancourt M, Raoult D, Fournier PE. Metagenomic analysis of brain abscesses identifies specific bacterial associations. Clin Infect Dis. 2012;54:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Maraki S, Papadakis IS, Chronakis E, Panagopoulos D, Vakis A. Aggregatibacter aphrophilus brain abscess secondary to primary tooth extraction: Case report and literature review. J Microbiol Immunol Infect. 2016;49:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Flynn TR. The swollen face. Severe odontogenic infections. Emerg Med Clin North Am. 2000;18:481-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Desseauve D, Fradet L, Pierre F. [Dessauve D et al in reply to the article by N Mottet et al Focus on the Odon Device™: "Technical improvements, mechanical principles and progress of the clinical research program", Gynecol Obstet Fertil 2020 March 14. https://doi.org/10.1016/j.gofs.2020.03.011]. Gynecol Obstet Fertil Senol. 2020;48:844. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Sah R, Nepal G, Sah S, Singla S, Upadhyay P, Rabaan AA, Dhama K, Rodriguez-Morales AJ, Ghimire R. A rare case of brain abscess caused by Actinomyces meyeri. BMC Infect Dis. 2020;20:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Lee HS, Kim JH, Kim YH, Lee S. Surgically Treated Community-Acquired Brain Abscess: Bacteriological Analysis Based on Predisposing Infections. Jpn J Infect Dis. 2018;71:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Xue H, Wang XH, Shi L, Wei Q, Zhang YM, Yang HF. Dental focal infection-induced ventricular and spinal canal empyema: A case report. World J Clin Cases. 2020;8:3114-3121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Crossley BM, Bai J, Glaser A, Maes R, Porter E, Killian ML, Clement T, Toohey-Kurth K. Guidelines for Sanger sequencing and molecular assay monitoring. J Vet Diagn Invest. 2020;32:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 25. | Tsai JC, Teng LJ, Hsueh PR. Direct detection of bacterial pathogens in brain abscesses by polymerase chain reaction amplification and sequencing of partial 16S ribosomal deoxyribonucleic acid fragments. Neurosurgery. 2004;55:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | van Dijk EL, Auger H, Jaszczyszyn Y, Thermes C. Ten years of next-generation sequencing technology. Trends Genet. 2014;30:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 907] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 27. | Woerther PL, d'Humières C, Lescure X, Dubreuil L, Rodriguez C, Barbier F, Fihman V, Ruppé E. Is the term "anti-anaerobic" still relevant? Int J Infect Dis. 2021;102:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Ma Z, Yan S, Dong H, Wang H, Luo Y, Wang X. Case Report: Metagenomics Next-Generation Sequencing Can Help Define the Best Therapeutic Strategy for Brain Abscesses Caused by Oral Pathogens. Front Med (Lausanne). 2021;8:644130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Tonon E, Scotton PG, Gallucci M, Vaglia A. Brain abscess: clinical aspects of 100 patients. Int J Infect Dis. 2006;10:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Ratnaike TE, Das S, Gregson BA, Mendelow AD. A review of brain abscess surgical treatment--78 years: aspiration vs excision. World Neurosurg. 2011;76:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |