Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.575
Peer-review started: October 26, 2023
First decision: November 8, 2023
Revised: November 21, 2023
Accepted: January 2, 2024
Article in press: January 2, 2024
Published online: January 26, 2024
Processing time: 84 Days and 5.9 Hours
Breast cancer brain metastasis (BCBM) is an advanced breast disease that is difficult to treat and is associated with a high risk of death. Patient prognosis is usually poor, with reduced quality of life. In this context, we report the case of a patient with HER-2-positive BCBM treated with a macromolecular mAb (ine
The patient was a 58-year-old woman with a 12-year history of type 2 diabetes. She was compliant with regular insulin treatment and had good blood glucose control. The patient was diagnosed with invasive carcinoma of the right breast (T3N1M0 stage IIIa, HER2-positive type) through aspiration biopsy of the ipsilateral breast due to the discovery of a breast tumor in February 2019. Immunohistochemistry showed ER (-), PR (-), HER-2 (3+), and Ki-67 (55-60%+). Preoperative neoadjuvant chemotherapy, i.e., the AC-TH regimen (epirubicin, cyclophosphamide, docetaxel-paclitaxel, and trastuzumab), was administered for 8 cycles. She underwent modified radical mastectomy of the right breast in November 2019 and received tocilizumab targeted therapy for 1 year. Brain metastasis was found 9 mo after surgery. She underwent brain metastasectomy in August 2020. Immunohistochemistry showed ER (-) and PR. (-), HER-2 (3+), and Ki-67 (10-20%+). In November 2020, the patient experienced headache symptoms. After an examination, tumor recurrence in the original surgical region of the brain was observed, and the patient was treated with inetetamab, pyrotinib, and capecitabine. Whole-brain radiotherapy was recommended. The patient and her family refused radiotherapy for personal reasons. In September 2021, a routine examination revealed that the brain tumor was considerably larger. The original systemic treatment was continued and combined with intensity-modulated radiation therapy for brain metastases, followed by regular hospitalization and routine examinations. The patient’s condition is generally stable, and she has a relatively high quality of life. This case report demonstrates that in patients with BCBM and resistance to trastuzumab, inetetamab combined with pyrotinib and chemotherapy can prolong survival.
Inetetamab combined with small molecule TKI drugs, chemotherapy and radiation may be an effective regimen for maintaining stable disease in patients with BCBM.
Core Tip: Herein, we report the case of a patient with complex breast cancer brain metastasis. She underwent brain metastasis resection and received macromolecular mAbs, small molecule tyrosine kinase inhibitors, combination chemotherapeutic drugs, and radiation therapy. The overall condition of the patient was controlled and stabilized. We propose that combined macromolecule and small molecule chemotherapy and radiotherapy can provide a new option for the treatment of patients with breast cancer brain metastases. Magnetic resonance imaging of the brain of the patient showed that the tumor size was stable, and all the tumor indicators were within the normal range.
- Citation: Dou QQ, Sun TT, Wang GQ, Tong WB. Inetetamab combined with pyrotinib and chemotherapy in the treatment of breast cancer brain metastasis: A case report. World J Clin Cases 2024; 12(3): 575-581
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/575.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.575
Among malignancies, breast cancer has the highest incidence among women worldwide[1], and most causes of death from breast cancer are attributed to distant metastasis. Common distant metastasis sites of breast cancer include the lungs, bones, liver and brain. In recent years, with improvements in diagnosis and treatment, an increase in the diversity of treatment drugs and an increase in patient survival, the incidence of breast cancer brain metastasis (BCBM) has increased each year, and the probabilities of brain metastases from Her-2-positive breast cancer and triple-negative breast cancer are increasing. Approximately 34% of HER2-positive breast cancers metastasize to the brain[2]. The prognosis of patients with BCBM is poor, with negative impacts on survival time and quality of life, for example, severe neurocognitive impairment and poor physical prognosis. The common treatments for BCBM include local treatment and systemic therapy. Local treatment includes surgery, whole-brain radiotherapy and stereotactic radiotherapy; systemic treatment includes chemotherapy, targeted therapy, endocrine therapy, immunotherapy and other systemic drug treatments. The selection of treatment for patients depends on neuropsychiatric symptoms, the number and size of brain metastatic lesions and previous treatments[3]. Although some progress has been made in the systemic treatment of BCBM, the blood-brain barrier still poses challenges for effective treatment, and the overall results of systemic treatment are unsatisfactory. Current treatments rarely result in substantial prolongation of the life of patients and only provide palliation. There are enormous treatment challenges, and the prognosis of and benefits to patients need to be considered as a whole.
We describe the case of a patient with BCBM who, after trastuzumab treatment failure, was administered inetetamab combined with pyrotinib, capecitabine and radiation therapy; the disease was controlled and remained stable.
A 58-year-old female patient with a 2-year history of breast cancer, a 3-mo history of BCBM and a history of type 2 diabetes mellitus was admitted to our hospital for further evaluation and treatment after modified radical mastectomy for breast cancer.
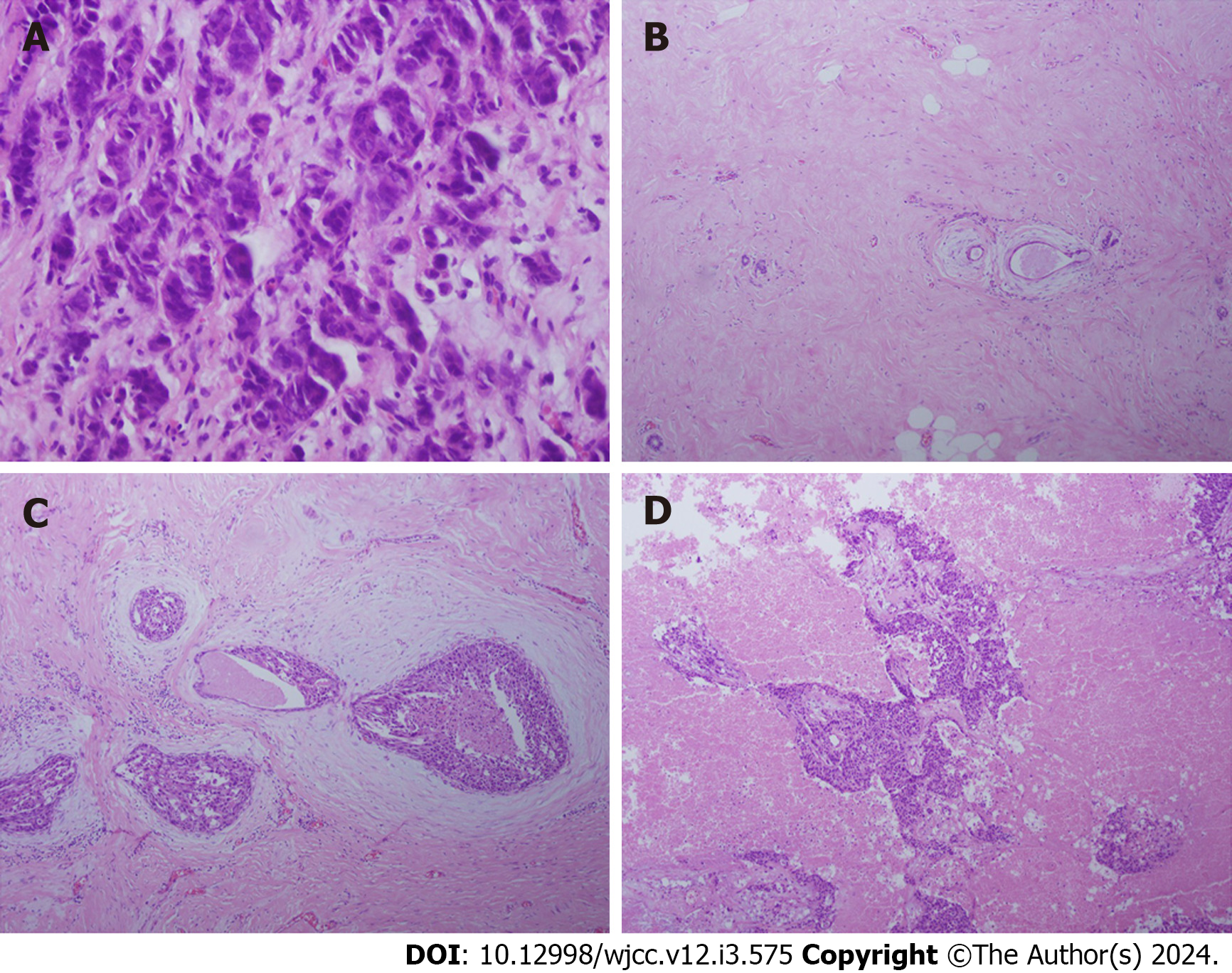
The patient was diagnosed with BCBM at the time of admission. One year prior, the patient underwent modified radical mastectomy and, 3 mo ago, underwent unilateral resection of BCBM. The results of an aspiration biopsy of the ipsilateral breast at the first visit were as follows: ER (-), PR (-), HER-2 (3+), HER-2 (3+), and Ki-67 (55%-60%+) (Figure 1A). The results of the biopsy after modified radical mastectomy were as follows: ER (1+, approximately 1%), PR (-), HER-2 (3+), and Ki-67 (approximately 60%+) (Figure 1B and C). The results of the pathological biopsy after brain metastasis resection were as follows: ER (-), PR (-), HER-2 (3+), and Ki-67 (10%-20%+) (Figure 1D). After the resection of brain metastases, routine magnetic resonance imaging (MRI) of the brain showed irregular and abnormal signals in the right cerebellar hemisphere (original surgical area) with unclear boundaries (size, approx. 25 mm × 20 mm). An enhanced scan showed heterogeneous enhancement. The signal in the right cerebellar hemisphere was abnormal, and metastasis was considered.
The patient had a 12-year history of diabetes, was compliant with diabetes treatment (regular insulin injections), and had good blood glucose control.
The patient denied a family history of malignant tumors.
The breasts were asymmetrical. The right breast was absent, and a well-healed surgical scar (approximately 20 cm in length) was visible on the right chest wall. The left breast showed normal development. There was no obvious tumor palpable in the left breast, and no superficial swollen lymph nodes were palpable. The patient had an unstable gait and slightly slurred speech, physiological reflexes were present, pathological reflexes were not elicited, and the meningeal irritation sign was weakly positive.
The levels of tumor markers in the blood did not markedly exceed the normal range during the entire treatment process.
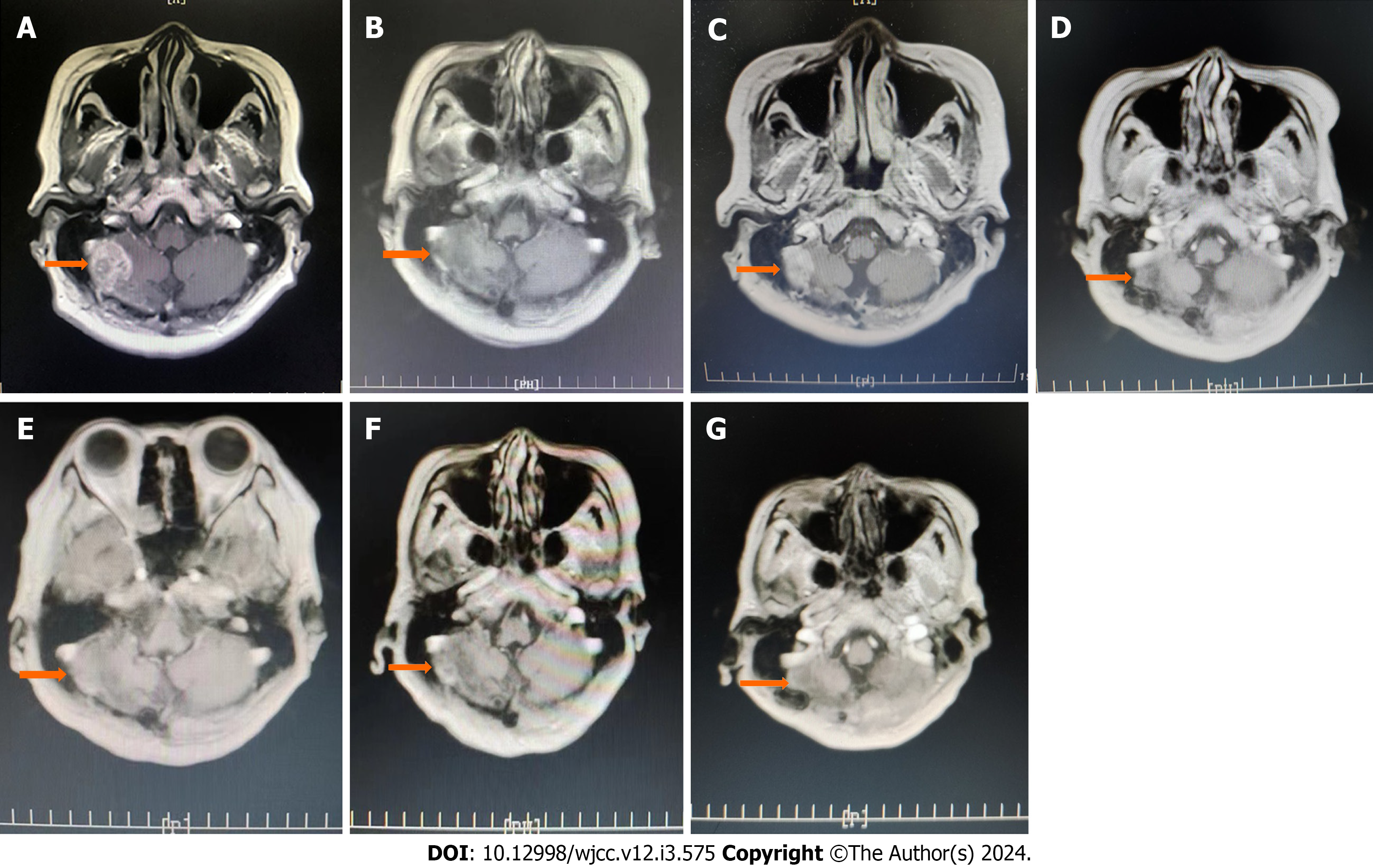
MRI of the brain metastasis, with recurrence in the original brain surgical area, is shown in Figure 2A. After 10 cycles of systemic drug treatment, the tumor size decreased considerably (Figure 2B). The brain tumor was larger after the 16th cycle of treatment (Figure 2C). Although the treatment plan was adjusted, the original systemic treatment regimen was generally unchanged; radiotherapy for 12 cycles was added. Brain MRI showed that the tumor was in a stable state (Figure 2D-G). The size of the tumor remained stable at 15 mm × 10 mm (Figure 2E).
After comprehensive laboratory and imaging examinations, the final diagnosis was BCBM (T2N2bM1 stage IV, Her-2 positive type).
The recommended regimen for patients with brain metastasis and recurrence of breast cancer is the intravenous injection of 400 mg of inetetamab for 21 d and 400 mg of oral pyrotinib; due to severe gastrointestinal reactions to the drug, the recommended regimen was changed to 320 mg of oral pyrotinib and capecitabine (2.0 g/time 3 times/day) for the patient in this case study. After consultation with the radiotherapy department, whole-brain radiotherapy was recommended, but the patient and her family refused this option.
Brain metastasis progressed, and the regimen was adjusted: intravenous injection of inetetamab 400 mg for 21 d, pyrotinib 320 mg, and capecitabine 1.5 g bid (reduced from 2.0 g bid because of patient intolerance). Intensity-modulated radiation therapy was administered to the intracranial metastatic lesions: 6 MV X-ray IGRT 95% PGTV 52.5 Gy/3.5 Gy/15F.
After 47 cycles of systemic drug treatment and 31 cycles of radiation therapy, no progression or recurrence of the primary lesions was observed, and the brain metastatic lesions were controlled. Brain MRI showed that the tumor was stable, with no substantial changes from the previous images of the tumor (Figure 2D-G).
Breast cancer is a highly heterogeneous disease. Compared with primary tumors, the HER2 and HR statuses of metastatic lesions are not completely the same[4]. Reevaluations of the molecular typing of brain metastases are key for treatment. Inconsistencies in molecular expression suggest that care needs to be taken when considering the treatment strategy. Additionally, a meta-analysis of patients with HER-2-positive breast cancer found that patients who had previously received adjuvant trastuzumab treatment were more likely than patients who did not receive treatment to develop metastatic recurrence in the central nervous system[5]. At present, anti-HER2 targeted drugs mainly include monoclonal antibodies, small molecule TKIs, and antibody-drug conjugates. For economic reasons in China, the former two are the main drugs used. Trastuzumab is a humanized anti-HER-2 monoclonal antibody that acts on the extracellular portion of the HER-2 receptor to prevent the activation of intracellular tyrosine kinases and inhibit the proliferation and survival of HER-2-dependent tumor cells; additionally, trastuzumab can mediate the antibody-dependent cellular cytotoxicity (ADCC) effect and kill tumor cells. The ADCC effect is a mechanism in which the Fab fragment of a mAb recognizes and binds to tumor cell surface antigens, and the Fc fragment binds to the Fcγ receptor of NK cells, thereby activating and mediating the killing of tumor cells by NK cells. Studies have shown[6] that compared with trastuzumab, the optimized Fc region has an enhanced ADCC effect on HER2+ tumor cells, including cells that are resistant to trastuzumab and have low HER2 expression. The SOPHIA study[7] showed that in a population with progression after trastuzumab, pertuzumab and T-DM1 treatment, margetuximab, with an optimized and modified Fc region, had a stronger ADCC effect. In patients with advanced breast cancer after multiple lines of treatment, margetuximab, compared with che
Inetetamab combined with small molecule TKI, chemotherapy and radiotherapy may be an effective adjuvant therapy for the treatment of BCBM. Clinicians should provide reasonable treatments based on the real-time condition of each patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nag A, India S-Editor: Gong ZM L-Editor: A P-Editor: Zhang YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64446] [Article Influence: 16111.5] [Reference Citation Analysis (176)] |
| 2. | Fecci PE, Champion CD, Hoj J, McKernan CM, Goodwin CR, Kirkpatrick JP, Anders CK, Pendergast AM, Sampson JH. The Evolving Modern Management of Brain Metastasis. Clin Cancer Res. 2019;25:6570-6580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Ramakrishna N, Temin S, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, Giordano SH, Kirshner JJ, Krop IE, Levinson J, Modi S, Patt DA, Perlmutter J, Winer EP, Lin NU. Recommendations on Disease Management for Patients With Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36:2804-2807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Chen X, Wang M, Yu K, Xu S, Qiu P, Lyu Z, Zhang X, Xu Y. Chronic stress-induced immune dysregulation in breast cancer: Implications of psychosocial factors. J Transl Int Med. 2023;11:226-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 5. | Lin NU. Better treatments needed for breast cancer brain metastases. Lancet Oncol. 2015;16:1583-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, Li H, Ciccarone V, Zhang T, Stavenhagen J, Koenig S, Stewart SJ, Moore PA, Johnson S, Bonvini E. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011;13:R123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Rugo HS, Im SA, Cardoso F, Cortés J, Curigliano G, Musolino A, Pegram MD, Wright GS, Saura C, Escrivá-de-Romaní S, De Laurentiis M, Levy C, Brown-Glaberman U, Ferrero JM, de Boer M, Kim SB, Petráková K, Yardley DA, Freedman O, Jakobsen EH, Kaufman B, Yerushalmi R, Fasching PA, Nordstrom JL, Bonvini E, Koenig S, Edlich S, Hong S, Rock EP, Gradishar WJ; SOPHIA Study Group. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7:573-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 8. | Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Wang T, Zhang P, Di LJ, Wang XJ, Yang JL, Tong ZS, Liu J, Feng JF, Liu DJ, Yu QT, Liu YP, Yu H, Jiang ZF. Efficacy and safety of inetetamab in combination with chemotherapy as first-line treatment of HER2-positive metastatic breast cancer: a subgroup analysis in the HOPES study. 2022; 3: 2218-6778. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Galluzzi L, Zitvogel L, Kroemer G. Immunological Mechanisms Underneath the Efficacy of Cancer Therapy. Cancer Immunol Res. 2016;4:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Mimura K, Kamiya T, Shiraishi K, Kua LF, Shabbir A, So J, Yong WP, Suzuki Y, Yoshimoto Y, Nakano T, Fujii H, Campana D, Kono K. Therapeutic potential of highly cytotoxic natural killer cells for gastric cancer. Int J Cancer. 2014;135:1390-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Curigliano G, Mueller V, Borges V, Hamilton E, Hurvitz S, Loi S, Murthy R, Okines A, Paplomata E, Cameron D, Carey LA, Gelmon K, Hortobagyi GN, Krop I, Loibl S, Pegram M, Slamon D, Ramos J, Feng W, Winer E. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 132] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 13. | Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, Tong Z, Li H, Zhang Q, Sun T, Wang X, Yin Y, Cheng Y, Li W, Gu Y, Chen Q, Liu J, Cheng J, Geng C, Qin S, Wang S, Lu J, Shen K, Liu Q, Wang H, Luo T, Yang J, Wu Y, Yu Z, Zhu X, Chen C, Zou J; PHOEBE Investigators. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 14. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 461] [Article Influence: 115.3] [Reference Citation Analysis (1)] |
| 15. | Miller JA, Kotecha R, Ahluwalia MS, Mohammadi AM, Chao ST, Barnett GH, Murphy ES, Vogelbaum MA, Angelov L, Peereboom DM, Suh JH. Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies. Cancer. 2017;123:2283-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Foreman PM, Jackson BE, Singh KP, Romeo AK, Guthrie BL, Fisher WS, Riley KO, Markert JM, Willey CD, Bredel M, Fiveash JB. Postoperative radiosurgery for the treatment of metastatic brain tumor: Evaluation of local failure and leptomeningeal disease. J Clin Neurosci. 2018;49:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |