Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.565
Peer-review started: September 8, 2023
First decision: November 9, 2023
Revised: November 22, 2023
Accepted: January 3, 2024
Article in press: January 3, 2024
Published online: January 26, 2024
Processing time: 131 Days and 21.5 Hours
Marginal zone lymphoma (MZL) is an indolent subtype of non-Hodgkin lym
This study reports a case of MZL with generalized skin rashes accompanied by pruritus and purulent discharge. First-line treatment with rituximab combined with zanubrutinib had poor effects. However, after switching to obinutuzumab combined with zanubrutinib, the case was alleviated, and the rashes disappeared.
For patients with advanced stage MZL not benefiting from type I anti-CD20 monoclonal antibody (mAb) combination therapy, switching to a type II anti-CD20 mAb combination regimen may be considered. This approach may provide a new perspective in the treatment of MZL.
Core Tip: This paper reports a case of Marginal zone lymphoma (MZL) with generalized skin rashes accompanied by pruritus and purulent discharge. First-line treatment with rituximab combined with zanubrutinib had poor effects. However, after switching to obinutuzumab combined with zanubrutinib, the case was alleviated, and the rashes disappeared. Therefore, in patients with advanced stage MZL not benefiting from type I anti-CD20 monoclonal antibody (mAb) combination therapy, switching to a type II anti-CD20 mAb combination regimen may be considered. This approach may provide a new perspective in the treatment of MZL.
- Citation: Bai SJ, Geng Y, Gao YN, Zhang CX, Mi Q, Zhang C, Yang JL, He SJ, Yan ZY, He JX. Marginal zone lymphoma with severe rashes: A case report. World J Clin Cases 2024; 12(3): 565-574
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/565.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.565
Marginal zone lymphoma (MZL) is a type of B-cell non-Hodgkin lymphoma (B-NHL) originating in the marginal zone of lymphoid follicles. MZL is the third most common type of B-NHL after diffuse large B-cell lymphoma and follicular lymphoma. MZL has low incidence, insidious onset, slow clinical progression and relatively good prognosis.
An epidemiological survey carried out in the United States in 2016 on 7460 patients diagnosed with non-Hodgkin lymphoma revealed that MZL accounted for 7% of all non-Hodgkin lymphomas[1]. Three subtypes have been proposed for MZL according to the site of origin, including extranodal MZL of mucosa-associated lymphoid tissue (MALT), splenic MZL (SMZL), and nodal MZL (NMZL). Although all three subtypes are indolent lymphomas, they have different clinical presentations, disease prognoses, and treatment options. According to the National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO) guidelines, rituximab-based regimens are preferred for first-line treatment in advanced and symptomatic MZL cases. However, MZL cases with extensive and severe rashes at onset are rare, and no consensus on relevant treatment options is available.
A 63-year-old man presented with superficial lymphadenopathy for more than 4 months and severe rashes for 3 months.
Symptoms and signs began 4 months ago, and the rashes recurred, gradually increasing in size and severity.
The 63-year-old man noticed a 1.5 cm × 1 cm mass on the right neck and a 2 cm × 1 cm mass under the right armpit 4 months before admission without overt causes. The patient experienced fatigue, poor appetite, acid regurgitation, heartburn, and nausea, but no vomiting, fever, or night sweats. A routine blood test at the local hospital revealed a platelet count of 55 × 109/L; a color Doppler ultrasound examination revealed multiple enlarged lymph nodes throughout the body. A biopsy of the right cervical lymph node was performed at the local hospital, and pathological analysis showed a tendency toward B-NHL. Following the recommendations of the local hospital, the patient was orally administered 80 doses of a traditional Chinese medicine preparation, but the symptoms did not significantly improve. Three months before admission, the patient developed red maculopapular skin rashes on both lower limbs, which gradually expanded to cover the head, face, trunk, and limbs with ulceration and pus. The patient self-administrated prednisone acetate at 20 mg/d for 3 d, but the symptoms continued to progressively worsen. Consequently, the patient was admitted to our department for a definitive diagnosis of lymphadenopathy and rash etiology. Patient weight was unchanged before the 1st half-year of admission.
The patient denied any family history of malignant tumors.
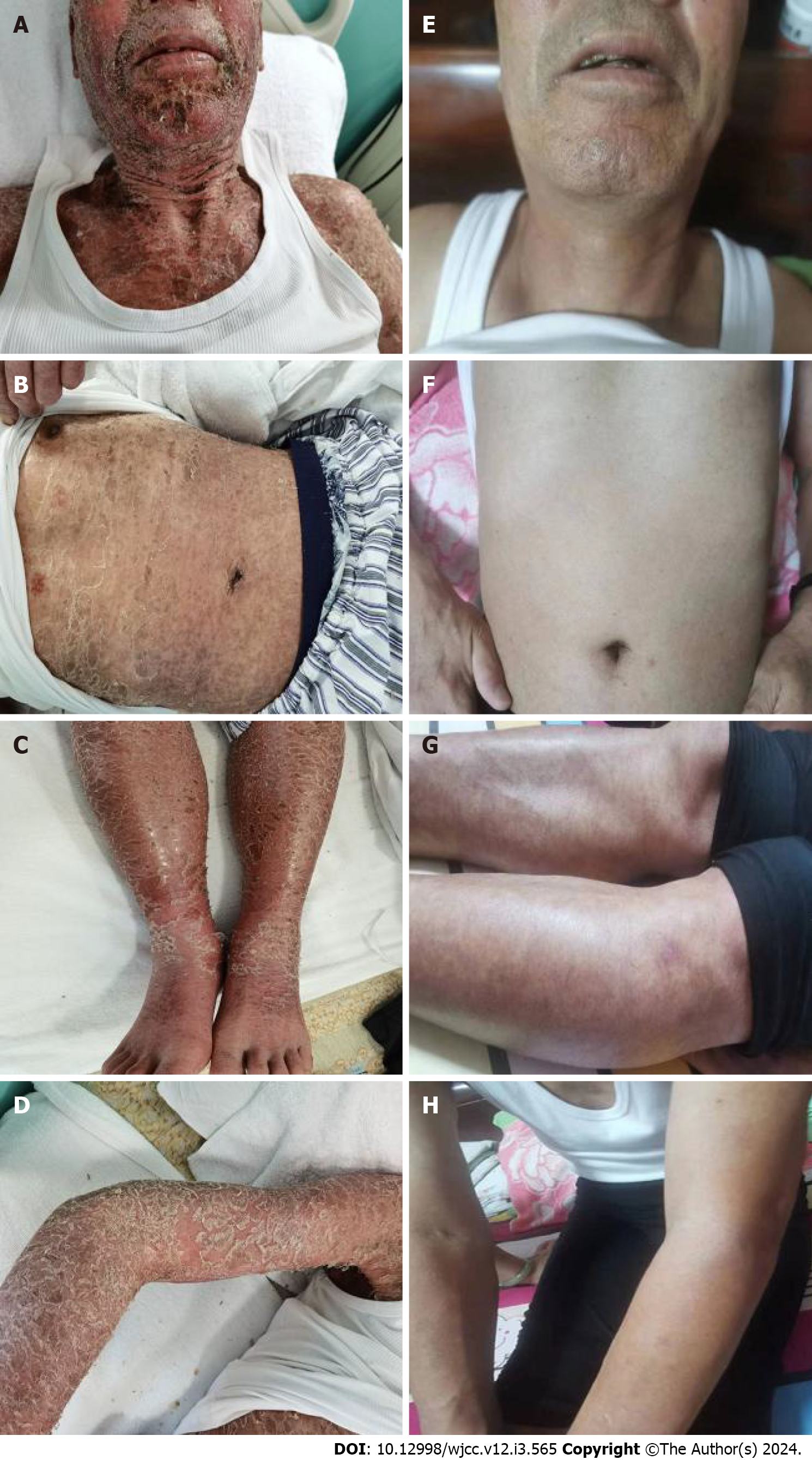
Large areas of dark red rashes were observed on the face, chest, abdomen, back, and limbs, with some being ruptured and purulent, and others scabbed, without tenderness (Figure 1A-D). The patient had multiple enlarged lymph nodes that were palpable in the neck, clavicular region, axilla and inguinal region of both sides, with the largest one of approximately 3 cm × 2 cm found on the right neck. The lymph nodes were hard, mobile, nontender, and nonadherent to the surrounding tissues. No specific positive signs were observed in the heart and lungs. The abdomen was flat and soft. The liver was not palpable under the ribs, while the spleen was palpable 8 cm below the ribs without tenderness. The patient also had bilateral lower limb edema.
Routine blood test revealed white blood cell (WBC) count at 7.15 × 109/L, hemoglobin at 142 g/L, lymphocyte count at 1.85 × 109/L, and platelet count at 112 × 109/L.
Biochemical analysis showed alanine aminotransferase at 46.56 IU/L, aspartate aminotransferase at 46.90 IU/L, serum albumin at 24.47 g/L, and β2 microglobulin at 6.48 mg/L. No obvious abnormality was detected in blood urea nitrogen, serum creatinine, lactate dehydrogenase, electrolytes, immunoglobulin (Ig) A, IgG, IgM, infectious diseases (including hepatitis B virus-DNA and hepatitis C virus-RNA), antinuclear antibody spectrum and hematuria immunofixation electrophoresis.
Inflammatory indicators were C-reactive protein at 44.84 mg/L, and procalcitonin level and erythrocyte sedimentation rate within normal ranges.
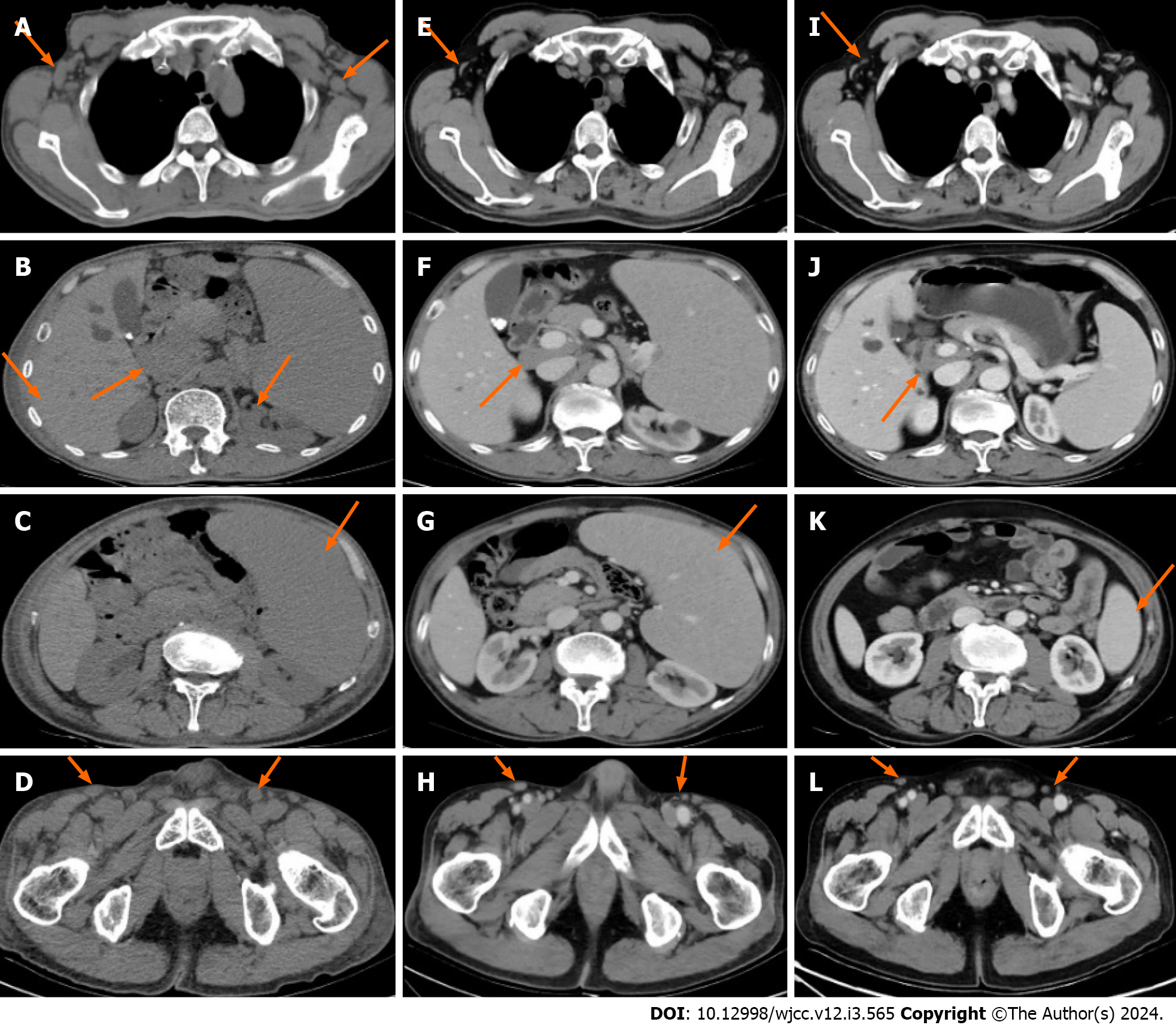
To avoid hypersensitivity in the patient’s skin due to enhancers, which would aggravate the rash symptoms, plain computed tomography (CT) of the neck, chest, abdomen, and pelvis was performed, revealing splenomegaly and multiple enlarged lymph nodes in the neck, clavicular region, axilla and inguinal region of both sides, as well as the mediastinum and retroperitoneal region (Figure 2A-D).
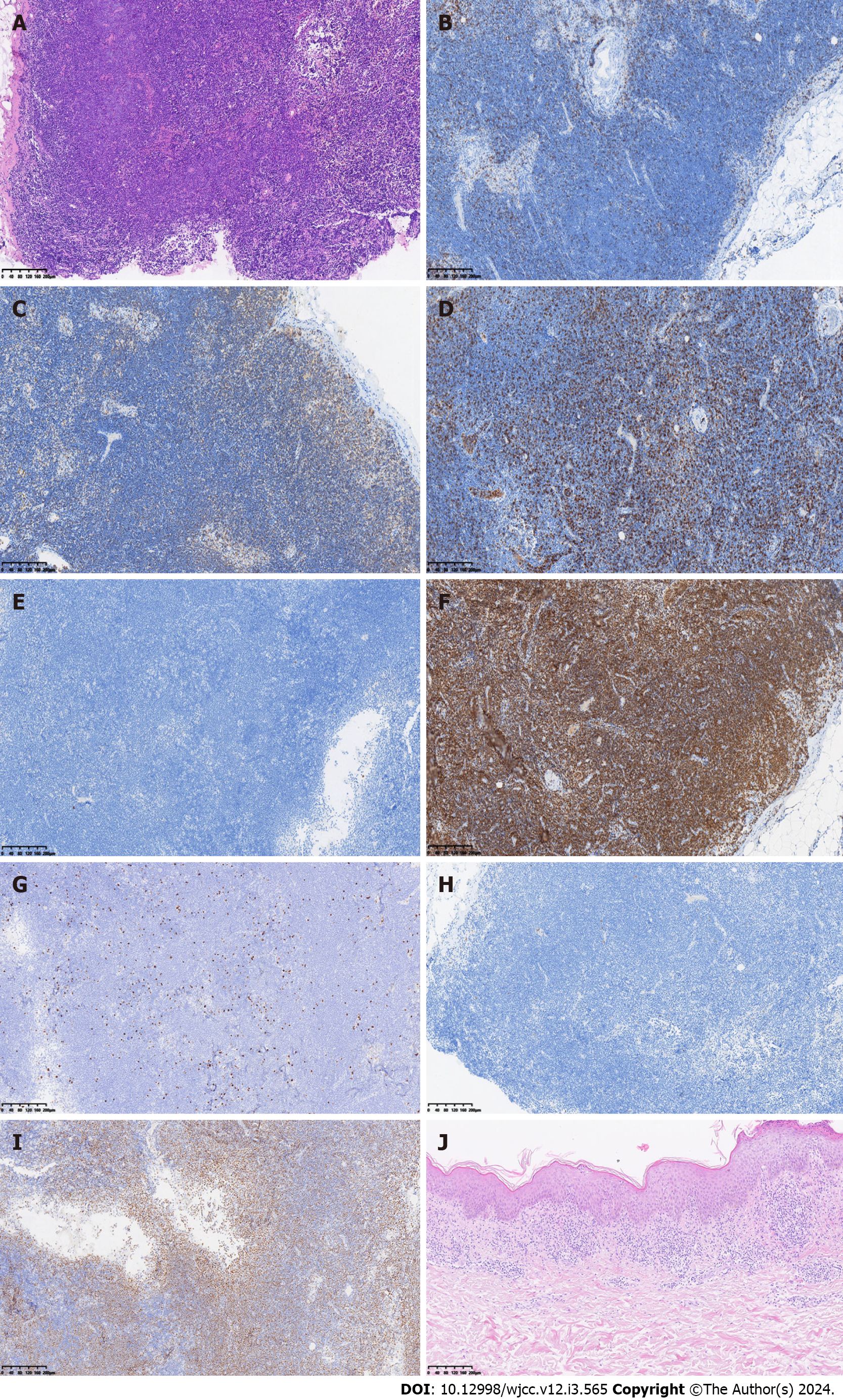
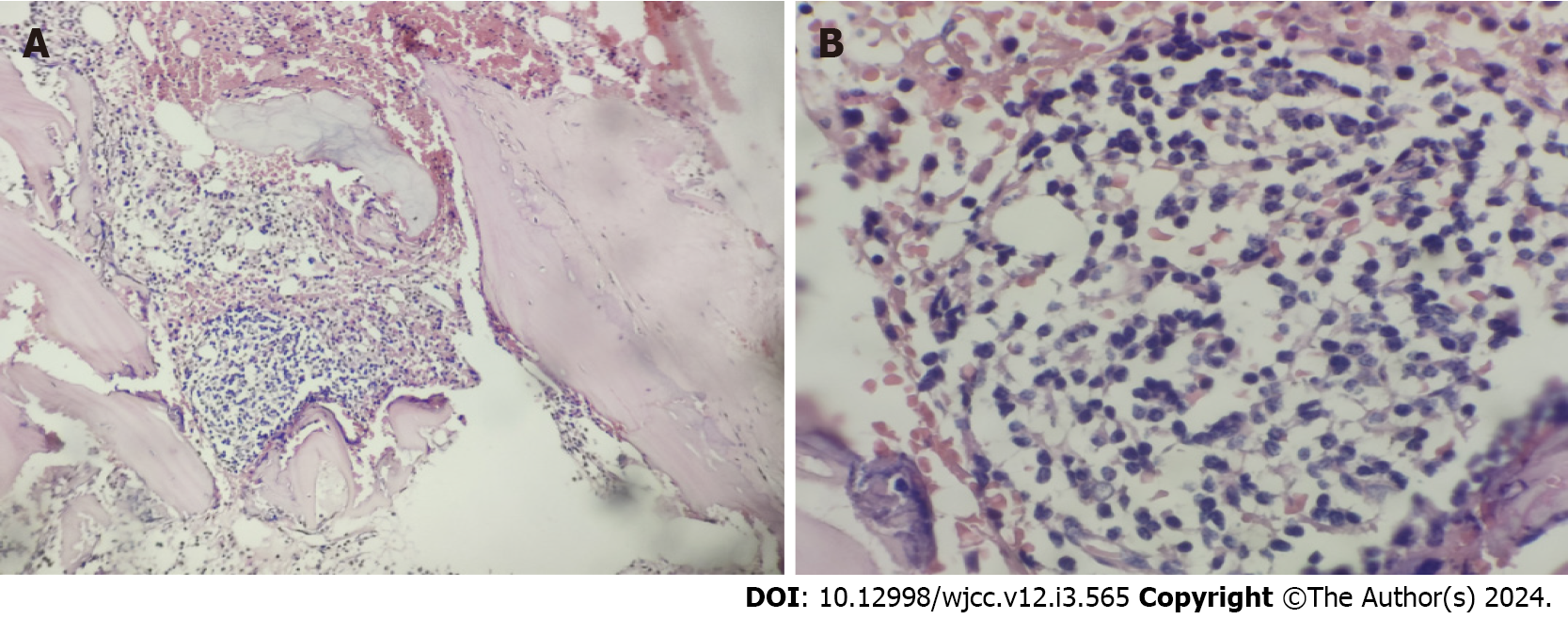
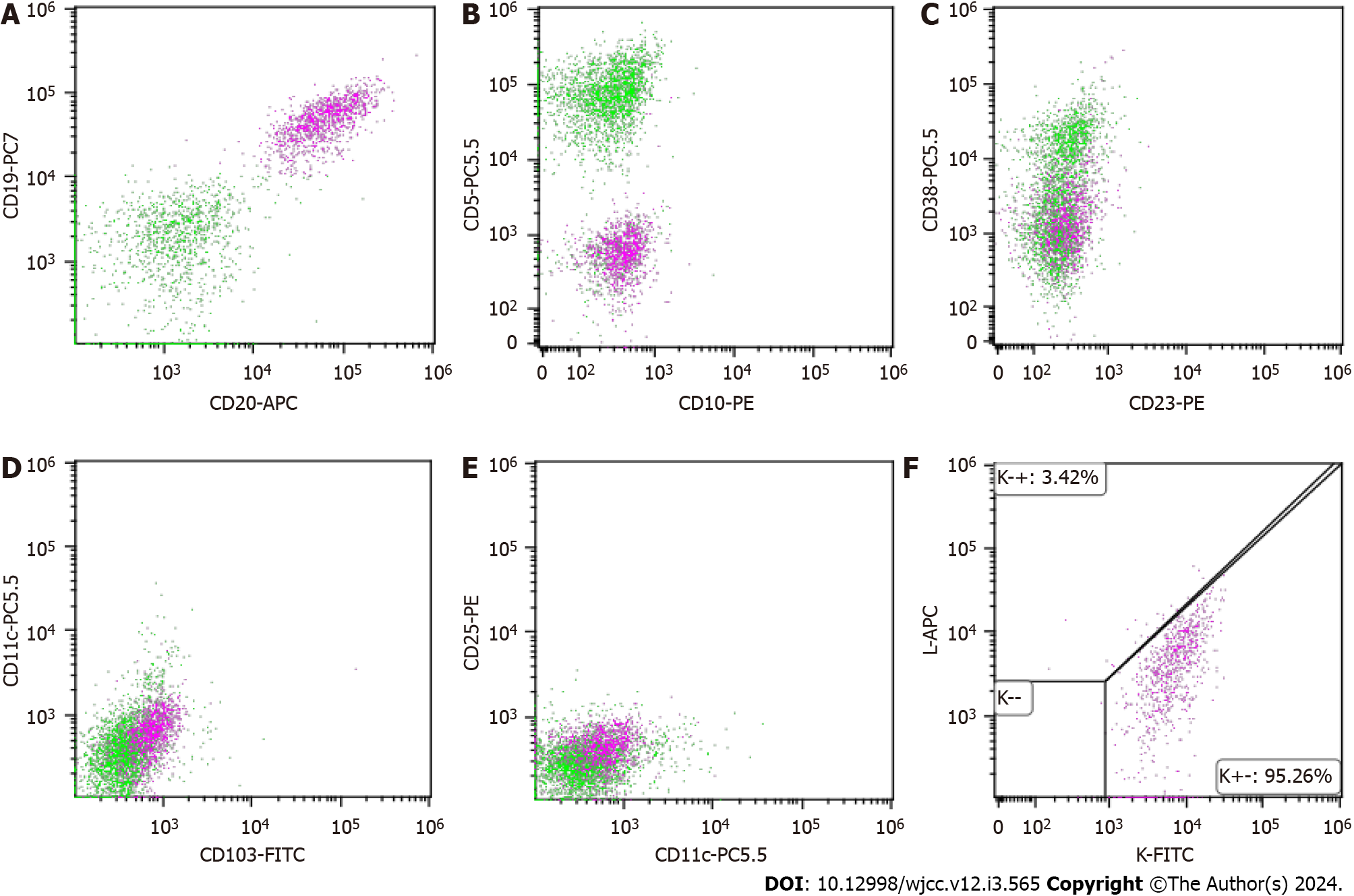
The pathological analysis of right cervical lymph nodes showed that the lymph node structure disappeared in most areas, with diffuse and consistent infiltration of small lymphocytes with predominantly small centroblasts. The cellular morphology suggested that atypia was not obvious; mitotic cells were rare, and a small number of lymphoid follicles remained locally, indicative of follicular implantation. Immunohistochemistry showed CD20 (diffuse +), CD3 (scattered low +), Ki67 (about 15%), CD10 (-), BCL-6 (-), CD5 (-), CD23 (-), CyclinD1 (-), MUM-1 (scattered low +), CD21 (low residual follicular dendritic reticulum), C-myc (-), BCL-2 (+), P53 (about 60%), and CD30 (scattered low +) (Figure 3A-I). Skin pathology suggested mild hyperplasia of stratified squamous epithelium with parakeratosis, scattered T cell-dominated lymphocyte infiltration in the epithelium, focal erosion and small abscess formation, and T cell-dominated lymphocyte infiltration in tissues under the epithelium. Combined with immunohistochemical data, there was insufficient evidence to diagnose lymphoma (Figure 3J). Immunohistochemistry suggested CD20 (partial +), CD3 (majority +), CD5 (majority +), CD23 (dendritic cell +), CyclinD1 (scattered +), Bcl-2 (partial +), p53 (-), CD30 (-), Ki-67 (20% +), CD43 (majority +) and CD79α (partial +). Interestingly, next-generation sequencing of lymph node samples detected a TP53 mutation (NM_000546.5 on exon 6) with the nucleotide change of c.659a > G resulting in the amino acid change of p.Tyr220cys (dbSNP, rs121912666; mutation frequency, 3.85%). However, BRAF/V600E and MYD88L265P mutations were not detected. Analysis of bone marrow morphology showed active proliferation, normal proportion and morphology of mature lymphocytes, and visible lymphoid histiocytes. Bone marrow biopsy showed focal or scattered distribution of lymphocytes and no morphologically abnormal lymphocytes (Figure 4). Bone marrow immunophenotyping revealed abnormal B lymphocytes, accounting for 6.67% of nuclear cells, expressing CD19, CD20, Kappa, IgM and CD81; partially expressing FMC7; and not expressing CD103, CD10, CD43, CD11c, CD200, CD25, CD23, CD38, CD5, IgD, and Lambda (Figure 5).
Summarizing the features of the current case, the patient not only had generalized skin rashes and splenomegaly, but also showed extensive lymph node involvement. Even though the WBC count was normal, we further assessed peripheral blood smear, bone marrow morphology, and flow cytometry to exclude leukemic change. Flow cytometry suggested abnormal B lymphocytes < 20%. Combined with immunophenotypic data, diagnostic criteria for lymphoma leukemia were insufficient. Furthermore, based on pathological biopsy, next-generation sequencing and whole-body CT, the diagnosis of stage IVA MZL (high-risk group with an international prognostic index IPI score of 4) involving the spleen, bone marrow, bilateral neck, clavicular, axillary, mediastinal, retroperitoneal and inguinal lymph nodes with a TP53 mutation was made. Skin infiltration of lymphoma was excluded.
Rituximab (375 mg/m2 once every 3 wk) plus zanubrutinib (160 mg twice daily) was given for three courses starting on November 13, 2021.Then, starting on April 8, 2022, the patient received three courses of obinutuzumab (1000 mg/3 wk) in combination with zanubrutinib (160 mg twice daily), followed by a switch to maintenance zanubrutinib (160 mg twice daily) to date.
The course of treatment involved efficacy assessment after three courses of rituximab (375 mg/m2 once every 3 wk) plus zanubrutinib (160 mg twice daily) starting on November 13, 2021, which suggested stable disease. Treatment efficacy was close to complete remission (CR) after three courses of treatment with a switch to obinutuzumab (1000 mg/3 wk) combined with zanubrutinib (160 mg twice daily) starting on April 8, 2022. The skin rashes did not significantly improve after three courses of treatment, but disappeared completely after six courses of treatment (Figure 1E-H). CT of the neck, chest, abdomen and pelvis after three courses of treatment showed that lymph nodes in the neck, clavicular, axillar, retroperitoneal and inguinal regions were smaller than pre-treatment, with no significant spleen shrinkage (Figure 2E-H). Contrast-enhanced CT of the neck, chest, abdomen and pelvis after six courses of treatment revealed significant retraction of lymph nodes on the neck, clavicular region, axilla, retroperitoneal region, inguinal region and spleen (Figure 2I-L). Morphological and pathological examinations of the bone marrow showed no significant abnormalities after three courses of treatment, but bone marrow immunophenotyping showed abnormal B lymphocytes accounting for 4.86% of nuclear cells in bone marrow samples, which expressed CD19, CD20 and Kappa, but not CD103, CD10, CD43, CD11c, FMC7, CD38, CD200, CD25, CD23, CD5, IgD, IgM, CD81 and Lambda. After six courses of treatment, a repeat blood routine analysis showed a white blood cell count of 5.37 × 109/L, hemoglobin at 136 g/L, a lymphocyte count of 2.36 × 109/L, and a platelet count of 76 × 109/L. Further examination of bone marrow morphology and immunophenotyping showed no abnormal B lymphocytes, so thrombocytopenia was considered to indicate myelosuppression caused by Bruton’s tyrosine kinase inhibitors. Subsequently, the patient was administered zanubrutinib (160 mg twice daily) as maintenance therapy, and the patient’s condition has remained stable to date.
MZL is an indolent disease that tends to have an insidious onset, with MALT lymphoma showing the highest rate among the three subtypes. This subtype is further divided into gastric, cutaneous, and non-gastric/non-cutaneous MALT lymphomas. NMZL comprises the smallest proportion of all MZL cases, representing about 10%, and less than 2% of all NHL cases[2]. About 10% of NMZL patients show abnormal IgM protein elevation[3], which needs to be further distinguished from Waldenstrom macroglobulinemia. Although NMZL mainly involves lymph nodes and occasionally the bone marrow and peripheral blood, a large proportion of patients have painless multiple lymphadenopathies. SMZL accounts for approximately 20% of MZL[2], and most patients present with splenomegaly, lymphocytosis, and cytopenia, which may induce autoimmune disorders[4]. Although our patient had skin rashes and superficial lymphadenopathy, and bone marrow and spleen involvement was considered according to relevant examinations such as blood count, bone marrow count, and imaging, extranodal MZL was considered in combination with different clinical manifestations and laboratory tests for the three MZL lymphoma subtypes.
The specificity of this case is that it was accompanied by a large area of rashes on the whole body in the early stage of onset, and further clinical symptoms were rare. Because the patient refused a second biopsy, the current evidence of lymphoma on skin biopsy was insufficient, but combined with his medical history, symptoms and treatment, the causes of rashes were considered to involve two aspects. On the one hand, the rashes may be non-specific skin manifestations caused by MZL; on the other hand, the pathological analysis may have be unsuccessful and failed to provide evidence of lymphoma infiltrating skin lesions. Even if the rashes are lymphoma infiltrating the skin, they still could not be diagnosed as primary cutaneous MZL (PCMZL), which belongs to extranodal non-Hodgkin lymphoma, accounting for about 25% of all cutaneous lymphomas[5]. The 2016 World Health Organization (WHO) classified PCMZL as a MALT lymphoma manifesting in the skin, which is currently considered of post-germinal center marginal zone B-cell origin, and the neoplastic infiltrates are composed of a varying admixture of small lymphocytes, plasma cells, and lymphoplasmacytoids[6]. By definition, essentially no evidence of extracutaneous disease is found at the time of presentation with symptoms, and relevant studies have also revealed bone marrow involvement in less than 1% of patients[7]. PCMZL cases present with solitary or multifocal nodules, plaques, or papules that are generally localized to the arms or trunk[6,8]. In terms of treatment, hormonal smearing and local radiotherapy may be considered for focal lesions, and rituximab may be administered to individuals with systemic lesions or recurrence[9]. Given the unique clinicopathologic features of primary cutaneous MZL, it was included as a separate entity in the 5th Edition WHO classification of lymphoid neoplasms[10]. The current patient had extensive lesions, not only involving multiple lymph nodes but also affecting the spleen and bone marrow. Because hormonal treatment and first-line rituximab combination regimens were ineffective, extranodal MZL was still considered for this case with a diagnosis other than PCMZL.
Due to extensive involvement, the disease was at an advanced stage, and rituximab-based regimens were preferred according to current guidelines. Next-generation sequencing of the pathological tissue revealed a TP53 mutation, which accounts for about 5.8% of MZL lymphomas[11]. As in chronic lymphocytic leukemia (CLL), this mutation not only shortens the time from diagnosis to first treatment but also impairs progression-free survival (PFS) and overall survival, suggesting an association with worse prognosis[12]. Therefore, addition of targeted agents that may overcome TP53 mutations in combination with rituximab is recommended. A phase II clinical trial[13] of newly diagnosed CLL cases with del17p and/or TP53 mutations suggested that first-line ibrutinib therapy could lead to long-term remission. The present study suggests that BTK inhibitors (BTKi) can partially overcome P53 abnormalities.
Although no clinical studies have confirmed the use of BTKi for first-line treatment of advanced high-risk MZL, the NCCN and CSCO guidelines only included BTKi (ibrutinib and zanubrutinib) as second-line treatment options for stage III/IV MZL lymphoma. In the early disease stage, the patient was generally in poor condition and could not tolerate chemotherapy, so we selected the chemotherapy-free modality of BTKi in combination with rituximab for the first time. Compared with ibrutinib that exerts off-target effects, zanubrutinib, a potent, irreversible next-generation BTK inhibitor, is more selective and has less adverse events due to off-target effects[14]. The superiority of zanubrutinib was further confirmed in the phase III ALPINE trial[15]. Besides, the MAGNOLIA phase II clinical trial[16] demonstrated that zanubrutinib monotherapy has high overall response rate and CR with durable disease control and safety in R/R MZL. Considering the efficacy and safety of zanubrutinib, the patient was administered zanubrutinib plus rituximab as the initial therapy.
After three treatment courses, lymph nodes and spleen in the patient had some regression, but the skin rashes did not significantly improve. Abnormal lymphocytes were still detected by bone marrow immunophenotyping, resulting in stable disease. Based on the treatment principle of indolent lymphoma, the original regimen could be considered. However, since the patient’s skin symptoms persisted, the treatment regimen was adjusted to control the clinical symptoms as soon as possible. Obinutuzumab, a glycoengineered, type II anti-CD20 monoclonal antibody (mAb), exerts antitumor effects primarily via antibody-dependent cell-mediated cytotoxicity, antibody-dependent cell-mediated phagocytosis and direct cell death. Antibody-dependent cell-mediated cytotoxicity and direct cell death are significantly enhanced by obinutuzumab compared to the type I anti-CD20 mAb rituximab[17]. In the CLL11 (NCT01010061) phase 3 trial, obinutuzumab plus chlorambucil showed a benefit in PFS compared to rituximab plus chlorambucil, extending follow-up by about 2 years. The trial also demonstrated a significant increase in overall survival[18-20]. Similarly, in the phase 3 GALLIUM trial (NCT01332968), obinutuzumab and rituximab were combined with cyclophosphamide, doxorubicin, vincristine, and prednisone, cyclophosphamide, vincristine, and prednisone or bendamustine, respectively, for the treatment of indolent non-Hodgkin lymphoma. The latter trial as a head-to-head clinical analysis also showed the superiority of obinutuzumab in the treatment of follicular lymphoma[21,22]. Although current studies have not reported significant differences in PFS and adverse events between obinutuzumab plus chemotherapy and rituximab plus chemotherapy in newly diagnosed MZL[23], no head-to-head study has compared the combination of two anti-CD20 mAbs and BTK inhibitors. In this case, the patient was switched to obinutuzumab instead of rituximab combined with zanubrutinib, and CR was achieved after three cycles.
MZL diagnosis requires a comprehensive examination. When the involved site is used for disease staging, especially the skin, it is recommended to further complete a pathological biopsy to exclude lymphoma infiltration. This case of severe MZL with massive rashes is rare in clinical practice, and the individualized treatment regimen with BTK inhibitor combined with obinutuzumab was applied after a failed first-line treatment. Although this combination has not been previously examined, it conferred benefits to the current patient, suggesting that the sample size should be increased to provide better treatment options for severe MZL.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chuang SS, Taiwan; Radenska-Lopovok SG, Russia S-Editor: Qu XL L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 814] [Article Influence: 90.4] [Reference Citation Analysis (1)] |
| 2. | Sriskandarajah P, Dearden CE. Epidemiology and environmental aspects of marginal zone lymphomas. Best Pract Res Clin Haematol. 2017;30:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Thieblemont C, Molina T, Davi F. Optimizing therapy for nodal marginal zone lymphoma. Blood. 2016;127:2064-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Santos TSD, Tavares RS, Farias DLC. Splenic marginal zone lymphoma: a literature review of diagnostic and therapeutic challenges. Rev Bras Hematol Hemoter. 2017;39:146-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2714] [Cited by in RCA: 2576] [Article Influence: 128.8] [Reference Citation Analysis (2)] |
| 6. | Swerdlow SH. Cutaneous marginal zone lymphomas. Semin Diagn Pathol. 2017;34:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Kheterpal MK, Dai J, Geller S, Pulitzer M, Ni A, Myskowski PL, Moskowitz A, Kim J, Hong EK, Fong S, Hoppe RT, Kim YH, Horwitz SM. Role of imaging in low-grade cutaneous B-cell lymphoma presenting in the skin. J Am Acad Dermatol. 2019;81:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, Jaffe ES. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 847] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 9. | Valencak J, Weihsengruber F, Rappersberger K, Trautinger F, Chott A, Streubel B, Muellauer L, Der-Petrossian M, Jonak C, Binder M, Raderer M. Rituximab monotherapy for primary cutaneous B-cell lymphoma: response and follow-up in 16 patients. Ann Oncol. 2009;20:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 1932] [Article Influence: 644.0] [Reference Citation Analysis (0)] |
| 11. | Davis AR, Stone SL, Oran AR, Sussman RT, Bhattacharyya S, Morrissette JJD, Bagg A. Targeted massively parallel sequencing of mature lymphoid neoplasms: assessment of empirical application and diagnostic utility in routine clinical practice. Mod Pathol. 2021;34:904-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Onaindia A, Medeiros LJ, Patel KP. Clinical utility of recently identified diagnostic, prognostic, and predictive molecular biomarkers in mature B-cell neoplasms. Mod Pathol. 2017;30:1338-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Sivina M, Kim E, Wierda WG, Ferrajoli A, Jain N, Thompson P, Kantarjian H, Keating M, Burger JA. Ibrutinib induces durable remissions in treatment-naïve patients with CLL and 17p deletion and/or TP53 mutations. Blood. 2021;138:2589-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, Harrup R, Johnston PB, Marlton P, Munoz J, Seymour JF, Simpson D, Tedeschi A, Elstrom R, Yu Y, Tang Z, Han L, Huang J, Novotny W, Wang L, Roberts AW. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 15. | Hillmen P, Brown JR, Eichhorst BF, Lamanna N, O'Brien SM, Qiu L, Salmi T, Hilger J, Wu K, Cohen A, Huang J, Tam CS. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2020;16:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Opat S, Tedeschi A, Linton K, McKay P, Hu B, Chan H, Jin J, Sobieraj-Teague M, Zinzani PL, Coleman M, Thieblemont C, Browett P, Ke X, Sun M, Marcus R, Portell CA, Ardeshna K, Bijou F, Walker P, Hawkes EA, Mapp S, Ho SJ, Talaulikar D, Zhou KS, Co M, Li X, Zhou W, Cappellini M, Tankersley C, Huang J, Trotman J. The MAGNOLIA Trial: Zanubrutinib, a Next-Generation Bruton Tyrosine Kinase Inhibitor, Demonstrates Safety and Efficacy in Relapsed/Refractory Marginal Zone Lymphoma. Clin Cancer Res. 2021;27:6323-6332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Klein C, Jamois C, Nielsen T. Anti-CD20 treatment for B-cell malignancies: current status and future directions. Expert Opin Biol Ther. 2021;21:161-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Goede V, Fischer K, Engelke A, Schlag R, Lepretre S, Montero LF, Montillo M, Fegan C, Asikanius E, Humphrey K, Fingerle-Rowson G, Hallek M. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia. 2015;29:1602-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, Opat S, Owen CJ, Samoylova O, Kreuzer KA, Stilgenbauer S, Döhner H, Langerak AW, Ritgen M, Kneba M, Asikanius E, Humphrey K, Wenger M, Hallek M. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1139] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 20. | Goede V, Fischer K, Dyer MJS, Eckart MJ, Muller L, Smolej L, Bernado MCD, Knapp A, Nieisen T, Hallek M. Overall survival benefit of obinutuzumab over rituximab when combined with chlorambucil in patients with chronic lymphocytic leukemia and comorbidities: final survival analysis of the CLL11 study. EHA Library. 2018; S151. Available from: https://library.ehaweb.org/eha/2018/stockholm/215923/valentin.goede.overall.survival.benefit.of.obinutuzumab.over.rituximab.when.html. |
| 21. | Seymour JF, Marcus R, Davies A, Gallop-Evans E, Grigg A, Haynes A, Herold M, Illmer T, Nilsson-Ehle H, Sökler M, Dünzinger U, Nielsen T, Launonen A, Hiddemann W. Association of early disease progression and very poor survival in the GALLIUM study in follicular lymphoma: benefit of obinutuzumab in reducing the rate of early progression. Haematologica. 2019;104:1202-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, Townsend W, Trněný M, Wenger M, Fingerle-Rowson G, Rufibach K, Moore T, Herold M, Hiddemann W. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med. 2017;377:1331-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 555] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 23. | Herold M, Hoster E, Janssens A, McCarthy H, Tedeschi A, Pocock C, Rosta A, Trněný M, Nielsen TG, Knapp A, Hiddemann W, Marcus R. Immunochemotherapy and Maintenance With Obinutuzumab or Rituximab in Patients With Previously Untreated Marginal Zone Lymphoma in the Randomized GALLIUM Trial. Hemasphere. 2022;6:e699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (1)] |