Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.479
Peer-review started: August 25, 2023
First decision: November 20, 2023
Revised: November 27, 2023
Accepted: January 2, 2024
Article in press: January 2, 2024
Published online: January 26, 2024
Processing time: 146 Days and 4.3 Hours
Premature ovarian insufficiency (POI) is a condition that causes secondary amenorrhea owing to ovarian hypofunction at an early stage. Early follicular depletion results in intractable infertility, thereby considerably reducing the quality of life of females. Given the continuum in weakened ovarian function, progressing from incipient ovarian failure (IOF) to transitional ovarian failure and further to POI, it is necessary to develop biomarkers for predicting POI. The oxidative stress states in IOF and POI were comprehensively evaluated via oxidative stress [diacron-reactive oxygen metabolites (d-ROMs)] test and anti
To explore the possibilities of oxidative stress and antioxidant capacity as biomarkers for the early detection of POI.
Females presenting with secondary amenorrhea over 4 mo and a follicle stimulating hormone level of > 40 mIU/mL were categorized into the POI group. Females presenting with a normal menstrual cycle and a follicle stimulating hormone level of > 10.2 mIU/mL were categorized into the IOF group. Healthy females without ovarian hypofunction were categorized into the control group. Among females aged < 40 years who visited our hospital from January 2021 to June 2022, we recruited 11 patients into both POI and IOF groups. For the potential antioxidant capacity, the relative oxidative stress index (BAP/d-ROMs × 100) was calculated, and the oxidative stress defense system was comprehensively evaluated.
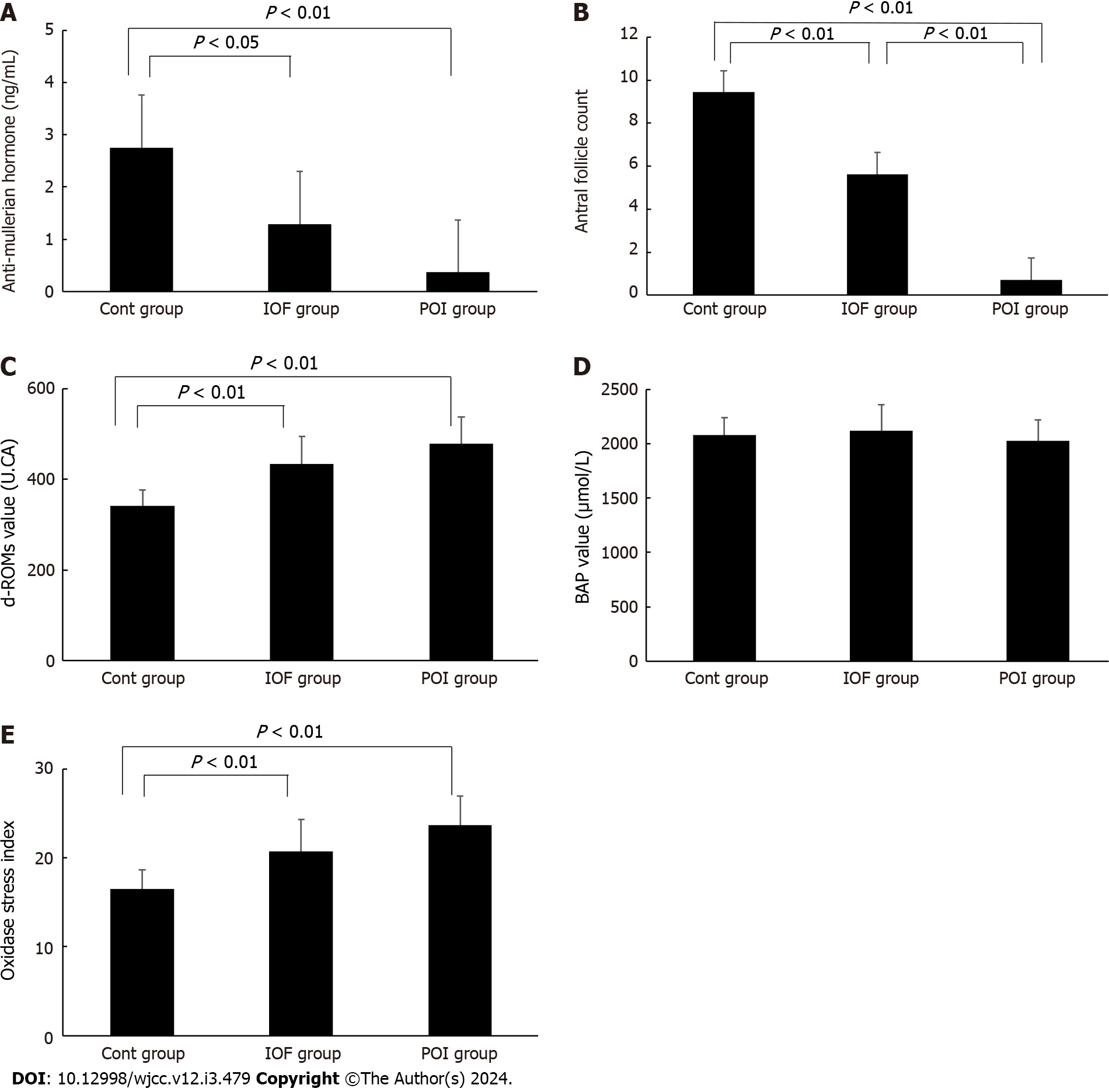
d-ROMs were significantly higher in the POI and IOF groups than in the control group, (478.2 ± 58.7 U.CARR, 434.5 ± 60.6 U.CARR, and 341.1 ± 35.1 U.CARR, respectively) (U.CARR is equivalent to 0.08 mg/dL of hydrogen peroxide). However, no significant difference was found between the POI and IOF groups. Regarding BAP, no significant difference was found between the control, IOF, and POI groups (2078.5 ± 157.4 μmol/L, 2116.2 ± 240.2 μmol/L, and 2029.0 ± 186.4 μmol/L, respectively). The oxidative stress index was significantly higher in the POI and IOF groups than in the control group (23.7 ± 3.3, 20.7 ± 3.6, and 16.5 ± 2.1, respectively). However, no significant difference was found between the POI and IOF groups.
High levels of oxidative stress suggest that evaluating the oxidative stress state may be a useful indicator for the early detection of POI.
Core Tip: The majority of premature ovarian insufficiency (POI) cases are idiopathic. Regardless of the cause, the disease causes a rapid decrease in the number of remaining follicles in the ovary and causes extremely intractable infertility. The process leading to POI is marked by a decline in ovarian function, starting with incipient ovarian failure, then transitional ovarian failure, and finally POI. Diacron-reactive oxygen metabolites and oxidative stress index were considerably higher in the incipient ovarian failure and POI groups than in the control group, indicating that evaluating oxidative stress status may be useful for the early diagnosis of POI.
- Citation: Kakinuma K, Kakinuma T. Significance of oxidative stress and antioxidant capacity tests as biomarkers of premature ovarian insufficiency: A case control study. World J Clin Cases 2024; 12(3): 479-487
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/479.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.479
Premature ovarian insufficiency (POI) is a disorder diagnosed as ovarian amenorrhea in patients under the age of 40 years. POI is characterized by the loss of normal ovarian function and is defined as hypergonadotropic amenorrhea[1]. Causes of POI include chromosome abnormalities, iatrogenicity, autoimmune disorders, metabolic disorders, infections, and environmental factors; however, most POI cases are idiopathic without a clear cause[2,3]. POI develops naturally in 1 out of 100 women[4], and its onset frequency by age is reported to be approximately 1% in patients aged < 40 years, 0.1% in patients aged < 30 years, and 0.01% in patients aged < 20 years[4]. However, in present-day society where trends of late marriage and childbirth are prevalent, patients with POI who achieve pregnancy before onset have been targets of infertility treatment, with recent reports indicating that the incidence of POI may be increasing[5,6].
Genetic factors account for approximately 10%-17% of POI cases, with many of the cases being abnormalities associated with the X chromosome; additionally, numerical, structural, and genetic abnormalities on the X chromosome have been reported[7-10]. Numerical abnormalities include 45, X (Turner syndrome), 47, XXX, and their mosaic. In addition, structural abnormalities include partial deletion, translocation, ring chromosome, isochromosome, and additional chromosome. Reportedly, DIAPH2, DACH2, XIST, etc., which are genes on the X chromosome, are involved in the onset of POI. Besides X chromosome abnormalities, blepharophimosis, ptosis, and epicanthus inversus syndrome, which develops due to mutations in FOXL2 found on an autosome, have also been reported[7-9]. In a study using genetically modified mice, numerous genetic abnormalities exhibiting the POI phenotype have been reported; in fact, NOBOX, BMP15, FSHR, etc have been confirmed in humans[10].
In POI, regardless of the cause, primordial follicle activation stops, and consequently, the recruitment of growing follicles vanishes. This usually occurs when the remaining follicle count in the ovary is < 1000, leading to lost ovulation. Because follicles leading to ovulation are lost owing to early follicular depletion, follicle development inhibition, and follicle pool destruction, the remaining eggs and follicles either extremely reduce in number or completely disappear[2,3]. Because there are no growing follicles, there are almost no granulosa cells, which are the main source of estrogen, thereby reducing blood estrogen levels. As a result, the endometrium does not thicken. Furthermore, as there is no post-ovulatory corpus luteum, progesterone secretion and withdrawal bleeding caused by the regression of the corpus luteum do not occur, resulting in amenorrhea. Therefore, in patients with POI, the loss of ovarian function causes low estradiol levels, and mental disorders such as climacteric symptoms, depression, anxiety, and cognitive disorders are likely to appear; thus, it is essential to appropriately manage it by improving the quality of life as well as preventing fractures caused by bone and circulatory diseases and decreased density[1,11].
Furthermore, major issues related to POI include extremely severe infertility. In this disorder, amenorrhea or anovulation associated with decreased follicle count is the main cause of infertility[12]. Approximately 25% of patients with this disorder have decreased ovulation; however, the lifetime pregnancy rate with own eggs is reportedly 5%-10%[2,3,12,13], leading to intractable infertility. Presently, there is no reliable reproductive healthcare for this disorder, and in the European Society of Human Reproduction and Embryology Guidelines, there is no recommended medical intervention other than egg donation[14,15]. This disorder is a progressive disorder that requires treatment before remaining follicles are depleted if pregnancy is desired, and early diagnosis before reaching POI is required.
Oxidative stress reportedly causes various diseases and is defined as a state where in vivo oxidative capacity exceeds antioxidant capacity[16,17]. Reactive oxygen species (ROS) is the general term for oxygen derivatives with high oxidative capacities, including superoxide anion, hydrogen peroxide, and hydroxyl radicals. There are numerous ROS-generating systems in vivo, such as the mitochondrial electron transport system, xanthine oxidative, and NADPH oxidase[18]. Presently, it has been clarified that redox signaling by ROS plays an important role in regulating various life phenomena, including immune response and wound healing[19]. Conversely, as ROS are highly toxic substances that cause nonspecific injury to cells owing to their high reactivity, in vivo ROS dysregulation-induced redox balance breakdown causes oxidative stress, and DNA damage, lipid peroxidation, and protein denaturation result in intractable diseases, such as senility, cardiovascular diseases, neurodegenerative diseases, and cancer[20]. Furthermore, in germ cells, ROS reportedly increase the risk of the incidence of hereditary diseases, infertility, and miscarriage[21,22]. Moreover, mitochondrial function in eggs and cytotoxicity owing to accompanying ROS may be factors involved in decreasing egg count or quality, which is observed in aging[23,24].
However, the body has an antioxidant mechanism that constantly scavenges generated ROS, thereby protecting the body from oxidative capacity induced by ROS. The ROS-scavenging mechanism includes endogenous antioxidant enzymes and exogenous antioxidants. Endogenous antioxidant enzymes include superoxide dismutase and catalase that scavenge superoxide and hydrogen peroxide as well as glutathione peroxidase, reducing lipid hydroperoxide levels[25]. There are numerous types of exogenous antioxidants, including carotenoids (lycopene and astaxanthin), polyphenols (carotenoids, catechin, and curcumin), and antioxidant vitamins (vitamin C, vitamin E, and β-carotene)[26-29]. Thus, oxidative and antioxidant capacities are balanced, maintaining homeostasis without developing oxidative stress and preventing injuries[16,17].
In recent years, tests for diacron-reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP), which can be easily measured, have been developed. The combination of d-ROMs and BAP tests can be used to comprehensively evaluate the blood oxidative stress[30,31]. The d-ROMs test can comprehensively assess the state of oxidative stress in vivo by primarily measuring the hydroperoxide levels in circulation caused by ROS and free radicals via color reaction in vivo[32]. The BAP test can evaluate the antioxidant capacity by measuring the reducing ability of antioxidants to donate electrons to ROS and free radicals to stop oxidation[32].
There exists a continuum in the weakened ovarian function of patients with POI. According to Knauff et al[33], this continuum begins with incipient ovarian failure (IOF) with normal menstruation but a slightly high follicle stimulating hormone (FSH) level (> 10.2 IU/L), progressing to transitional ovarian failure with a high FSH level and irregular menstruation, further progressing to amenorrhea for > 4 mo and an FSH level of > 40 IU/L, finally transitioning to a condition where follicles are depleted or follicular growth stops.
This study aimed to comprehensively evaluate the oxidative stress state in patients with IOF and POI by assessing oxidative stress and antioxidant capacity and investigate whether they can be used as biomarkers to aid in the early detection of POI.
This study was conducted with the approval of the ethics committee of our hospital (Ethics Review Committee, International University of Health and Welfare, approval number: 21-Im-075, approval date: March 22, 2022). The participants included patients aged < 40 years who visited the International University of Health and Welfare Hospital from January 2021 to June 2022. They were provided written and oral explanations regarding the contents of this study, and the participants provided their consent. Considering the effects on oxidative stress and antioxidant capacity, patients with gynecological diseases and severe paramenstrual symptoms requiring analgesics, patients taking other medications and supplements, and smokers were excluded.
Females presenting with secondary amenorrhea for ≥ 4 mo and an FSH level of > 40 mIU/mL were categorized into the POI group. Females presenting with a normal menstrual cycle and an FSH level of > 10.2 mIU/mL were categorized into the IOF group. Healthy females without ovarian hypofunction were categorized into the control group.
Blood collection was performed during the follicular phase (within 5 d from the start of menstruation). For ovarian function, FSH and anti-Mullerian hormone (AMH) levels were measured, and antral follicle count was determined via a transvaginal ultrasound imaging diagnostic machine (Voluson S10 Expert; GE Healthcare Japan, Tokyo, Japan). For evaluating the oxidative stress state, d-ROMs, the marker for oxidative stress, and BAP, the marker for antioxidant capacity, were measured. For potential antioxidant capacity, the oxidative stress index [oxidative stress index; BAP/d-ROMs × 100] was calculated, and the oxidative stress defense system was comprehensively evaluated.
The FSH levels were measured via chemiluminescent immunoassay (CL AIA-PACK®FSH Test; Tosoh Corporation, Tokyo, Japan). In addition, AMH levels were measured using the chemiluminescent enzyme immunoassay (Access AMH®; Beckman Coulter, Tokyo, Japan).
Blood oxidative stress (d-ROMs) and antioxidant capacity (BAP) in the follicular phase were measured using a free radical analyzer (FREE Carrio Duo; Diacron International, Grosseto, Italy). The validity and reproducibility of the measurement method using this instrument have previously been reported in another study[34].
To measure d-ROMs, 20 µL of plasma was collected from the post centrifugation blood and mixed into an acidic buffer of pH 4.8. Thereafter, colorless aromatic amine aqueous solution (color liquid chromogen) was added and mixed, the mixture was transferred into the photometer in the instrument, the decrease in absorbance at 505 nm was measured after 5 min, and plasma hydroperoxide concentration was calculated and measured from the rate of change. d-ROMs can assess the degree of the oxidative stress index by measuring blood hydroperoxide (ROOH) levels produced via in vivo ROS and free radicals through color reaction[34]. The unit U.CARR is equivalent to 0.08 mg/dL of hydrogen peroxide. The reference values applied are as follows: normal values, 200-300 U.CARR; borderline, 301-320 U.CARR; mild oxidative stress, 321-340 U.CARR; moderate oxidative stress, 341-400 U.CARR; severe oxidative stress, 401-500 U.CARR; and markedly severe oxidative stress, > 501 U.CARR[35].
To measure BAP, a reagent containing thiocyanic acid derivatives and a reagent containing iron ions were mixed and placed in the photometer of the instrument and absorbance at 505 nm was measured. Furthermore, 10 µL of plasma was added to this mixture, which was subsequently incubated for 5 min at 37 °C, and the absorbance was measured again. The oxidized iron ion concentration was calculated from the change in absorbance in 5 min. Assessing BAP can help determine the ability of the plasma solution to reduce trivalent iron (Fe3+) to bivalent iron (Fe2+; redox). The unit is µmol/L, and the reference values applied are as follows: optimal values, > 2200; borderline, 2000-2200; mildly deficient antioxidant capacity, 1800-2000; deficient antioxidant capacity, 1600-1800; markedly deficient antioxidant capacity, 1400-1600; and severely deficient antioxidant capacity, < 1400[36,37].
All measurement values were expressed as mean ± standard deviation. One-way analysis of variance and Tukey-Kramer multiple comparison test were used for statistical analysis. JMP® version 14.2 (SAS Institute Japan Co., Ltd., Tokyo Japan) statistical processing software (SPSS Statistics version 21; IBM Corp., Armonk, NY, United States) was used for statistical processing. A P value < 0.05 was considered statistically significant.
Among the 35 participants, two were smokers who were excluded. Thus, there were 11 participants in the POI group with secondary amenorrhea of > 4 mo and an FSH level of > 40 mIU/mL, 11 participants in the IOF group with a normal menstrual cycle and an FSH level of > 10.2 mIU/mL, and 11 participants in the control group without ovarian hypofunction. Table 1 shows the background information of the participants. The mean age and body mass index of the participants were 35.8 ± 3.0 years and 20.1 ± 1.9 years in the control group, 37.5 ± 1.7 years and 20.1 ± 2.1 years in the IOF group, and 35.8 ± 2.7 years and 19.4 ± 2.5 years in the POI group. In addition, gravidity and parity were 0.6 ± 0.7 and 0.5 ± 0.5 in the control group, 0.4 ± 0.5 and 0.2 ± 0.4 in the IOF group, and 0.6 ± 0.9 and 0.3 ± 0.5 in the POI group. No significant difference was found among the groups in terms of mean age, body mass index, gravidity, and parity (Table 1).
| Parameter | Control group, n = 11 | IOF group, n = 11 | POI group, n = 11 | P value |
| Age (yr) | 35.8 ± 3.0 | 37.5 ± 1.7 | 35.8 ± 2.7 | 0.195 |
| BMI in kg/m2 | 20.1 ± 1.9 | 20.1 ± 2.1 | 19.4 ± 2.5 | 0.678 |
| Gravidity as number of times | 0.6 ± 0.7 | 0.4 ± 0.5 | 0.6 ± 0.9 | 0.598 |
| Parity as number of times | 0.4 ± 0.5 | 0.2 ± 0.4 | 0.3 ± 0.5 | 0.655 |
AMH levels were significantly lower in the IOF and POI groups than in the control group (2.8 ± 1.4 ng/mL, 1.3 ± 1.3 ng/mL, and 0.4 ± 0.3 ng/mL in the control, IOF, and POI groups, respectively); however, no significant difference was found between the POI and IOF groups (Figure 1A). The antral follicle count was significantly lower in the POI and IOF groups than in the control group and significantly less in the POI group than in the IOF group (9.5 ± 2.4, 5.6 ± 1.8, and 0.7 ± 0.9 in the control, IOF, and POI group, respectively) (Figure 1B). d-ROMs were significantly higher in the POI and IOF groups than in the control group (341.1 ± 35.1 U.CARR, 434.5 ± 60.6 U.CARR, and 478.2 ± 58.7 U.CARR in the control, IOF, and POI groups, respectively); however, no significant difference was found between the POI and IOF groups (Figure 1C). Regarding BAP, no significant difference was found among the three groups (2078.5 ± 157.4 μmol/L, 2116.2 ± 240.2 μmol/L, and 2029.0 ± 186.4 μmol/L in the control, IOF, and POI groups, respectively) (Figure 1D). The oxidative stress index was significantly higher in the POI and IOF groups than in the control group (16.5 ± 2.1, 23.7 ± 3.3, and 20.7 ± 3.6 in the control, POI, and IOF groups, respectively); however, no significant difference was found between the POI and IOF groups (Figure 1F).
POI is a disorder diagnosed as ovarian amenorrhea in patients under the age of 40 years. When normal ovarian function is lost, decreased ovarian function causes reduced estradiol levels, infertility, climacteric symptoms, low bone density and accompanying fracture, circulatory diseases, and mental disorders such as depression, anxiety, and cognitive disorders; thus, appropriate management is essential. The causes of POI include heredity, iatrogenicity, autoimmunity, metabolism, infection, and environmental factors. However, most POI cases are idiopathic without a clear cause[2,3].
However, regardless of the cause, the remaining follicle count in the ovary decreases rapidly, exceeding the physiological level and resulting in extremely intractable infertility. In patients with POI, the lifetime pregnancy rate with own eggs is extremely low, and presently, infertility treatment using eggs donated by a third party instead of own eggs is the most effective and the only evidence-based infertility treatment in terms of time, efficiency, and live birth rate[2,3]. Follicle reduction in patients with POI is progressive, and there is no means to increase the remaining follicle count. For patients who wish to have children, in addition to guidance to begin infertility treatment as soon as possible, an early diagnosis before transitioning to POI is desirable.
The ovary is considered one of the organs with the earliest decrease in function, and decreased ovarian function reduces the remaining follicle count and egg quality, leading to infertility[35,38]. Apoptotic pathways have been suggested to be involved in these physiological changes[39,40]. Reportedly, mitochondrial function in eggs and cytotoxicity due to accompanying ROS are factors involved in inducing apoptosis[28,29].
Oxidative stress is defined as the difference between the ability of ROS generated in vivo to cause oxidative damage and the antioxidant potential of the in vivo antioxidant system[16,17]. It is thought that most ROS is generated in mitochondria, impairing lipids, proteins, and DNA as well as the mitochondria themselves, consequently causing various diseases including senility at the cellular level, arteriosclerosis, diabetes mellitus, and malignant diseases[20].
In a study regarding the mitochondrial membrane potential of human eggs, eggs harvested from older females with decreased remaining follicle count and egg quality owing to reduced ovarian function was considerably lower than that of the eggs harvested from young females[36]. Mitochondria have mtDNA, which is a double-stranded circular DNA with its own genome, and reportedly the percentage of deletions and point mutations in mtDNA is higher in eggs harvested from older females than that in eggs harvested from young females[41,42]. In a study regarding differential gene expression via microarray analysis using ovulation eggs of older and young mice, the expression of genes involved in mitochondrial function, oxidative stress regulation, and DNA and chromosome stabilization was decreased in the eggs of the elderly mice[43]. In a similar study involving human eggs, the expression of genes belonging to the same categories was decreased[44]. These studies suggest that mitochondrial dysfunction occurs. Mitochondria are intracellular organelles that play a central role in energy metabolism. They supply intracellular ATP via oxidative phosphorylation, and mitochondrial dysfunction causes various diseases[45,46]. In mitochondria, ROS is generated during oxidative phosphorylation; however, it is promptly scavenged and regulated to avoid excessive oxidative stress. Nevertheless, molecules and enzymes associated with the ROS-scavenging system decrease or become dysfunctional owing to the aging of eggs[43,44,47].
In addition, increased oxidative stress exacerbates intracellular Ca2+ regulatory mechanisms[48]. Eggs exhibit a cyclical increase/decrease in intracellular Ca2+ concentrations during fertilization, which is known as Ca2+ oscillation. Ca2+ oscillation during fertilization in eggs is associated with the release of surface granules, meiosis resumption, and egg activation. Increased oxidative stress reduces mitochondrial function and ATP production, and changes in the intracellular Ca2+ regulatory mechanism centering on the endoplasmic reticulum are associated with abnormal embryo growth[49,50].
In recent years, it has become possible to comprehensively and conveniently evaluate the blood oxidative stress state by combining d-ROMs and BAP. In the transition to POI, there is a continuum of weakened ovarian function, starting with IOF, to transitional ovarian failure, and finally POI[16]. Thus, herein, by comprehensively evaluating the blood oxidative stress of patients with IOF and POI, we examined whether it can be a biomarker for the early diagnosis of POI. d-ROMs, an indicator of oxidative stress, were compared in the control group to the stage of IOF. Conversely, BAP was assessed to examine the antioxidant capacity. BAP was constant regardless of disease progression; no significant difference was found among the three groups (the control, IOF, and POI groups), and no decrease in antioxidant capacity was observed. Furthermore, relative oxidative stress index increased from the stage of IOF, as with d-ROMs, suggesting that the combination of blood d-ROMs and BAP can be an indicator for the early diagnosis of POI. As ROS and free radicals are unstable, their measurement is complex and difficult. Herein, rather than directly measuring ROS and free radicals in the examined d-ROMs, we measured the ROOH produced by them, which more clearly reflects the state of blood oxidative stress. It is thought that the increase in oxidative stress could be evaluated from the state of IOF exhibiting mild ovarian function.
In the future, it is necessary to examine in detail the type of influence the blood oxidative stress state reported in this study has on the pathological conditions of IOF and POI and as a local factor how it reflects cytotoxicity caused by ROS, which can trigger the decrease in the remaining follicle count and egg quality associated with ovarian hypofunction.
Compared with the control group, d-ROMs and oxidative stress index were substantially higher in the IOF group and in the POI group, suggesting that evaluating oxidative stress state is useful as a biomarker for the early diagnosis of POI. In addition, these biomarkers are expected to be useful for the early intervention of treatment, including infertility treatment.
Premature ovarian insufficiency (POI) is a condition that causes secondary amenorrhea owing to decreased ovarian function before the age of 40 years. Early follicle depletion causes intractable infertility and considerably reduces a woman’s quality of life. Mitochondrial function within the egg and cytotoxicity caused by the accompanying reactive oxygen species have been suggested to be one of the factors involved in contributing to the decrease in the number of remaining follicles and the decline in oocyte quality owing to the decline in ovarian function. There is a continuum in the decline of ovarian function, including incipient ovarian failure (IOF), transitional ovarian failure, and POI.
There is a need to discover biomarkers for the early detection of POI and investigate the underlying etiology.
This study aimed to comprehensively evaluate the oxidative stress state in IOF and POI by determining oxidative stress [diacron-reactive oxygen metabolites (d-ROMs)] and antioxidant potential [biological antioxidant potential BAP)] to explore their potential as biomarkers for the early detection of POI.
This study included 11 women with secondary amenorrhea for ≥ 4 mo and an FSH level of ≥ 40 mIU/mL in the POI group and 11 women with normal menstrual cycles and an FSH level of ≥ 10.2 mIU/mL in the IOF group. d-ROMs and BAP in the plasma of each group were measured with healthy women of the same age without ovarian function decline as the control group.
In the POI and IOF groups, d-ROMs and oxidative stress index were significantly higher than those in the control group. Regarding BAP, no significant difference was observed between the three groups.
Oxidative stress (d-ROMs, oxidative stress index) in the IOF and POI groups was considerably higher than that in the control group, suggesting that evaluating oxidative stress status is a useful indicator for the early detection of POI.
d-ROMs and OSI were significantly higher in the IOF group, which is the pre-POI transition stage as well as in the POI group compared with that in the control group, and the evaluation of oxidative stress status may be a useful biomarker for the early diagnosis of POI. It is expected that this finding will be useful for early intervention in treatments such as infertility treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Sabetian S, Iran S-Editor: Lin C L-Editor: Filipodia P-Editor: Yuan YY
| 1. | National Collaborating Centre for Women’s and Children’s Health. Menopause. Nov 12, 2015. [cited 26 October 2022]. Available from: https://www.nice.org.uk/guidance/ng23/evidence/full-guideline-559549261. |
| 2. | van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 219] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Fraison E, Crawford G, Casper G, Harris V, Ledger W. Pregnancy following diagnosis of premature ovarian insufficiency: a systematic review. Reprod Biomed Online. 2019;39:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Lagergren K, Hammar M, Nedstrand E, Bladh M, Sydsjö G. The prevalence of primary ovarian insufficiency in Sweden; a national register study. BMC Womens Health. 2018;18:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019;22:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 7. | Chapman C, Cree L, Shelling AN. The genetics of premature ovarian failure: current perspectives. Int J Womens Health. 2015;7:799-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Fortuño C, Labarta E. Genetics of primary ovarian insufficiency: a review. J Assist Reprod Genet. 2014;31:1573-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure. J Mol Endocrinol. 2010;45:257-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Bonnet A, Bevilacqua C, Benne F, Bodin L, Cotinot C, Liaubet L, Sancristobal M, Sarry J, Terenina E, Martin P, Tosser-Klopp G, Mandon-Pepin B. Transcriptome profiling of sheep granulosa cells and oocytes during early follicular development obtained by laser capture microdissection. BMC Genomics. 2011;12:417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106:1588-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 12. | Panay N, Anderson RA, Nappi RE, Vincent AJ, Vujovic S, Webber L, Wolfman W. Premature ovarian insufficiency: an International Menopause Society White Paper. Climacteric. 2020;23:426-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 13. | Męczekalski B, Maciejewska-Jeske M, Podfigurna A. Reproduction in premature ovarian insufficiency patients - from latest studies to therapeutic approach. Prz Menopauzalny. 2018;17:117-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI; Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervorst E, Janse F, Liao L, Vlaisavljevic V, Zillikens C, Vermeulen N. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 612] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 15. | Pinelli S, Artini PG, Basile S, Obino MER, Sergiampietri C, Giannarelli D, Simi G, Cela V. Estrogen treatment in infertile women with premature ovarian insufficiency in transitional phase: a retrospective analysis. J Assist Reprod Genet. 2018;35:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:31S-38S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 715] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 17. | Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6531] [Cited by in RCA: 6504] [Article Influence: 260.2] [Reference Citation Analysis (0)] |
| 18. | Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181-H2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 872] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 19. | Miranda-Vizuete A, Veal EA. Caenorhabditis elegans as a model for understanding ROS function in physiology and disease. Redox Biol. 2017;11:708-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Brieger K, Schiavone S, Miller FJ Jr, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 385] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 21. | Ohno M, Sakumi K, Fukumura R, Furuichi M, Iwasaki Y, Hokama M, Ikemura T, Tsuzuki T, Gondo Y, Nakabeppu Y. 8-oxoguanine causes spontaneous de novo germline mutations in mice. Sci Rep. 2014;4:4689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 22. | Uchimura A, Higuchi M, Minakuchi Y, Ohno M, Toyoda A, Fujiyama A, Miura I, Wakana S, Nishino J, Yagi T. Germline mutation rates and the long-term phenotypic effects of mutation accumulation in wild-type laboratory mice and mutator mice. Genome Res. 2015;25:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Grøndahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod. 2010;25:957-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Bentov Y, Casper RF. The aging oocyte--can mitochondrial function be improved? Fertil Steril. 2013;99:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol (1985). 1995;79:675-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 350] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 26. | Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2006] [Cited by in RCA: 1938] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 27. | Nakagawa K, Ninomiya M, Okubo T, Aoi N, Juneja LR, Kim M, Yamanaka K, Miyazawa T. Tea catechin supplementation increases antioxidant capacity and prevents phospholipid hydroperoxidation in plasma of humans. J Agric Food Chem. 1999;47:3967-3973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Nielsen BR, Jørgensen K, Skibsted LH. Triplet—triplet extinction coefficients, rate constants of triplet decay and rate constant of anthracene triplet sensitization by laser flash photolysis of astaxanthin, β-carotene, canthaxanthin and zeaxanthin in deaerated toluene at 298 K. J Photochem Photobiol A. 1998;112:127-133. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Martin HD, Ruck C, Schmidt M, Sell S, Beutner S, Mayer B, Walsh R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl Chem. 1999;71:2253-2262. [RCA] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, Barsotti A, Terranova R, Nicolaides A. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127-130. [PubMed] |
| 31. | Trotti R, Carratelli M, Barbieri M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002;44:37-40. [PubMed] |
| 32. | Fukui T, Yamauchi K, Maruyama M, Yasuda T, Kohno M, Abe Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res. 2011;34:1041-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Knauff EA, Eijkemans MJ, Lambalk CB, ten Kate-Booij MJ, Hoek A, Beerendonk CC, Laven JS, Goverde AJ, Broekmans FJ, Themmen AP, de Jong FH, Fauser BC; Dutch Premature Ovarian Failure Consortium. Anti-Mullerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. J Clin Endocrinol Metab. 2009;94:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Nakayama K, Terawaki H, Nakayama M, Iwabuchi M, Sato T, Ito S. Reduction of serum antioxidative capacity during hemodialysis. Clin Exp Nephrol. 2007;11:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 777] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 36. | Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 371] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 37. | Iorio EL. The BAP test and the global assessment of oxidative stress in clinical practice. 2010. [cited 26 October 2022]. Available from: http://www.medial.cz/content/files/medial/download/prospekty/HaD/2010_4_1_BAP_TEST_PRESENTATION.pdf#search=%27Eugenio+Luigi+Iorio++BAP+test%27. |
| 38. | Gougeon A. Ovarian follicular growth in humans: ovarian ageing and population of growing follicles. Maturitas. 1998;30:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 268] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 40. | Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 818] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 41. | Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 185] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Barritt JA, Cohen J, Brenner CA. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reprod Biomed Online. 2000;1:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13:2263-2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 401] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 44. | Steuerwald NM, Bermúdez MG, Wells D, Munné S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online. 2007;14:700-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update. 2009;15:553-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 46. | Romano AD, Serviddio G, de Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. J Nephrol. 2010;23 Suppl 15:S29-S36. [PubMed] |
| 47. | Tarín JJ, Gómez-Piquer V, Pertusa JF, Hermenegildo C, Cano A. Association of female aging with decreased parthenogenetic activation, raised MPF, and MAPKs activities and reduced levels of glutathione S-transferases activity and thiols in mouse oocytes. Mol Reprod Dev. 2004;69:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 630] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 49. | Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006;17:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Rogers NT, Halet G, Piao Y, Carroll J, Ko MS, Swann K. The absence of a Ca(2+) signal during mouse egg activation can affect parthenogenetic preimplantation development, gene expression patterns, and blastocyst quality. Reproduction. 2006;132:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |