Published online Sep 26, 2024. doi: 10.12998/wjcc.v12.i27.6045
Revised: July 3, 2024
Accepted: July 10, 2024
Published online: September 26, 2024
Processing time: 124 Days and 24 Hours
Liver failure (LF) is prevalent in China and is characterized by complex path
Core Tip: This review highlights the potential of interleukins (Ils), particularly IL-21, IL-22, and IL-31, as the prognostic biomarkers of liver failure. The article emphasizes the crucial role of immune responses in liver failure pathogenesis and explores the complex interplay between these Ils and various immune cells, signaling pathways, and liver diseases. Moreover, it critically analyzes existing literature, identifying limitations and suggesting future directions for further research.
- Citation: Lin Y, Yan GJ, Liu MY, Cao Y, Zhang K, Wang N, Long FL, Mao DW. Review of the potential value of serum interleukin levels as prognostic biomarkers of liver failure. World J Clin Cases 2024; 12(27): 6045-6056
- URL: https://www.wjgnet.com/2307-8960/full/v12/i27/6045.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i27.6045
Liver failure (LF) is the end result of various acute or chronic liver diseases. Its pathological essence is the complete loss of hepatic metabolic and detoxification functions due to the extensive death of hepatocytes[1]. Liver transplantation is a fast and effective treatment for advanced LF. However, the stark incongruity between the substantial demand for liver donors and the limited supply is not expected to be effectively resolved in the near future. Active and effective comprehensive medical intervention, encompassing energy and nutrition supplement, electrolyte acid-base balance maintenance, anti-inflammatory liver protection, immunomodulation, artificial liver support, and complication prevention and treatment, is still the preferred approach for the treatment of this disorder[2,3]. However, the massive consumption of medical resources and high medical costs because of comprehensive treatment, difficulty in fully controlling inflammatory reactions, and complication prevention and management lead to a heavy burden on patients and society. Consequently, LF has emerged as a critical and challenging condition in the realm of liver diseases and needs to be comprehensively analyzed with regard to its complex pathogenesis. In addition, it is necessary to develop early assessment methods and formulation for evidence-based diagnostic and therapeutic strategies targeting its core pathological processes. In LF treatment plans, numerous countries emphasize the importance of comprehensive prognostic evaluation throughout the diagnosis and treatment course, particularly emphasizing early assessment. Kuroda et al[4] used the time interval between the time to peak of hepatic artery and liver parenchyma to accurately predict the prognosis of acute LF (ALF) patients, and this strategy may be helpful for clinical decision-making. Bernal et al[5] used the admission variables of age, Glasgow coma scale, arterial pH and lactate levels, creatinine level, international normalized ratio, and cardiovascular failure to develop an initial predictive model. It accurately predicts the quality of life for patients with paracetamol-induced ALF at an early stage and thus aids in emergency liver transplantation and medical decision-making. Xiao et al[6] found that patients receiving the treatment of artificial liver support had a higher short-term survival rate than those receiving standard drug treatment. Notably, the authors observed that among the patients who received artificial liver support, those receiving mixed artificial liver support demonstrated a higher short-term survival rate than those undergoing plasma exchange, confirming the efficacy of the artificial liver support system. Clearly, early recognition and proactive intervention may improve the prognosis of patients with ALF. Therefore, reliable prognostic markers that can distinguish between patients at high risk of death and those with the potential for recovery following treatment may help reduce ALF-associated mortality, and these biological indicators are mostly closely related to the disease mechanism of LF[7]. The underlying mechanism of LF fundamentally involves a sequence of critical clinical conditions that emerge when a substantial number of hepatocytes undergo pathological passive death, compromising the structure and function of the liver[8]. Concurrently, the rapid and massive death of hepatocytes is accompanied by hepatic injury caused by hyperimmunity (immune responses that are heightened beyond normal physiological levels) and inflammatory stress[9]. Moreover, an Increasing amount of research evidence suggests that immune hyperactivity may be the main factor leading to the rapid hepatocyte death in the early stages of LF[10-16]. Kupffer cells, which are resident macrophages in the liver, produce large amounts of interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin 1 (IL-1), capable of inducing rapid hepatocyte death and causing hepatic damage[10]. Additionally, natural killer (NK) and NKT cells have been shown to induce hepatocyte death through the Fas/Fas ligand (FasL) and NKG2D/NKG2DL signaling pathways[11]. Likewise, cytotoxic T cells can induce rapid hepatocyte death through the perforin-1/granzyme, Fas/FasL, TNFR1/TNF, and receptor interaction protein kinase 1 (RIP-1)/RIP-3 signaling pathways[12]. Moreover, T helper 17 cells (Th17) cells can contribute to hepatocyte death and hepatic inflammatory damage by secreting the key inflammatory factor IL-17, which enhances the function of tumor necrosis factor and expression of cell adhesion molecule[13]. Hyperimmunity combined with cytokine storm triggers a "domino effect" of rapid hepatocyte death in LF[14]. These findings suggest that the development and exacerbation of LF are related to severe systemic inflammation. Systemic inflammatory responses are recognized as the indicators of LF that affect the prognosis of LF[15,16]. In this context, the recruitment and differentiation of immune cells at inflammatory sites are facilitated by pro-inflammatory cytokines[17]. As a type of proinflammatory factor, IL often interacts with white blood cells and immune cells, thereby participating in intercellular information transmission, activating and regulating immune cells, and mediating inflammatory reactions such as activation, proliferation, and differentiation of T and B cells[18]. Therefore, ILs may be a kind of mediator that promotes the occurrence of immune response in LF.
The present article explores the relationship between the levels of IL-21, IL-22, and IL-31 and prognosis of LF, shedding light on the potential of ILs as prognostic biomarkers of LF.
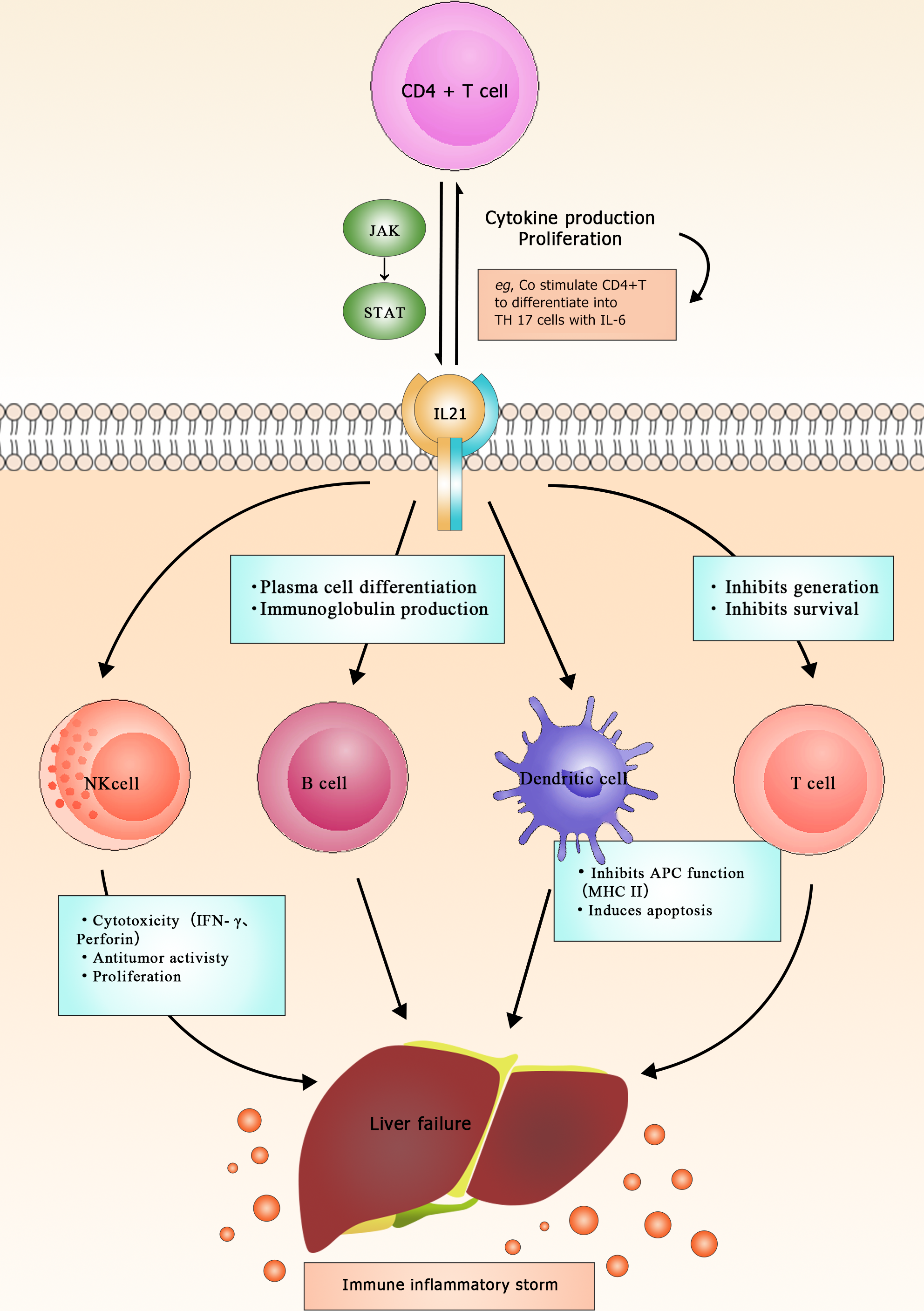
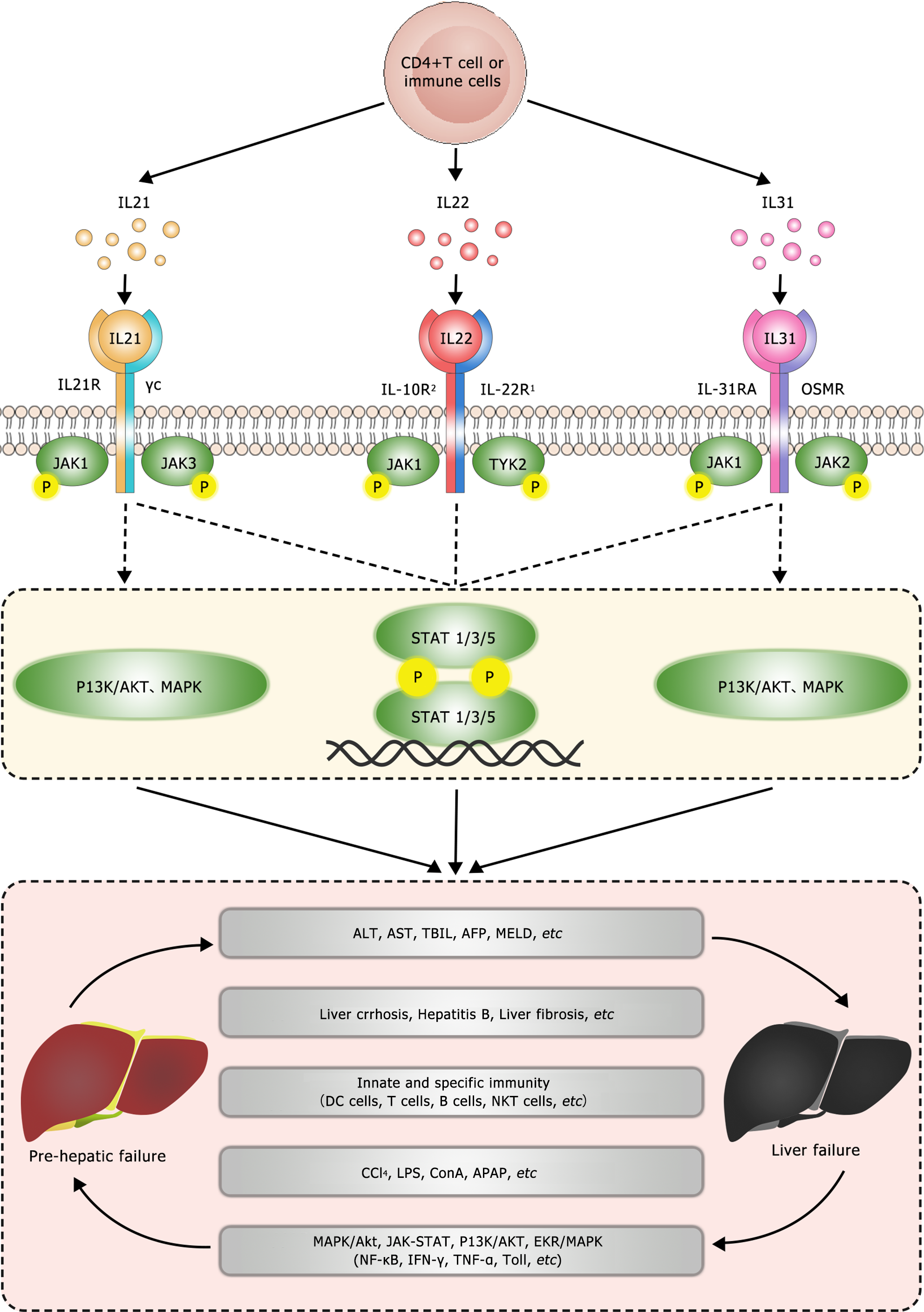
Research has found that IL-21, as one of the main immune modulators, plays a crucial role in the induction, initiation, progression, and deterioration of various diseases[19]. It is mainly expressed in activated CD4+ T cells and regulates various immune responses by modulating many immune cells[20] (Figure 1). The IL-21R gene encodes the receptor for IL-21. IL-21 receptor (IL-21R) belongs to the type I cytokine receptor family and complexes with the common γ chain (γc), forming a heterodimer receptor complex. The subunit γc is also a common receptor for IL-2, IL-4, IL-7, IL-9, and IL-15[21]. The IL-21R transduces the growth-promoting signal of IL-21, which is of great significance for the proliferation and differentiation of T, B, and NK cells[22]. Binding of the ligand to the receptor results in the activation of multiple downstream signaling molecules, including Janus kinase 1 (JAK1), JAK3, signal transducer and activator of transcription 1 (STAT1), and STAT3[23] (Figure 2). Studies have demonstrated that IL-21 can be upregulated in various subsets of T helper cells, including Th2, Th17, and follicular T cells[24]. In addition, IL-21 is expressed in NKT cells, which oversee the functions of these cells[22]. Investigations on IL-21R have revealed that this receptor plays a vital role in regulating immunoglobulin production in both animals and humans[25,26]. Notably, IL-21 has been linked to inflammatory diseases, and studies suggest the existence of a positive autocrine loop that can enhance and stabilize IL-21-driven, T cell-mediated responses[27,28]. Moreover, IL-21 exhibits anticancer properties and stimulates autoimmune reactions[29]. IL-21 has garnered significant attention as a potential therapeutic and predictive target of LF. A study has shown that IL-21 may participate in the regulation of inflammatory mediators of liver injury by modulating the functions of cells associated with innate and adaptive immunities[30]. Additionally, it may contribute to LF by changing the expression of other pro-inflammatory cytokines. Specifically, research has revealed that IL-21 can increase IL-1β, IL-6, IL-10, IFN-γ, and TNF-α levels in peripheral blood monocytes, potentially contributing to the development of ALF triggered by the hepatitis B virus (HBV)[30]. However, in a mouse model of ALF induced by the hepatitis virus 3 strain, significant upregulation of the Th17-related cytokine IL-21 was observed after 72 h of infection[31]. Importantly, this upregulation demonstrated a positive correlation with the degree of liver function impairment in the infected mice. Liu et al[32] used high-resolution melting to genotype five single nucleotide polymorphisms in 546 HBV-infected Chinese patients and 353 healthy subjects, and the results revealed that the IL-21R (rs2285452 AA) genotype was associated with an increased risk of developing HBV-related cirrhosis and liver cancer in Chinese patients. Chen et al[33] observed significantly higher serum IL-21 levels in patients with chronic hepatitis B (CHB) or HBV-related acute-on-chronic LF (ACLF) than in healthy individuals, whereas there was no such increase in patients with liver cirrhosis. The relatively high serum IL-21 levels in CHB patients may play a causal role in the persistence of HBV infection. Additionally, the proportion of CD4+ T cells in the peripheral blood of HBV-ACLF patients was higher than in healthy individuals and correlated with the number and proportion of lymphocytes in the blood. This suggests that elevated serum IL-21 levels lead to the activation of T and B cells, stimulating them to release pro-inflammatory cytokines to combat the virus, although this immune response can inadvertently lead to liver damage. The same study compared patients with moderate CHB (M-CHB), severe CHB (S-CHB), and HBV-ACLF. Compared with M-CHB patients and the control group, both HBV-ACLF and S-CHB patients showed higher frequencies of IL-21 secretion by CD4+ T cells. HBV-ACLF patients had the highest mean serum IL-21 levels, which positively correlated with the “Model for End-Stage Liver Disease”(MELD) score and mortality rate. The recovery of the HBV-ACLF patients was found to be associated with decreased serum IL-21 level and decreased CD4+ T cell proportion. Moreover, IL-21 stimulation has been shown to significantly increase serum IL-6, IL-10, and TNF-α levels[31]. Pan et al[34] showed a positive correlation between serum IL-21 level and alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL) levels in HBV-ACLF patients and a negative correlation with albumin (ALB) level. These observations suggest that IL-21 plays a causative role in the progression of severe hepatic inflammation and is associated with the severity of liver diseases. T follicular helper cells (Tfh) are considered a unique subset of CD4+ T cells that mediate the development of long-term humoral immunity and play an important role in the process of hepatic viral infection[35,36]. CD4+ T cells in the immune system produce IL-21, which is crucial for the development of CD8+ tissue-resident memory cells during persistent viral infections, particularly in the central nervous system[37,38]. A recent study showed that HBV-ACLF patients have elevated proportions of Tfh cells and high serum IL-21 levels, displaying a strong correlation with MELD scores. Notably, in HBV-ACLF patients, CD4+ T cells tend to differentiate into Tfh cells under the influence of the IL-21-rich serum, a process effectively suppressed by IL-21 antibodies. Furthermore, the induced Tfh cells facilitate the proliferation and IgG production of B cells, as indicated by significant increases in the number of CD19+ B cells and in serum and hepatic IgG/M levels[39]. These findings underscore the importance of IL-21 in the pathogenesis of liver diseases and its potential as a therapeutic target in LF.
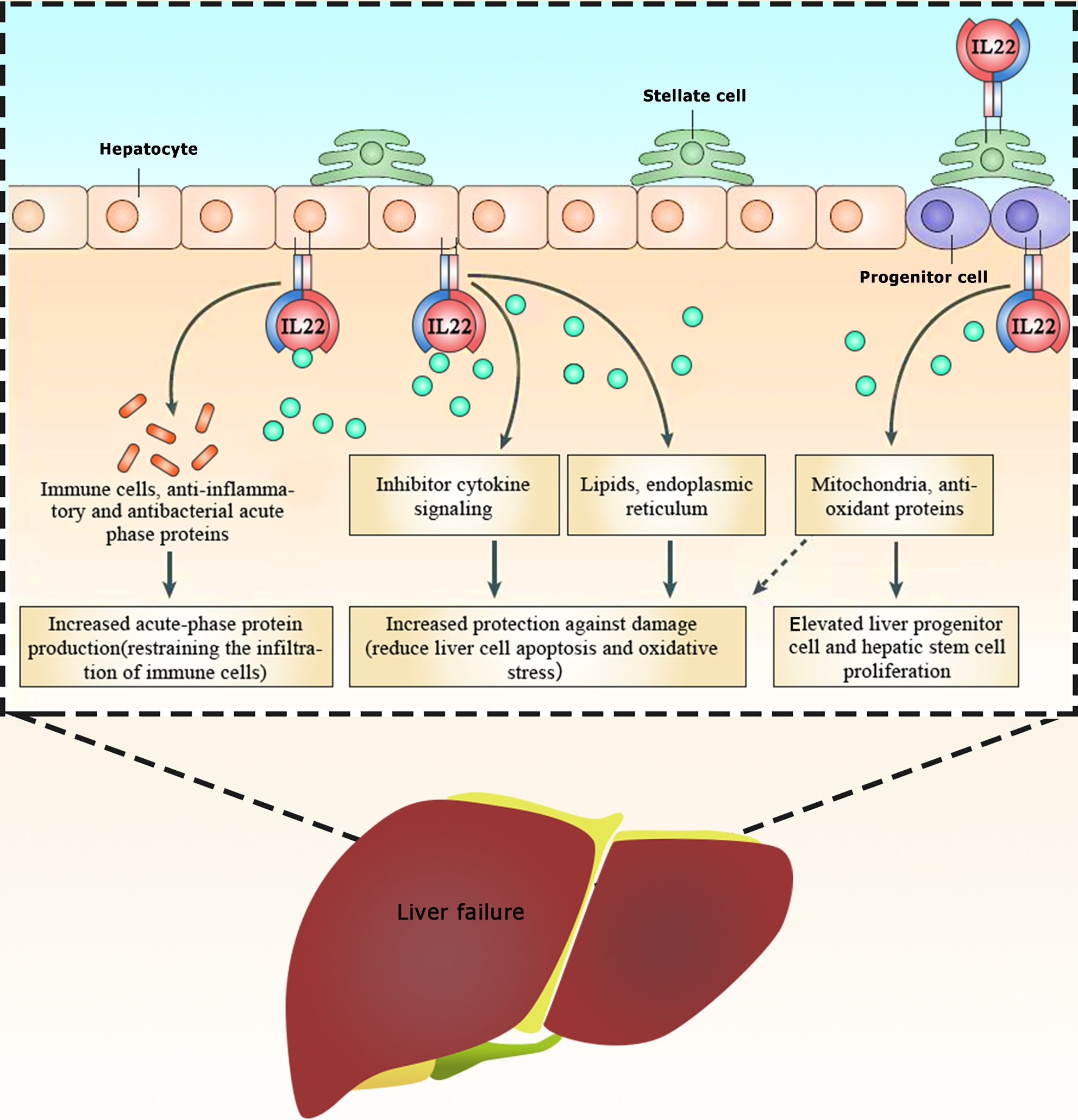
IL-22, discovered in 2000, is secreted by activated T cells. It is a member of the IL-10 family of cytokines, which consists of IL-19, IL-20, IL-24, IL-26, IL-28, and IL-29. IL-22 is expressed by various types of lymphocytes, including innate and acquired immune cells, such as CD4+ T cells (especially Th17 cells), δγ T cells, and NK cells[40]. All of these different cell subpopulations express IL-22 with similar features (e.g., similar activation receptors and transcription factors) as well as unique features. For example, IL-22 is produced by activated immune cells during inflammation or infection. Its primary mechanism involves binding to IL-22Rs specifically expressed on the surface of epithelial and stromal cells, thereby exerting its effects on these cell types[41]. Upon binding to the IL-22R, IL-22 triggers processes such as cell proliferation and tissue remodeling and repair in diverse tissues and organs. This process supports the innate defense mechanism of the host against pathogenic intrusion, thereby playing a crucial role in fostering antibacterial immunity, inflammation, and tissue repair at barrier surfaces[42]. The IL-22R is a heterodimer complex composed of IL-22R1 and IL-10R2, which belong to the type II cytokine receptor family[43]. IL-22R1 is expressed in various non-immune organs, such as the liver, skin, lungs, small intestine, colon, kidneys, and pancreas, whereas IL-10R2 is widely expressed in immune cells[44]. In addition to its functional receptor, IL-22 has an endogenous antagonist called IL-22-binding protein (IL-22BP, also known as IL-22RA2)[45]. Upon binding to its receptor, IL-22 activates STAT3 primarily through the JAK-STAT pathway, in addition to STAT1 and STAT5. It can also trigger the nuclear factor kappa B (NF-kB), mitogen-activated protein kinase, and phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathways[46-48] (Figure 2). IL-22-induced signaling pathways can upregulate genes that counteract inflammation or apoptosis and those promoting cell proliferation, thereby contributing to the cellular mechanisms associated with local tissue regeneration and host defense[49,50]. Clinical studies have indicated that patients with chronic HBV or hepatitis C virus infections exhibit elevated levels of IL-22 and an abundance of IL-22-producing cells in the liver compared with healthy individuals[51,52]. Numerous in vitro and animal experiments have shown that IL-22 has a strong protective effect against hepatocyte damage[53,54]. The specific actions of IL-22 include stimulating STAT3-dependent cell division in injured hepatocytes and liver stem cells, enhancing hepatocyte fibroblast growth factor 3 expression and restoring hepatocyte function. It has several mechanisms of action. First, it prevents hepatocyte apoptosis by regulating lipid metabolism, alleviating oxidation, and attenuating endoplasmic reticulum stress[57]. Second, it shields hepatocytes from oxidative stress by inhibiting mitochondrial apoptosis and boosting antioxidant protein levels[58]. Third, it regulates immune cells via certain pathways such as nuclear factor erythroid 2-related factor 2/heme oxygenase 1, TNF-α/NF-κB, and JNK/STAT3 signaling to induce the production of anti-inflammatory and antibacterial proteins, thereby restraining immune cell infiltration[59,60]. Finally, it binds to the highly expressed receptors IL-22R1 and IL-10R2 to activate STAT3 in hepatic stellate cells, thereby upregulating inhibitory factors in these cells, reducing liver fibrosis, and ameliorating alcoholic liver injury[61,62] (Figure 3). LF mainly encompasses ALF and ACLF. In a rat model of ALF induced by d-galactosamine-positive (D-GalN+) lipopolysaccharides (LPS), the mortality rate of the rats was 20% after 48 h of D-GalN/LPS administration, along with increased levels of indicators such as AST, ALT, alkaline phosphatase, TBIL, prothrombin time, TNF-α, and cyclooxygenase-2 (COX-2). However, IL-22 treatment completely reversed the mortality, improved the abnormal biochemical and serological parameters, and reduced TNF-α and COX-2 levels in the liver[63]. Similarly, IL-22 has shown promise in acetaminophen (APAP)-induced acute liver injury, notably reducing serum ALT levels and liver necrosis in mice with early intervention. These processes may be related to factors that induce the IL-22/STAT3 axis, including cyclin signal-3, lipocalin-2, and alpha 1-antichymotrypsin. Furthermore, after 2 h of APAP administration, IL-22 showed unique antioxidant and anti-inflammatory effects[64], possibly due to IL-22 controlling the activity of metabolic regulators and enzymes by inducing AMP-activated protein kinase, AKT, and mTOR. This process aids in restoring mitochondrial integrity, reducing reactive oxygen species accumulation, and suppressing mitochondrial dysfunction[65] (Figure 3). IL-22 has shown a similar effect in cases of concanavalin A (ConA)-induced ALF[66,67]. A study showed that recombinant IL-22 could suppress ConA-induced ALF, whereas IL-22 deficient mice were more sensitive to ConA-induced ALF than wild-type mice. This pattern is also evident in the liver-injury model induced by carbon tetrachloride (CCl4)[68]. Similarly, IL-22Fc treatment 24 h after CCl4-induced acute liver injury in rats increased their survival rate, reduced serum ALT levels, and improved the bacterial load. This effect is achieved by IL-22Fc reprogramming the liver-regeneration signaling pathway, i.e. boosting liver regeneration by activating the STAT3 pathway while suppressing the STAT1 pathway. In addition, Schwarzkopf et al[69] observed in clinical practice that high levels of IL-22 and low IL-22BP/IL-22 ratios are associated with ACLF and mortality in patients with liver cirrhosis. High levels of IL-22BP in ACLF patients may inhibit hepatocyte IL-22 signaling, where excessive secretion of IL-22BP can neutralize IL-22, potentially preventing the hepatoprotective effects of IL-22 in patients with liver cirrhosis[69]. Mo et al[70] found that patients with HBV-ACLF had increased peripheral blood Th22/Th17 cells as well as elevated plasma IL-22 and IL-17 levels but decreased Th1 cells compared with healthy controls. Subsequent analyses have confirmed these findings, showing that elevated plasma levels of IL-22 were associated with lower survival rates in HBV-associated ACLF patients[70]. A study by Khanam et al[71] supported these conclusions but also indicated that serum IL-22 levels was positively correlated with CCR6 and ALT levels in HBV-ACLF[71-75].
IL-31, a four-helix cytokine discovered by Dillon et al[75] in 2004, belongs to the IL-6 cytokine family. It is primarily secreted by activated CD4+ T lymphocytes, especially activated helper Th2 cells, mast cells, monocytes/macrophages, and dendritic cells[72]. Studies have shown that monocytes can express the IL-31RA subunit when co-stimulated with IFN-γ and LPS[73,74]. Notably, LPS can activate cells via Toll-like receptor 4, which is crucial for driving adaptive Th1 responses. Moreover, IL-31 has been observed to induce Th2 responses in mouse models of allergic sensitization[75], suggesting that it aids antigen presentation through monocytes or dendritic cells. Furthermore, IL-31 has been identified as a significant player in cell proliferation, activation, migration, inflammation, and allergic reactions, engaging with signaling pathways such as MAPK/Akt, JAK/STAT, PI3K/AKT, and extracellular signal-regulated kinase (ERK)/MAPK[76-78] (Figure 2). In studies on LF, IL-31 was positively correlated with the serum levels of transforming growth factor-β1 (TGF-β1), TBIL, and alpha-fetoprotein (AFP) in patients with HBV-ACLF. Conversely, recovery of the liver damage in HBV-ACLF was accompanied by decreased levels of IL-31 and TGF-β1, which were significantly upregulated in deceased HBV-ACLF patients. Notably, the levels of IL-31/TGF-β1 exhibited high sensitivity and specificity in predicting the survival rates of patients with HBV-ACLF[79]. Furthermore, the TGF-β/IL-31 axis has been implicated in HBV-associated cirrhosis. Increased serum levels of TGF-β1 and IL-31 have been observed, demonstrating a positive correlation with albumin, AFP, creatinine, white blood cell count, and platelet level, in individuals with HBV-associated cirrhosis. This association serves as a potential early warning indicator in cirrhotic patients without esophageal varices[80]. Recent studies have connected serum IL-31 levels to cholestatic diseases complicated by nonalcoholic steatohepatitis, where IL-31 mRNA levels were closely related to the concentration of bile acid and determined the degree of pruritus in such patients[81]. In terms of its function, TGF-β1 is a 25-kDa homologous dimer protein consisting of two subunits linked by disulfide bonds and is a potent inhibitor of DNA synthesis and cell proliferation[82]. Relevant studies have found that serum and hepatic TGF-β1 levels are significantly increased in patients with fulminant LF compared with healthy individuals[83]. Additionally, TGF-β1 has been shown to inhibit liver regeneration in mice with fulminant LF by promoting fibrosis and hepatocyte apoptosis[84].
The liver has a strong regenerative capacity, offering potential for intervention at various stages of LF through timely warnings and appropriate treatments. However, the management of LF is currently challenging due to its intricate pathogenesis and diverse etiologies, leading to limitations in diagnostic and therapeutic approaches. IL-21, IL-22, and IL-31 have emerged as promising candidates for diagnosing, predicting, and treating LF, with correlations observed in liver biochemical function (ALT, AST, TBIL, and AFP), underlying liver diseases (cirrhosis, hepatitis B, and liver fibrosis), immune system dynamics (DC, T, B, NKT, and CD4+ T cells), LF inducers (CCl4, LPS, ConA, and APAP), and inflammatory pathways (MAPK/Akt, JAK/STAT, PI3K/AKT, and ERK/MAPK) that involve factors such as NF-κB, IFN-γ, TNF-α, and Toll-like receptors. To enhance our understanding of LF, future efforts should focus on identifying effective early warning signs and therapeutic indicators. The exhaustive exploration and validation of IL-21, IL-22, and IL-31 and their associations with liver and inflammatory disorders may provide promising avenues for advancing our comprehension and devising efficient interventions.
I would like to thank Xing Lin for her assistance in the digital compilation of this paper.
| 1. | Wang J, Liu Y, Ding H, Shi X, Ren H. Mesenchymal stem cell-secreted prostaglandin E(2) ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization. Stem Cell Res Ther. 2021;12:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Shah S, Goldberg DS. Acute-on-chronic liver failure: update on pathogenesis, therapeutic targets, predictive models, and liver transplantation. Curr Opin Gastroenterol. 2021;37:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Kok B, Karvellas CJ. Management of Cerebral Edema in Acute Liver Failure. Semin Respir Crit Care Med. 2017;38:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Kuroda H, Abe T, Fujiwara Y, Nagasawa T, Suzuki Y, Kakisaka K, Takikawa Y. Contrast-Enhanced Ultrasonography-Based Hepatic Perfusion for Early Prediction of Prognosis in Acute Liver Failure. Hepatology. 2021;73:2455-2467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Bernal W, Wang Y, Maggs J, Willars C, Sizer E, Auzinger G, Murphy N, Harding D, Elsharkawy A, Simpson K, Larsen FS, Heaton N, O'Grady J, Williams R, Wendon J. Development and validation of a dynamic outcome prediction model for paracetamol-induced acute liver failure: a cohort study. Lancet Gastroenterol Hepatol. 2016;1:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Xiao LL, Wu XX, Chen JJ, Yan D, Shi DY, Huang JR, Xu XW, Li LJ. Progress in hepatitis B virus-related acute-on-chronic liver failure treatment in China: A large, multicenter, retrospective cohort study using a propensity score matching analysis. Hepatobiliary Pancreat Dis Int. 2021;20:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3074] [Cited by in RCA: 3361] [Article Influence: 168.1] [Reference Citation Analysis (0)] |
| 8. | Raudenská M, Balvan J, Masařík M. Cell death in head and neck cancer pathogenesis and treatment. Cell Death Dis. 2021;12:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Castillo-Dela Cruz P, Wanek AG, Kumar P, An X, Elsegeiny W, Horne W, Fitch A, Burr AHP, Gopalakrishna KP, Chen K, Methé BA, Canna SW, Hand TW, Kolls JK. Intestinal IL-17R Signaling Constrains IL-18-Driven Liver Inflammation by the Regulation of Microbiome-Derived Products. Cell Rep. 2019;29:2270-2283.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Dutta S, Chakraborty AK, Dey P, Kar P, Guha P, Sen S, Kumar A, Sen A, Chaudhuri TK. Amelioration of CCl4 induced liver injury in swiss albino mice by antioxidant rich leaf extract of Croton bonplandianus Baill. PLoS One. 2018;13:e0196411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Das R, Tripathy A. Increased expressions of NKp44, NKp46 on NK/NKT-like cells are associated with impaired cytolytic function in self-limiting hepatitis E infection. Med Microbiol Immunol. 2014;203:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Nagai K. Co-inhibitory Receptor Signaling in T-Cell-Mediated Autoimmune Glomerulonephritis. Front Med (Lausanne). 2020;7:584382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Hasan I, Gani RA, Lesmana LA, Kresno SB, Pandelaki J, Suwarto S. The Association between Peripheral Th17, Th1, IL-17, and IFN-γ Levels and TACE Response in Patients with Unresectable Hepatocellular Carcinoma with or without Cirrhosis. Acta Med Indones. 2020;52:326-333. [PubMed] |
| 14. | Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1330] [Cited by in RCA: 1539] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 15. | Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, Zhang S, Xu Z, Wu Y, Yan H, Chen Z. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 16. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 559] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 17. | Balmasova IP, Yushchuk ND, Mynbaev OA, Alla NR, Malova ES, Shi Z, Gao CL. Immunopathogenesis of chronic hepatitis B. World J Gastroenterol. 2014;20:14156-14171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 18. | Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701-21.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 579] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 19. | Chang L, Wu H, Huang W, Li Y, Chen Y, Li X, Yao Z, Chen X, Lai X, Zheng R, Huang Z, Wu X, Zhang G. IL-21 induces pyroptosis of Treg cells via Akt-mTOR-NLRP3-caspase 1 axis in eosinophilic chronic rhinosinusitis. J Allergy Clin Immunol. 2023;152:641-655.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 20. | Abhiraman GC, Bruun TUJ, Caveney NA, Su LL, Saxton RA, Yin Q, Tang S, Davis MM, Jude KM, Garcia KC. A structural blueprint for interleukin-21 signal modulation. Cell Rep. 2023;42:112657. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Cagdas D, Mayr D, Baris S, Worley L, Langley DB, Metin A, Aytekin ES, Atan R, Kasap N, Bal SK, Dmytrus J, Heredia RJ, Karasu G, Torun SH, Toyran M, Karakoc-Aydiner E, Christ D, Kuskonmaz B, Uçkan-Çetinkaya D, Uner A, Oberndorfer F, Schiefer AI, Uzel G, Deenick EK, Keller B, Warnatz K, Neven B, Durandy A, Sanal O, Ma CS, Özen A, Stepensky P, Tezcan I, Boztug K, Tangye SG. Genomic Spectrum and Phenotypic Heterogeneity of Human IL-21 Receptor Deficiency. J Clin Immunol. 2021;41:1272-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Ahmad SF, Ansari MA, Nadeem A, Bakheet SA, Alsanea S, Al-Hosaini KA, Mahmood HM, Alzahrani MZ, Attia SM. Inhibition of tyrosine kinase signaling by tyrphostin AG126 downregulates the IL-21/IL-21R and JAK/STAT pathway in the BTBR mouse model of autism. Neurotoxicology. 2020;77:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Vallières F, Girard D. Mechanism involved in interleukin-21-induced phagocytosis in human monocytes and macrophages. Clin Exp Immunol. 2017;187:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Jen HY, Yang YH, Chiang BL, Chuang YH. Upregulated interleukin-21 receptor on B cells associated with the downregulation of IgE in patients with allergic rhinitis. J Interferon Cytokine Res. 2015;35:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Ryu JG, Lee J, Kim EK, Seo HB, Park JS, Lee SY, Moon YM, Yoo SH, Park YW, Park SH, Cho ML, Kim HY. Treatment of IL-21R-Fc control autoimmune arthritis via suppression of STAT3 signal pathway mediated regulation of the Th17/Treg balance and plasma B cells. Immunol Lett. 2015;163:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Caprioli F, Sarra M, Caruso R, Stolfi C, Fina D, Sica G, MacDonald TT, Pallone F, Monteleone G. Autocrine regulation of IL-21 production in human T lymphocytes. J Immunol. 2008;180:1800-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Davis ID, Skak K, Smyth MJ, Kristjansen PE, Miller DM, Sivakumar PV. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin Cancer Res. 2007;13:6926-6932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Ren HM, Lukacher AE, Rahman ZSM, Olsen NJ. New developments implicating IL-21 in autoimmune disease. J Autoimmun. 2021;122:102689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Spolski R, Leonard WJ. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol. 2008;20:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Hu X, Ma S, Huang X, Jiang X, Zhu X, Gao H, Xu M, Sun J, Abbott WG, Hou J. Interleukin-21 is upregulated in hepatitis B-related acute-on-chronic liver failure and associated with severity of liver disease. J Viral Hepat. 2011;18:458-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Zhu L, Chen T, Lu Y, Wu D, Luo X, Ning Q. Contribution of IL-17 to mouse hepatitis virus strain 3-induced acute liver failure. J Huazhong Univ Sci Technolog Med Sci. 2012;32:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Liu T, Song J, Zhang M, Li S, Zhang J, Hu X, Zhao Z, Peng W, Wu Q, Bai H, Li Y, Lu X, Ying B. Interleukin-21 receptor gene polymorphism is associated with hepatitis B virus-related hepatocellular carcinoma in Chinese patients. J Clin Lab Anal. 2019;33:e22860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 33. | Chen HM, Liu HL, Yang YC, Cheng XL, Wang YF, Xing FF, Zhao YR. Serum IL-21 levels associated with chronic hepatitis B and hepatitis B-related liver failure. Exp Ther Med. 2014;7:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Pan XF, Zhang K, Yang XA, Li XJ, Xu QH. [Interleukin-21 expression in serum of patients with acute-on-chronic liver failure and its significance]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2012;26:477-479. [PubMed] |
| 35. | Islam M, Sevak JK, Sharma MK, Jindal A, Vyas AK, Bajpai M, Ramakrishna G, Sarin SK, Trehanpati N. Immune predictors of hepatitis B surface antigen seroconversion in patients with hepatitis B reactivation. Aliment Pharmacol Ther. 2023;57:689-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Liu Y, Hu X, Hu X, Yu L, Ji H, Li W, Cai Y, Cheng G, Jiang Y. T follicular helper cells improve the response of patients with chronic hepatitis B to interferon by promoting HBsAb production. J Gastroenterol. 2022;57:30-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Ren HM, Kolawole EM, Ren M, Jin G, Netherby-Winslow CS, Wade Q, Shwetank, Rahman ZSM, Evavold BD, Lukacher AE. IL-21 from high-affinity CD4 T cells drives differentiation of brain-resident CD8 T cells during persistent viral infection. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 38. | Chen T, Ding X, Liao Q, Gao N, Chen Y, Zhao C, Zhang X, Xu J. IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Du B, Teng J, Yin R, Tian Y, Jiang T, Du Y, Cai W. Increased Circulating T Follicular Helper Cells Induced via IL-12/21 in Patients With Acute on Chronic Hepatitis B Liver Failure. Front Immunol. 2021;12:641362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 326] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 41. | Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, Wherry EJ, Koni PA, Bushman FD, Elson CO, Eberl G, Artis D, Sonnenberg GF. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 618] [Cited by in RCA: 617] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 42. | Sakamoto K, Kim YG, Hara H, Kamada N, Caballero-Flores G, Tolosano E, Soares MP, Puente JL, Inohara N, Núñez G. IL-22 Controls Iron-Dependent Nutritional Immunity Against Systemic Bacterial Infections. Sci Immunol. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Kumar P, Rajasekaran K, Palmer JM, Thakar MS, Malarkannan S. IL-22: An Evolutionary Missing-Link Authenticating the Role of the Immune System in Tissue Regeneration. J Cancer. 2013;4:57-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Xin N, Namaka MP, Dou C, Zhang Y. Exploring the role of interleukin-22 in neurological and autoimmune disorders. Int Immunopharmacol. 2015;28:1076-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Hebert KD, Mclaughlin N, Galeas-Pena M, Zhang Z, Eddens T, Govero A, Pilewski JM, Kolls JK, Pociask DA. Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection. Mucosal Immunol. 2020;13:64-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Gluud M, Pallesen EMH, Buus TB, Gjerdrum LMR, Lindahl LM, Kamstrup MR, Bzorek M, Danielsen M, Bech R, Monteiro MN, Blümel E, Willerslev-Olsen A, Lykkebo-Valløe A, Vadivel CK, Krejsgaard T, Bonefeld CM, Geisler C, Becker JC, Koralov SB, Iversen L, Litman T, Woetmann A, Ødum N. Malignant T cells induce skin barrier defects through cytokine-mediated JAK/STAT signaling in cutaneous T-cell lymphoma. Blood. 2023;141:180-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 47. | Chamcheu JC, Adhami VM, Esnault S, Sechi M, Siddiqui IA, Satyshur KA, Syed DN, Dodwad SM, Chaves-Rodriquez MI, Longley BJ, Wood GS, Mukhtar H. Dual Inhibition of PI3K/Akt and mTOR by the Dietary Antioxidant, Delphinidin, Ameliorates Psoriatic Features In Vitro and in an Imiquimod-Induced Psoriasis-Like Disease in Mice. Antioxid Redox Signal. 2017;26:49-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 48. | Sudha Yalamarthi S, Puppala ER, Abubakar M, Saha P, Challa VS, Np S, Usn M, Gangasani JK, Naidu VGM. Perillyl alcohol inhibits keratinocyte proliferation and attenuates imiquimod-induced psoriasis like skin-inflammation by modulating NF-κB and STAT3 signaling pathways. Int Immunopharmacol. 2022;103:108436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Zai W, Chen W, Liu H, Ju D. Therapeutic Opportunities of IL-22 in Non-Alcoholic Fatty Liver Disease: From Molecular Mechanisms to Clinical Applications. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Wang P, Chan WK, Wang J, Yang Z, Wang Y. Role of IL-22 in intestinal microenvironment and potential targeted therapy through diet. Immunol Res. 2023;71:121-129. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Cobleigh MA, Robek MD. Protective and pathological properties of IL-22 in liver disease: implications for viral hepatitis. Am J Pathol. 2013;182:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Sertorio M, Hou X, Carmo RF, Dessein H, Cabantous S, Abdelwahed M, Romano A, Albuquerque F, Vasconcelos L, Carmo T, Li J, Varoquaux A, Arnaud V, Oliveira P, Hamdoun A, He H, Adbelmaboud S, Mergani A, Zhou J, Monis A, Pereira LB, Halfon P, Bourlière M, Parana R, Dos Reis M, Gonnelli D, Moura P, Elwali NE, Argiro L, Li Y, Dessein A. IL-22 and IL-22 binding protein (IL-22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. Hepatology. 2015;61:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 53. | Lai R, Xiang X, Mo R, Bao R, Wang P, Guo S, Zhao G, Gui H, Wang H, Bao S, Xie Q. Protective effect of Th22 cells and intrahepatic IL-22 in drug induced hepatocellular injury. J Hepatol. 2015;63:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Fernandez-Botran R, Plankey MW, Ware D, Bordon J. Changes in liver steatosis in HIV-positive women are associated with the BMI, but not with biomarkers. Cytokine. 2021;144:155573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, Gershwin ME, Gao B. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012;143:188-98.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 56. | Tang TT, Li YY, Li JJ, Wang K, Han Y, Dong WY, Zhu ZF, Xia N, Nie SF, Zhang M, Zeng ZP, Lv BJ, Jiao J, Liu H, Xian ZS, Yang XP, Hu Y, Liao YH, Wang Q, Tu X, Mallat Z, Huang Y, Shi GP, Cheng X. Liver-heart crosstalk controls IL-22 activity in cardiac protection after myocardial infarction. Theranostics. 2018;8:4552-4562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Hwang S, He Y, Xiang X, Seo W, Kim SJ, Ma J, Ren T, Park SH, Zhou Z, Feng D, Kunos G, Gao B. Interleukin-22 Ameliorates Neutrophil-Driven Nonalcoholic Steatohepatitis Through Multiple Targets. Hepatology. 2020;72:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 58. | Li W, Jiang H, Bai C, Yu S, Pan Y, Wang C, Li H, Li M, Sheng Y, Chu F, Wang J, Chen Y, Li J, Jiang J. Ac2-26 attenuates hepatic ischemia-reperfusion injury in mice via regulating IL-22/IL-22R1/STAT3 signaling. PeerJ. 2022;10:e14086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Elshal M, Hazem SH. Escin suppresses immune cell infiltration and selectively modulates Nrf2/HO-1, TNF-α/JNK, and IL-22/STAT3 signaling pathways in concanavalin A-induced autoimmune hepatitis in mice. Inflammopharmacology. 2022;30:2317-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 60. | Feng D, Wang Y, Wang H, Weng H, Kong X, Martin-Murphy BV, Li Y, Park O, Dooley S, Ju C, Gao B. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol. 2014;193:2512-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Kong X, Feng D, Mathews S, Gao B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol. 2013;28 Suppl 1:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 63. | Ashour TH. Therapy with interleukin-22 alleviates hepatic injury and hemostasis dysregulation in rat model of acute liver failure. Adv Hematol. 2014;2014:705290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Scheiermann P, Bachmann M, Goren I, Zwissler B, Pfeilschifter J, Mühl H. Application of interleukin-22 mediates protection in experimental acetaminophen-induced acute liver injury. Am J Pathol. 2013;182:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 65. | Chen W, Zai W, Fan J, Zhang X, Zeng X, Luan J, Wang Y, Shen Y, Wang Z, Dai S, Fang S, Zhao Z, Ju D. Interleukin-22 drives a metabolic adaptive reprogramming to maintain mitochondrial fitness and treat liver injury. Theranostics. 2020;10:5879-5894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Rani R, Tandon A, Wang J, Kumar S, Gandhi CR. Stellate Cells Orchestrate Concanavalin A-Induced Acute Liver Damage. Am J Pathol. 2017;187:2008-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Yang F, Lou G, Zhou X, Zheng M, He J, Chen Z. MicroRNA-223 acts as an important regulator to Kupffer cells activation at the early stage of Con A-induced acute liver failure via AIM2 signaling pathway. Cell Physiol Biochem. 2014;34:2137-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 547] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 69. | Schwarzkopf K, Rüschenbaum S, Barat S, Cai C, Mücke MM, Fitting D, Weigert A, Brüne B, Zeuzem S, Welsch C, Lange CM. IL-22 and IL-22-Binding Protein Are Associated With Development of and Mortality From Acute-on-Chronic Liver Failure. Hepatol Commun. 2019;3:392-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Mo R, Wang P, Lai R, Li F, Liu Y, Jiang S, Zhao G, Guo S, Zhou H, Lin L, Lu J, Cai W, Wang H, Yu H, Bao S, Xiang X, Xie Q. Persistently elevated circulating Th22 reversely correlates with prognosis in HBV-related acute-on-chronic liver failure. J Gastroenterol Hepatol. 2017;32:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Khanam A, Trehanpati N, Sarin SK. Increased interleukin-23 receptor (IL-23R) expression is associated with disease severity in acute-on-chronic liver failure. Liver Int. 2019;39:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Alkon N, Assen FP, Arnoldner T, Bauer WM, Medjimorec MA, Shaw LE, Rindler K, Holzer G, Weber P, Weninger W, Freystätter C, Chennareddy S, Kinaciyan T, Farlik M, Jonak C, Griss J, Bangert C, Brunner PM. Single-cell RNA sequencing defines disease-specific differences between chronic nodular prurigo and atopic dermatitis. J Allergy Clin Immunol. 2023;152:420-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 73. | Mena J, Alloza I, Tulloch Navarro R, Aldekoa A, Díez García J, Villanueva Etxebarria A, Lindskog C, Antigüedad A, Boyero S, Mendibe-Bilbao MDM, Álvarez de Arcaya A, Sánchez Menoyo JL, Midaglia L, Villarrubia N, Malhotra S, Montalban X, Villar LM, Comabella M, Vandenbroeck K. Genomic Multiple Sclerosis Risk Variants Modulate the Expression of the ANKRD55-IL6ST Gene Region in Immature Dendritic Cells. Front Immunol. 2021;12:816930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Samorano LP, Manfrere KCG, Pereira NV, Takaoka R, Valente NYS, Sotto MN, Silva LFF, Sato MN, Aoki V. Methotrexate for refractory adult atopic dermatitis leads to alterations in cutaneous IL-31 and IL-31RA expression. An Bras Dermatol. 2024;99:72-79. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 75. | Edukulla R, Singh B, Jegga AG, Sontake V, Dillon SR, Madala SK. Th2 Cytokines Augment IL-31/IL-31RA Interactions via STAT6-dependent IL-31RA Expression. J Biol Chem. 2015;290:13510-13520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Che DN, Cho BO, Kim JS, Shin JY, Kang HJ, Jang SI. Luteolin and Apigenin Attenuate LPS-Induced Astrocyte Activation and Cytokine Production by Targeting MAPK, STAT3, and NF-κB Signaling Pathways. Inflammation. 2020;43:1716-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 77. | Ferretti E, Corcione A, Pistoia V. The IL-31/IL-31 receptor axis: general features and role in tumor microenvironment. J Leukoc Biol. 2017;102:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 78. | Che DN, Shin JY, Kang HJ, Cho BO, Kim YS, Jang SI. Luteolin suppresses IL-31 production in IL-33-stimulated mast cells through MAPK and NF-κB signaling pathways. Int Immunopharmacol. 2020;83:106403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Yu X, Guo R, Ming D, Deng Y, Su M, Lin C, Li J, Lin Z, Su Z. The Transforming Growth Factor β1/Interleukin-31 Pathway Is Upregulated in Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure and Is Associated with Disease Severity and Survival. Clin Vaccine Immunol. 2015;22:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 80. | Ming D, Yu X, Guo R, Deng Y, Li J, Lin C, Su M, Lin Z, Su Z. Elevated TGF-β1/IL-31 Pathway Is Associated with the Disease Severity of Hepatitis B Virus-Related Liver Cirrhosis. Viral Immunol. 2015;28:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Xu J, Wang Y, Khoshdeli M, Peach M, Chuang JC, Lin J, Tsai WW, Mahadevan S, Minto W, Diehl L, Gupta R, Trauner M, Patel K, Noureddin M, Kowdley KV, Gulamhusein A, Bowlus CL, Huss RS, Myers RP, Chung C, Billin AN. IL-31 levels correlate with pruritus in patients with cholestatic and metabolic liver diseases and is farnesoid X receptor responsive in NASH. Hepatology. 2023;77:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 82. | Strain AJ, Frazer A, Hill DJ, Milner RD. Transforming growth factor beta inhibits DNA synthesis in hepatocytes isolated from normal and regenerating rat liver. Biochem Biophys Res Commun. 1987;145:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Miwa Y, Harrison PM, Farzaneh F, Langley PG, Williams R, Hughes RD. Plasma levels and hepatic mRNA expression of transforming growth factor-beta1 in patients with fulminant hepatic failure. J Hepatol. 1997;27:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Yoshimoto N, Togo S, Kubota T, Kamimukai N, Saito S, Nagano Y, Endo I, Sekido H, Nagashima Y, Shimada H. Role of transforming growth factor-beta1 (TGF-beta1) in endotoxin-induced hepatic failure after extensive hepatectomy in rats. J Endotoxin Res. 2005;11:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |