Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5431
Revised: June 6, 2024
Accepted: June 26, 2024
Published online: August 16, 2024
Processing time: 81 Days and 20.4 Hours
Primary renal Ewing’s sarcoma (ES) is extremely rare, and only two cases causing Cushing’s syndrome (CS) have been reported to date. We report that the case of an 18-year-old patient is diagnosed primary renal ES with typical CS characterized by purple stripes, weight gain, and hypertension.
CS was first diagnosed by laboratory testing. A huge tumor was revealed in the kidney following an imaging examination. Moreover, brain and bone metastases were observed. After comprehensive treatment, primarily based on surgery, primary renal ES was pathologically diagnosed with a typical EWSR1-FLI1 genetic mutation through genetic testing. Furthermore, the glucocorticoid level returned to normal. By the ninth postoperative month of follow-up, the patient was recovering well. Cushing-related symptoms had improved, and a satisfactory curative effect was achieved.
Primary renal ES, a rare adult malignant tumor, can cause CS and a poor prognosis.
Core Tip: Primary renal Ewing's sarcoma (ES), a rare adult malignant tumor, can cause Cushing syndrome and a poor prognosis. The "gold standard" for diagnosing primary renal ES remains pathological examination, even though gene studies can aid in diagnosis and therapy planning. When treating advanced metastatic primary renal ES, a comprehensive surgical approach centered on surgery can produce specific outcomes.
- Citation: Dong GF, Hou YK, Ma Q, Ma SY, Wang YJ, Rexiati M, Wang WG. Cushing's syndrome caused by giant Ewing's sarcoma of the kidney: A case report and review of literature. World J Clin Cases 2024; 12(23): 5431-5440
- URL: https://www.wjgnet.com/2307-8960/full/v12/i23/5431.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i23.5431
Ewing’s sarcoma (ES) is a highly malignant tumor originating from the neuroectoderm and mainly occurs in children’s long bones, with an incidence rate of 1 case per 1.5 million[1]. ES in other tissues is even rarer. Related reports of relevant cases show that, to date, there have been no more than 200 cases of renal ES[2].
Cushing’s syndrome (CS) is a series of symptoms caused by excessive glucocorticoid secretion from the adrenal cortex. In general, some dispersed neuroendocrine system can secrete adrenocor ticotropic hormore (ACTH) or corticotropin releasing hormone (CRH), leading to CS, most commonly in small-cell bronchogenic carcinoma. CS is the rare symptom of primary renal ES, and only two cases have been reported. Herein, we report the case of an 18-year-old patient with typical CS and review related literature.
Weight gain and dizziness for three months.
The weight of the increased by 15 kg in three months, accompanied by purple lines in the bilateral armpits, abdomen, and thigh; dizziness; fatigue; and facial pigmentation.
The patient was in good physical health.
The patient had five siblings and no family history of the presentation.
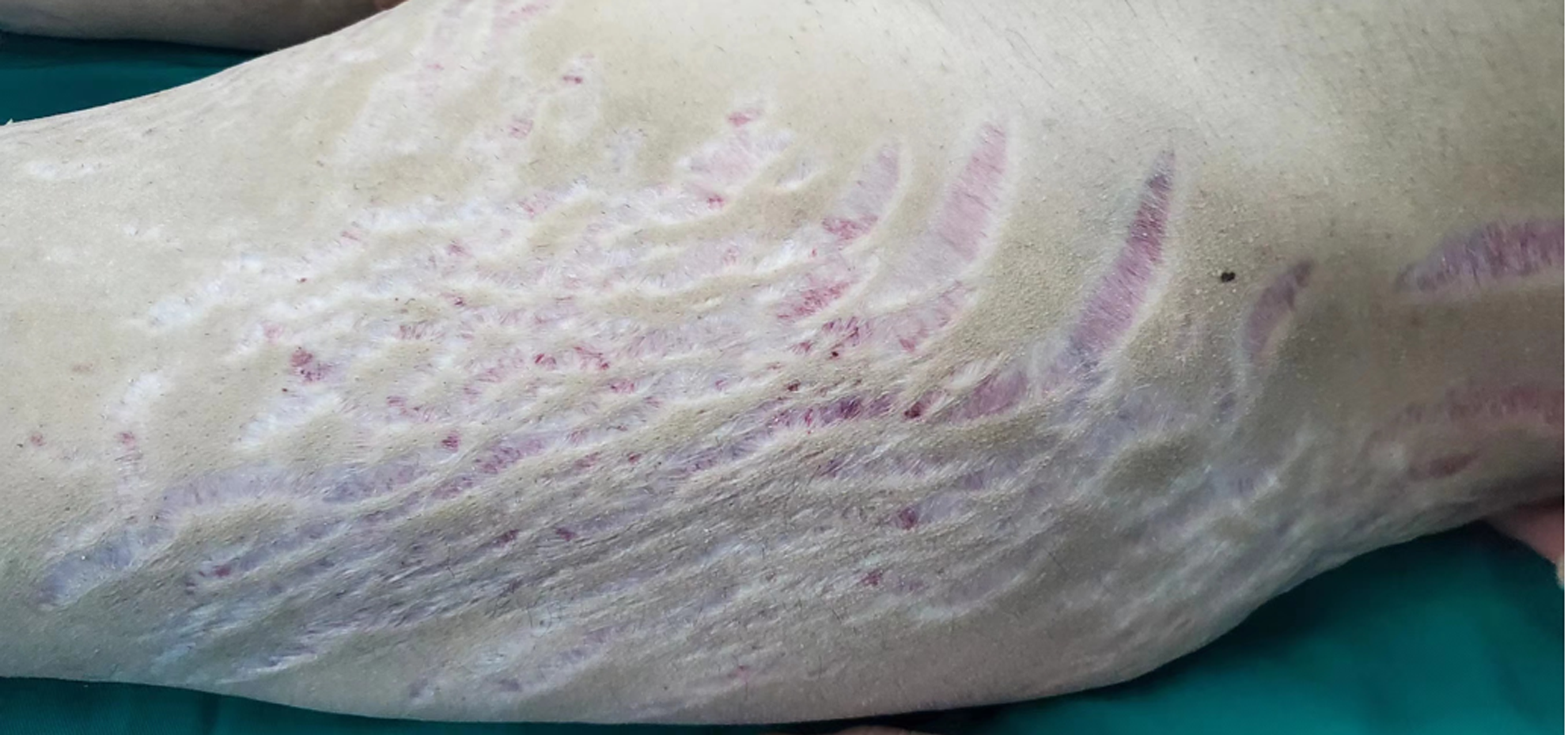
Physical examination revealed stable vital signs and a blood pressure, p(B) = 20.6/14.4 kpa. The patient also had a full-moon face, acne, buffalo back, obvious centripetal obesity, and relatively thin limbs. Pigmented, dark red, scattered ecchymosis could be observed on the left upper limb. Large purple stripes could be observed from the bilateral armpits, hips, and thighs to the knees (Figure 1).
The patient’s cortisol level was examined (Table 1). An increased cortisol secretion was observed, alongside a disordered rhythm and increased adrenocorticotropic hormone levels. Both low- and large-dose dexamethasone suppression tests were negative. The fasting blood glucose was 5.28 mmol/L, and the blood glucose level was 13.29 mmol/L 120 min after an oral glucose tolerance test (OGTT). This indicated that the patient had diabetes. His blood potassium level was 2.50 mmol/L and his blood calcium level was 2.05 mmol/L.
| Blood cortisol level at 0:00 in the morning (nmol/L) | Blood cortisol level at 8:00 in the morning (nmol/L) | Blood cortisol level at 16:00 in the evening (nmol/L) | Adrenocorticotropic hormone level (pg/mL) | 24-hour urinary free cortisol level (nmol/24 h) | |
| Reference range | 101.2–535.7 (before 8:00) | 79.0–477.8 (after 15:00) | 7.2–63.3 | 11.8–485.6 | |
| Patient baseline | 1293.2↑ | 1329.5↑ | 1479.1↑ | 96.5↑ | 10524.0↑ |
| Low-dose dexamethasone suppression test | 1526.6↑ | ||||
| High-dose dexamethasone suppression test | 1782.5↑ | ||||
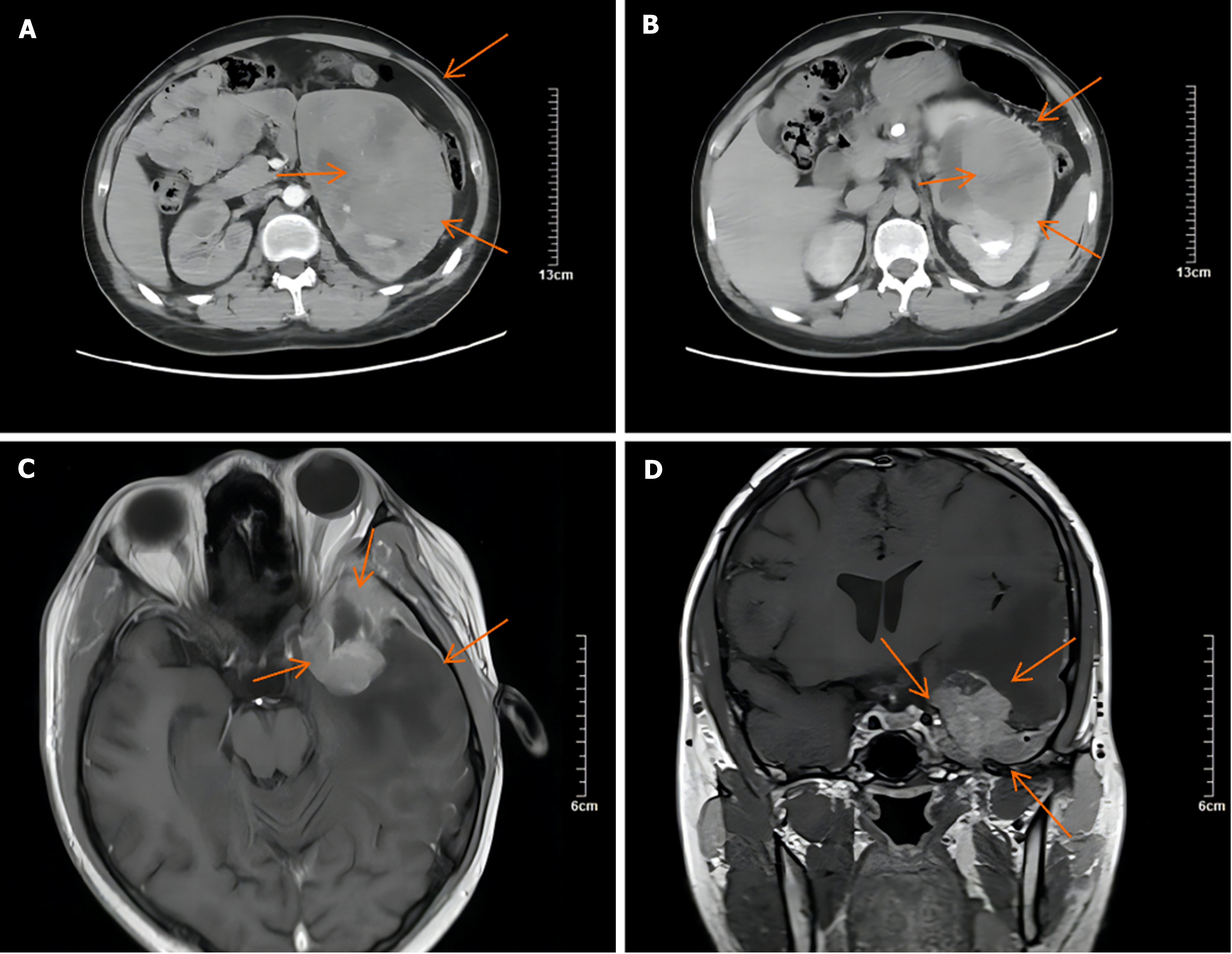
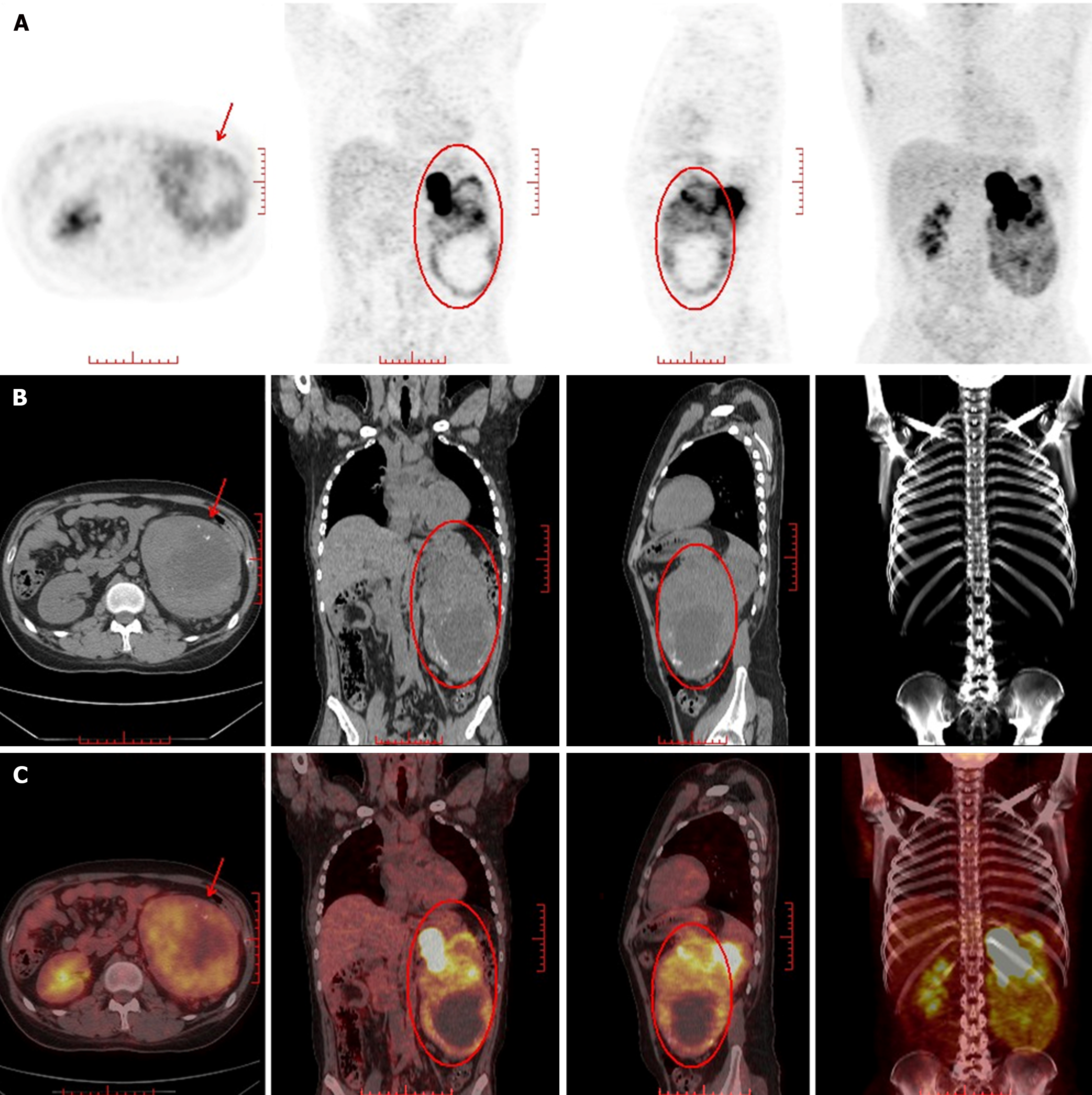
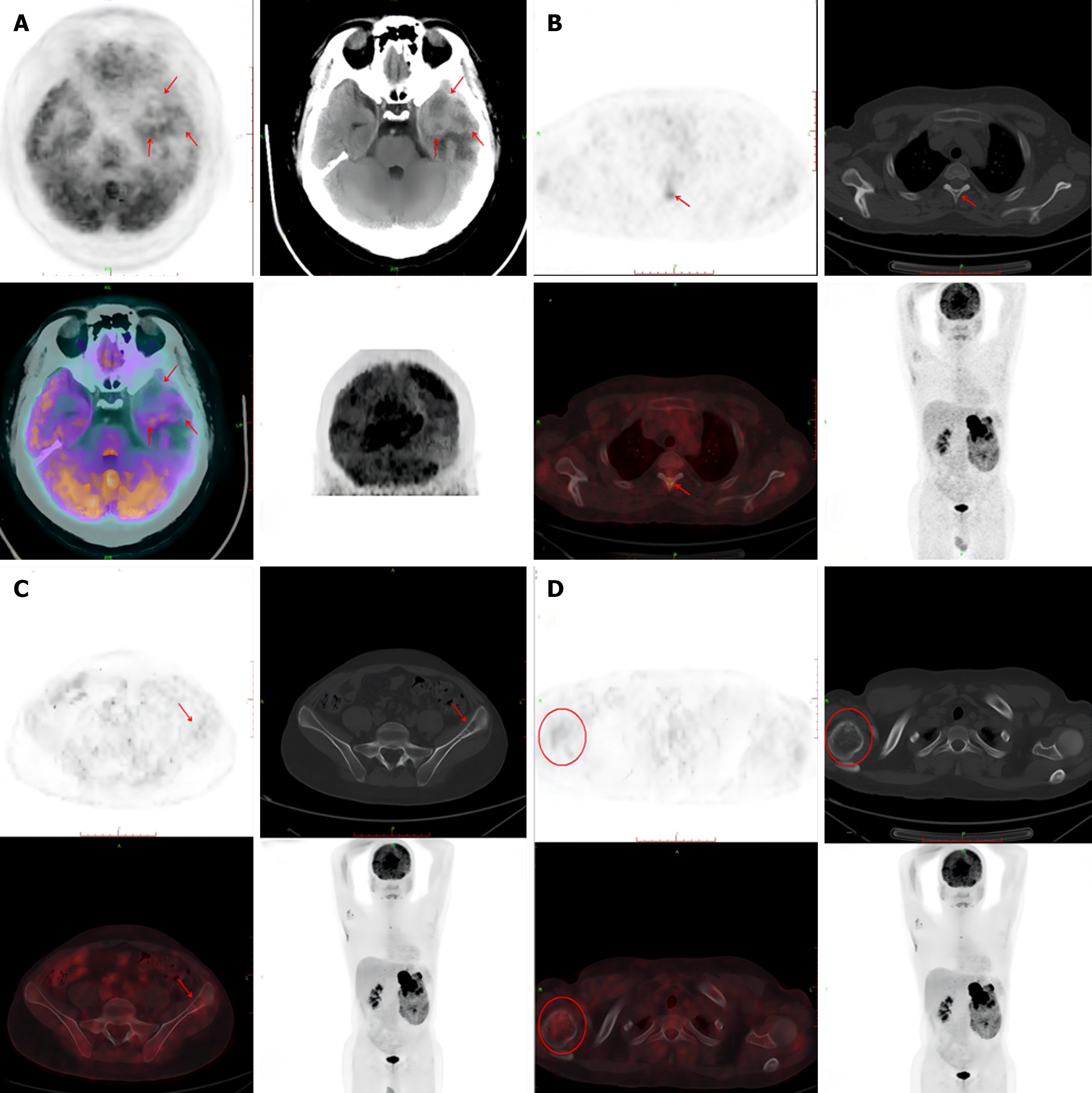
A computed tomography (CT) scan revealed an 11.65 cm × 10.91 cm nonuniform enhanced occupation in the middle and lower part of the left kidney. Magnetic resonance imaging (MRI) revealed that the left temporal lobe was occupied, and the pituitary gland was normal (Figure 2). Fluorine-18 fluorodeoxyglucose (FDG) PET/CT revealed a huge mixed space occupation in the left kidney (Figure 3), unevenly high uptake of the solid component FDG, and high uptake of FDG in the left temporal pole and thoracic 3 laminae of the vertebral arch. This indicated that the left kidney had a malignant tumor, and there were brain, vertebral body, and right femur metastases (Figure 4).
After a multidisciplinary discussion in the hospital, the preoperative diagnosis was a malignant tumor of the left kidney, bone metastasis, left temporal lobe secondary malignant tumor, secondary diabetes, secondary hypertension, CS, and hypokalemia. Comprehensive treatment based on surgery was determined.
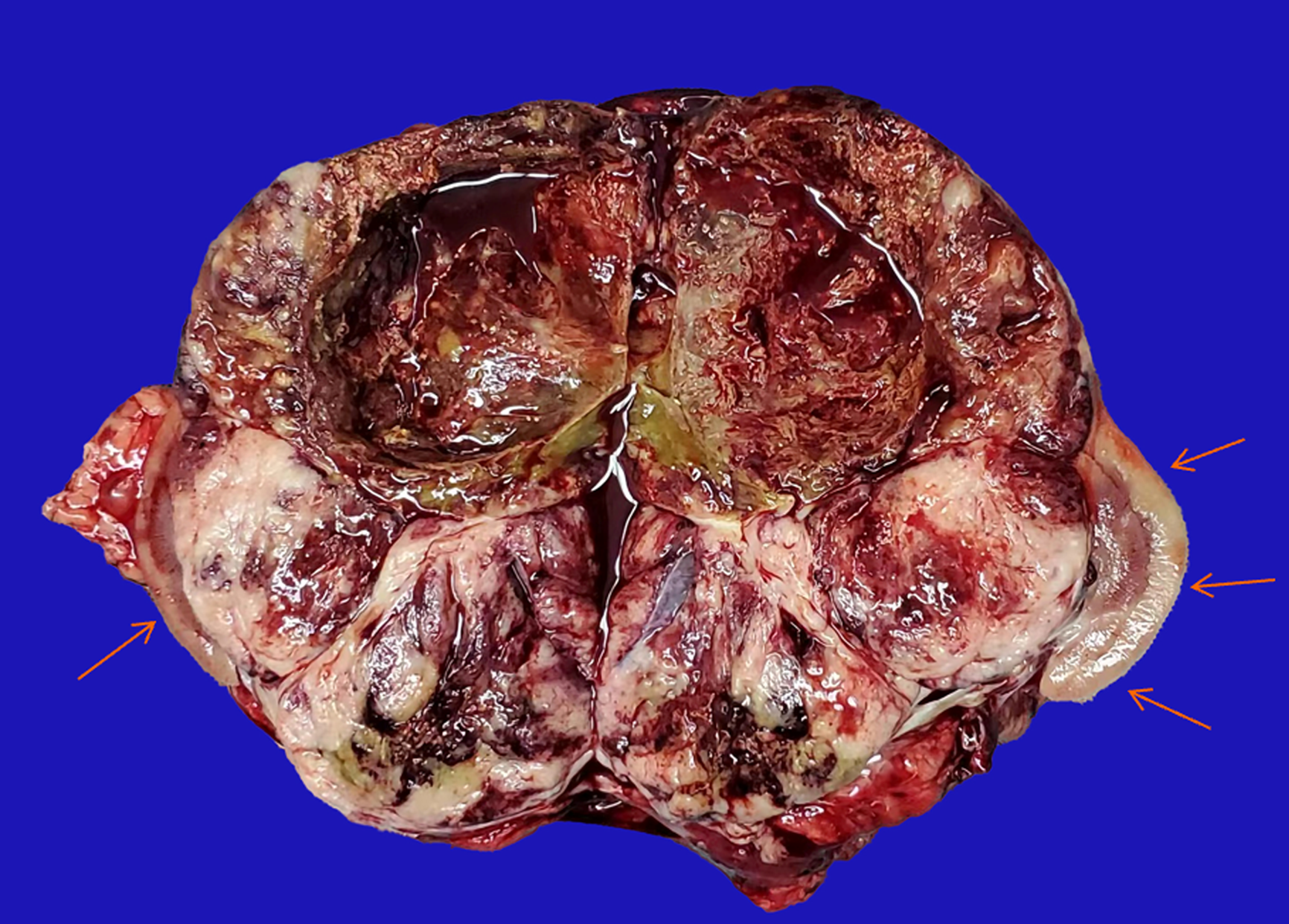
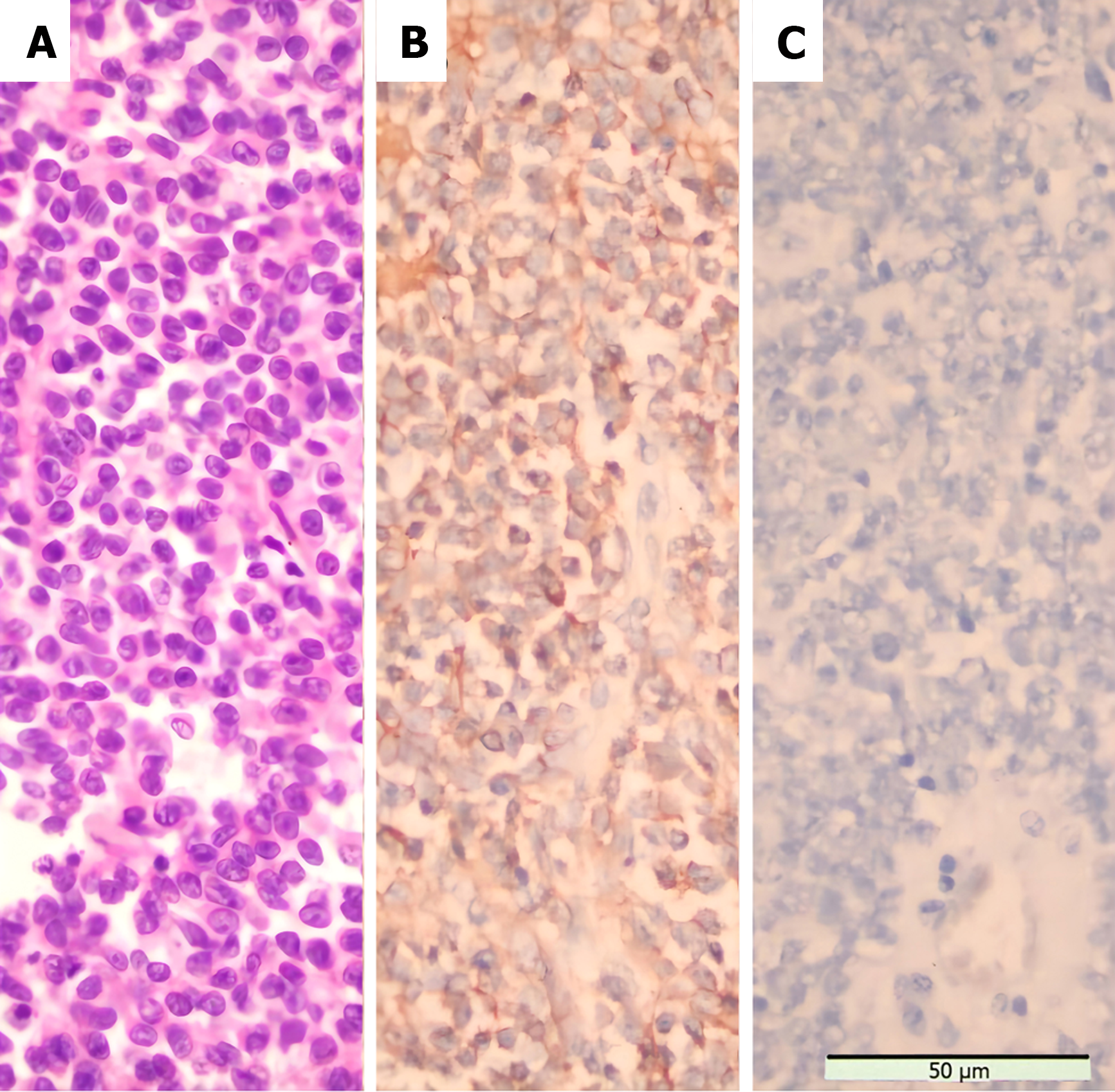
The patient underwent a Palliative nephrectomy (Figure 5). The relevant tissues were sent for pathological examination (Figure 6A), and all biochemical indicators recovered postoperatively (Table 2). On the second postoperative day, the cortisol levels were reviewed. On the third postoperative day, cortisol and ACTH levels returned to normal, and the blood potassium level recovered to 3.41 mmol/L. Primary renal ES (extraosseous ES) was confirmed by histopathological examination of the mass. No tumor accumulation was found in the perirenal fat and ureteral stump. Immunohistochemistry (Figure 6B) revealed CD99 (+), vimentin (VIM) (+), CD56 (+), synaptophysin (Syn) (less +), Ki-67 (hot spot 50% +), neuron-specific enolase (NSE) (focus +), CD34 (vascular +), AE1/AE3 (focus +), WT (-), D2-40 (-), Desmin (-), chromaffin A (CgA) (-), S-100 (-), epithelial membrane antigen (EMA) (-), and ACTH (-) (Figure 6C). Genetic test: Exactly 808 tumor-related hot spot genes (605 whole exon coding regions and 203 hot spot mutation regions) were analyzed. The mutation with clear clinical significance (primary mutation) (NTRK1, NTRK1-ZPBP) and the locus with potential clinical significance (secondary mutation) (CHEK2 c.444 + 1G > A, EWSR1 EWSR1-FLI1) were observed.
| The second day after surgery | The third day after surgery | The fourth day after surgery | |
| Blood cortisol level at 8:00 in the morning (nmol/L) | 1649.9↑ | 157.2 | 183.2 |
| Adrenocorticotropic hormone level (pg/mL) | 20.9 | 27.3 | |
| K+ | 2.83 | 3.41 |
The diagnosis was primary renal ES with CS.
The patient was transferred to the oncology department for postoperative radiotherapy and chemotherapy 1 month postoperatively. The chemotherapy regimen consisted of vincristine 2 mg d1 plus doxorubicin 50 mg d1-d2 plus cyclophosphamide 2 g d1. The chemotherapy cycle was 21 days. Due to the continuous increase in intracranial pressure in the patient alongside brain metastases, mannitol dehydration was ensured. Intensity-modulated radiotherapy was administered to the metastatic tumors in the left temporal lobe of the brain (3 Gy per fraction of 17 fractions of a total dose of 51 Gy). The patient continued chemotherapy for two courses. Owing to economic problems, chemotherapy was discontinued. The patient was followed up at 4 and 9 months after surgery.
The patient was followed up at 4 and 9 months post-surgery. Up to 9 months post-surgery, the patient was well-nourished, clear-minded, and free to move physically, with a weight loss of 2 kg. The patient reported no dizziness, nausea, vomiting, or facial acne. The purple stripes on the abdomen disappeared, and those from other body parts had reduced. The patient could perform day-to-day activities such as driving. The patient and his family were satisfied with the effectiveness of the treatment. However, the patient was eventually lost to follow-up two years after surgery
Primary renal ES/primary neuroectodermal tumors (PNETs) are extremely rare and highly malignant. Since Seemayer et al[3], the first reported renal PNET was in 1975, and the number of cases reported worldwide was less than 200. The pathological type of CS caused by ectopic ACTH/CRH in Primary renal ES is rare and is usually a neuroendocrine tumor and individual nephroblastoma[4]. The most prominent feature of our case was that the primary renal ES had neuroendocrine function and secreted ectopic ACTH/CRH, which led to CS. In many reports, primary renal ES does not involve neuroendocrine function. Moreover, most of the symptoms are nonspecific, majorly involving lumbar pain. Some patients also have macroscopic or microscopic hematuria[5-7]. According to the PubMed database, only a few studies have reported the terms “Ewing's sarcoma and Cushing’s syndrome” (four reports)[8-11], and even fewer studies have reported the term “Renal Ewing's sarcoma and Cushing’s syndrome” (two studies)[8,12].
Renal ES is difficult to diagnose and usually does not have specific imaging manifestations. CT usually reveals large heterogeneous masses accompanied by large necrosis and uneven enhancement. As renal ES is highly malignant, approximately 57% of patients already have metastasis at the time of diagnosis[13]. Therefore, it is necessary to perform enhanced CT and bone scans to detect metastasis in patients with suspected renal ES. PET-CT is more advantageous for identifying small metastases, through which we identified many small bone metastases in our case. All the above examinations lack specificity, and the final diagnosis requires histopathological manifestations and immunophenotyping. When it is necessary to differentiate between the pathological types of tumors, such as nephroblastoma and neuroblastoma, a clear diagnosis can be made by adding the corresponding molecular pathological tests. Using immunophenotyping, the examiner can specifically judge whether the tumor produces ectopic ACTH or CRH. Histologically, the tumor cells are distributed in slices or lobules under a microscope and are uniformly round and oval. The nuclei are large and hyperchromatic, and different degrees of karyokinesis are observed. The cytoplasm is clear, and some cells can be vacuolated. Homer-Wright chrysanthemums have relatively characteristic structures with varying degrees of neural differentiation[14].
Regarding immunohistochemistry, most primary renal ESs are positive for the transmembrane glycoprotein CD99, encoded by MIC2. In addition, the endocrine and neuroendocrine system markers NSE, CgA, and Syn can be positive. VIM, CD56, and hemophilia transcription integration factor 1 are also observed in some primary renal ESs. Moreover, epithelial markers, such as cytokeratin and EMA, are usually negative[14,15].
The first challenge the patient must face is the difficulty in diagnosis, where the patient first exhibits severe CS and then seeks the primary focus according to the principle of quality action before positioning. Low-dose dexamethasone failed to inhibit the secretion of cortisol, indicating that the patient had ACTH-dependent CS. The high-dose dexamethasone suppression test revealed a failure to inhibit cortisol secretion, suggesting that Cushing’s disease could be excluded. Therefore, the patient may have had ectopic ACTH syndrome or ectopic CRH syndrome. Immunohistochemistry revealed negative ACTH levels. Due to the limitations of technical conditions, immunohistochemical detection of CRH could not be performed; therefore, the tumor may have produced ectopic CRH or ACTH analogs.
Only 20 cases of ectopic CRH syndrome have been reported worldwide, mostly due to medullary thyroid carcinoma (33%) and pheochromocytoma (19%), as well as carcinoid (5%) and small cell lung cancer (9.5%), which are relatively rare. If a patient produces ectopic CRH, it is generally believed that a high-dose dexamethasone suppression test can reduce the cortisol levels[16]. However, the patient showed the opposite result when high-dose dexamethasone was administered, possibly because the auxo-action of ectopic CRH on ACTH exceeded the inhibition of high-dose dexamethasone on pituitary ACTH. Only three of the 20 reported cases presented the same situation as this patient[17-19].
Pro-opiomelanocortin (POMC), the precursor of ACTH, produces ACTH, melanocyte-stimulating hormone, and other products via a series of processes[20]. Incorrect processing of POMC in tumor cells is likely to produce derivatives with ACTH functions. In addition, false-negative immunohistochemical reactions may be caused by the inability to store and release ACTH rapidly after production.
The relevant reports are mostly case reports owing to the low incidence of primary renal ES. Currently, there is a lack of standard treatment plans, and the treatment mainly involves exaires is combined with radiotherapy, chemotherapy, or targeted therapy. Owing to the malignant degree of primary renal ES and the large volume of the tumor, radical nephrectomy is the first choice of surgery. There is a significant correlation between chemotherapy and overall survival (OS). Neoadjuvant chemotherapy can contribute to tumor downstaging and resect ability, improving the prognosis of patients[21-24]. In comparison, adjuvant chemotherapy can prevent tumor recurrence and metastasis[25]. However, owing to the lack of contrast tests, the existing evidence cannot determine which method is most beneficial to patients. The most commonly used treatments are VDC (vincristine + doxorubicin + decacyclophosphamide), IE (ifosfamide + etoposide), etc.[2,21,26]. In addition, in the EURO-E.W.I.N.G.99 experiment in Europe, the researchers used the VIDE protocol (vincristine + ifosfamide + doxorubicin + etoposide) during the induction period and the VAI protocol (vincristine + actinomycin + ifosfamide) or a large dose of busulfan/ melphalan followed by autologous stem cell transplantation according to risk stratification during the consolidation period for 24 patients with primary renal ES[18]. It is difficult to judge whether radiotherapy can benefit patients according to existing research; however, some researchers use radiotherapy to irradiate the surgical area after surgery, and reducing the residual focus significantly[21,27].
Furthermore, there have been few reports on targeted drug treatments for primary renal ES. To date, there are only a few reports on the treatment of ES using pazopanib[28]. Zhao et al[29]reported a case of partial remission after the use of apatinib. The prognosis of primary renal ES was poor. In a retrospective analysis of 48 patients, the average survival time of patients with metastatic diseases was 26.14 months[30]. The Anderson Cancer Center in the United States analyzed the 4-year median event-free survival (EFS) and OS rates of 30 patients with primary renal ES in a single center with no metastasis at the time of diagnosis. These were 54% and 85%, respectively. However, the incidence rates for patients with metastasis were 35% and 47%, respectively[21]. A previous study analyzed the 3-year median EFS and OS of 22 patients with primary renal ES in EURO-E.W.I.N.G.99, diagnosed as non-metastatic. These were 78% and 92%, respectively. For patients with metastasis, these were 45% and 58%, respectively[27]. Therefore, early detection and comprehensive treatment are key to improving the prognosis.
The second challenge is treatment. Our patient had primary renal ES with CS and extensive brain and bone metastases at the time of diagnosis. Considering that the patient had severe CS and metabolic disorder, an open total left nephrectomy was performed to relieve symptoms, identify pathological types, and eradicate the tumors. Cortisol and ACTH levels returned to normal on the third day after surgery. The patient was discharged six days after the surgery. Subsequently, two courses of VDC chemotherapy were administered; the patient’s symptoms improved, and the intracranial metastatic tumors were significantly reduced. However, the long-term effects require further follow-up.
At the gene level, gene mutation caused by chromosome heterotopia is the main cause of ES. The types include t (11; 22) (q24; q12), t (21; 22) (q22; q12), t (7; 22) (p22; q12), t (17; 22) (q12; q12), and t (2; 22) (q33; q12), which produce
In summary, primary renal ES is a rare adult malignant tumor with a poor prognosis. CS is its rare clinical manifestation. When assessing the patient, the principle of prioritization before quality must be followed in diagnosis. While gene tests are helpful for diagnosis and can guide treatment (although they are not a necessary examination method for this disease), the “gold standard” for diagnosing primary renal ES is still pathological examination. Comprehensive surgical treatment based on surgery can achieve certain effects in advanced metastatic primary renal ES.
The authors are grateful for invaluable support and useful discussions with other members of the urology department.
| 1. | Riggi N, Suvà ML, Stamenkovic I. Ewing's Sarcoma. N Engl J Med. 2021;384:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 192] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 2. | Liang L, Song H, Ma B, Zhang Z, Zhu K, Li Q, Zhou C, Li A, Liu J, Zhang Q, Zhu S, Zhang Q. Renal Ewing's sarcoma/primitive neuroectodermal tumor (PNET): a case series of 7 patients and literature review. Transl Androl Urol. 2021;10:548-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Seemayer TA, Thelmo WL, Bolande RP, Wiglesworth FW. Peripheral neuroectodermal tumors. Perspect Pediatr Pathol. 1975;2:151-172. [PubMed] |
| 4. | Arlt A, Harbeck B, Anlauf M, Alkatout I, Klöppel G, Fölsch UR, Bewig B, Mönig H. Fatal pneumocystis jirovecii pneumonia in a case of ectopic Cushing's syndrome due to neuroendocrine carcinoma of the kidney. Exp Clin Endocrinol Diabetes. 2008;116:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Cochetti G, Paladini A, de Vermandois JAR, Fatigoni S, Zanelli M, Ascani S, Mearini E. Metastatic renal Ewing's sarcoma in adult woman: Case report and review of the literature. Open Med (Wars). 2021;16:397-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Kozel ZM, Reifsnyder JE, Griffiths L, Gitlin JS, Kavoussi LR. Primary renal Ewing Sarcoma masquerading as Wilms in an adolescent female. Urol Case Rep. 2020;31:101187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Doroudinia A, Ahmadi S, Mehrian P, Pourabdollah M. Primary Ewing sarcoma of the kidney. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Karguppikar MB, Oza CM, Khadilkar V, Khadilkar A. Rare case of renal Ewing sarcoma presenting as ectopic Cushing syndrome in a 12-year-old girl. BMJ Case Rep. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Di Ruscio V, Del Baldo G, De Pasquale MD, De Vito R, Miele E, Colafati GS, Deodati A, De Ioris MA, Tornesello A, Milano GM, Mastronuzzi A. Ectopic ACTH Secretion in a Child With Metastatic Ewing's Sarcoma: A Case Report. Front Oncol. 2020;10:574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Guran T, Turan S, Ozkan B, Berrak SG, Canpolat C, Dagli T, Eren FS, Bereket A. Cushing's syndrome due to a non-adrenal ectopic adrenocorticotropin-secreting Ewing's sarcoma in a child. J Pediatr Endocrinol Metab. 2009;22:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 11. | Preeyasombat C, Sirikulchayanonta V, Mahachokelertwattana P, Sriphrapradang A, Boonpucknavig S. Cushing's syndrome caused by Ewing's sarcoma secreting corticotropin releasing factor-like peptide. Am J Dis Child. 1992;146:1103-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Mao W, Xu J, Lu H, Wang Y, Zhang L, Chen M. A rare case report of renal ewing sarcoma/primitive neuroectodermal tumor with ACTH production. BMC Urol. 2022;22:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 13. | Ellinger J, Bastian PJ, Hauser S, Biermann K, Müller SC. Primitive neuroectodermal tumor: rare, highly aggressive differential diagnosis in urologic malignancies. Urology. 2006;68:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Bing Z, Zhang P, Tomaszewski JE, Maclennan GT. Primary Ewing sarcoma/primitive neuroectodermal tumor of the kidney. J Urol. 2009;181:1341-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Risi E, Iacovelli R, Altavilla A, Alesini D, Palazzo A, Mosillo C, Trenta P, Cortesi E. Clinical and pathological features of primary neuroectodermal tumor/Ewing sarcoma of the kidney. Urology. 2013;82:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. [Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2019;40:52-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 17. | Shahani S, Nudelman RJ, Nalini R, Kim HS, Samson SL. Ectopic corticotropin-releasing hormone (CRH) syndrome from metastatic small cell carcinoma: a case report and review of the literature. Diagn Pathol. 2010;5:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Chrisoulidou A, Pazaitou-Panayiotou K, Georgiou E, Boudina M, Kontogeorgos G, Iakovou I, Efstratiou I, Patakiouta F, Vainas I. Ectopic Cushing's syndrome due to CRH secreting liver metastasis in a patient with medullary thyroid carcinoma. Hormones (Athens). 2008;7:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Auchus RJ, Mastorakos G, Friedman TC, Chrousos GP. Corticotropin-releasing hormone production by a small cell carcinoma in a patient with ACTH-dependent Cushing's syndrome. J Endocrinol Invest. 1994;17:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Beuschlein F, Hammer GD. Ectopic pro-opiomelanocortin syndrome. Endocrinol Metab Clin North Am. 2002;31:191-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Tarek N, Said R, Andersen CR, Suki TS, Foglesong J, Herzog CE, Tannir NM, Patel S, Ratan R, Ludwig JA, Daw NC. Primary Ewing Sarcoma/Primitive Neuroectodermal Tumor of the Kidney: The MD Anderson Cancer Center Experience. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Perer E, Shanberg AM, Matsunaga G, Finklestein JZ. Laparoscopic removal of extraosseous Ewing's sarcoma of the kidney in a pediatric patient. J Laparoendosc Adv Surg Tech A. 2006;16:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Abolhasani M, Salarinejad S, Moslemi MK. Ewing sarcoma/primitive neuroectodermal tumor of the kidney: A report of three cases. Int J Surg Case Rep. 2016;28:330-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Rowe RG, Thomas DG, Schuetze SM, Hafez KS, Lawlor ER, Chugh R. Ewing sarcoma of the kidney: case series and literature review of an often overlooked entity in the diagnosis of primary renal tumors. Urology. 2013;81:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Ohgaki K, Horiuchi K, Mizutani S, Sato M, Kondo Y. Primary Ewing's sarcoma/primitive neuroectodermal tumor of the kidney that responded to low-dose chemotherapy with ifosfamide, etoposide, and doxorubicin. Int J Clin Oncol. 2010;15:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Yoshihara H, Kamiya T, Hosoya Y, Hasegawa D, Ogawa C, Asanuma H, Mizuno R, Hosoya R, Manabe A. Ewing sarcoma/primitive neuroectodermal tumor of the kidney treated with chemotherapy including ifosfamide. Pediatr Int. 2016;58:766-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Zöllner S, Dirksen U, Jürgens H, Ranft A. Renal Ewing tumors. Ann Oncol. 2013;24:2455-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Attia S, Okuno SH, Robinson SI, Webber NP, Indelicato DJ, Jones RL, Bagaria SP, Jones RL, Sherman C, Kozak KR, Cortese CM, McFarland T, Trent JC, Maki RG. Clinical Activity of Pazopanib in Metastatic Extraosseous Ewing Sarcoma. Rare Tumors. 2015;7:5992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Zhao Y, Chen Y, Cheng K, Li ZP, Zeng H, Liu JY. Renal Ewing sarcoma treated with apatinib. Anticancer Drugs. 2018;29:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Hakky TS, Gonzalvo AA, Lockhart JL, Rodriguez AR. Primary Ewing sarcoma of the kidney: a symptomatic presentation and review of the literature. Ther Adv Urol. 2013;5:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Sorensen PH, Lessnick SL, Lopez-Terrada D, Liu XF, Triche TJ, Denny CT. A second Ewing's sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat Genet. 1994;6:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 536] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 32. | Ventura S, Aryee DN, Felicetti F, De Feo A, Mancarella C, Manara MC, Picci P, Colombo MP, Kovar H, Carè A, Scotlandi K. CD99 regulates neural differentiation of Ewing sarcoma cells through miR-34a-Notch-mediated control of NF-κB signaling. Oncogene. 2016;35:3944-3954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Franzetti GA, Laud-Duval K, Bellanger D, Stern MH, Sastre-Garau X, Delattre O. MiR-30a-5p connects EWS-FLI1 and CD99, two major therapeutic targets in Ewing tumor. Oncogene. 2013;32:3915-3921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |