Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5366
Revised: May 25, 2024
Accepted: June 12, 2024
Published online: August 16, 2024
Processing time: 104 Days and 22.9 Hours
Neonatal respiratory distress syndrome (NRDS) is one of the most common diseases in neonatal intensive care units, with an incidence rate of about 7% among infants. Additionally, it is a leading cause of neonatal death in hospitals in China. The main mechanism of the disease is hypoxemia and hypercapnia caused by lack of surfactant
To explore the effect of pulmonary surfactant (PS) combined with noninvasive positive pressure ventilation on keratin-14 (KRT-14) and endothelin-1 (ET-1) levels in peripheral blood and the effectiveness in treating NRDS.
Altogether 137 neonates with respiratory distress syndrome treated in our hospital from April 2019 to July 2021 were included. Of these, 64 control cases were treated with noninvasive positive pressure ventilation and 73 observation cases were treated with PS combined with noninvasive positive pressure ventilation. The expression of KRT-14 and ET-1 in the two groups was compared. The deaths, complications, and PaO2, PaCO2, and PaO2/FiO2 blood gas indexes in the two groups were compared. Receiver operating characteristic curve (ROC) analysis was used to determine the diagnostic value of KRT-14 and ET-1 in the treatment of NRDS.
The observation group had a significantly higher effectiveness rate than the control group. There was no significant difference between the two groups in terms of neonatal mortality and adverse reactions, such as bronchial dysplasia, cyanosis, and shortness of breath. After treatment, the levels of PaO2 and PaO2/FiO2 in both groups were significantly higher than before treatment, while the level of PaCO2 was significantly lower. After treatment, the observation group had significantly higher levels of PaO2 and PaO2/FiO2 than the control group, while PaCO2 was notably lower in the observation group. After treatment, the KRT-14 and ET-1 levels in both groups were significantly decreased compared with the pre-treatment levels. The observation group had a reduction of KRT-14 and ET-1 levels than the control group. ROC curve analysis showed that the area under the curve (AUC) of KRT-14 was 0.791, and the AUC of ET-1 was 0.816.
Combining PS with noninvasive positive pressure ventilation significantly improved the effectiveness of NRDS therapy. KRT-14 and ET-1 levels may have potential as therapeutic and diagnostic indicators.
Core Tip: The purpose of this study was to explore the effect of pulmonary surfactant (PS) combined with noninvasive positive pressure ventilation on the levels of keratin-14 (KRT-14) and endothelin-1 (ET-1) in peripheral blood and the effectiveness for treating neonatal respiratory distress syndrome (NRDS). KRT-14 and ET-1 expression in the two groups was compared. The therapeutic effectiveness, occurrence of death and complications, and the blood gas indexes PaO2, PaCO2 and PaO2/FiO2 in the two groups were compared. Receiver operating characteristic curve analysis was used to determine the diagnostic value of KRT-14 and ET-1 for the effectiveness of NRDS therapy. PS combined with noninvasive positive pressure ventilation significantly improved the effectiveness of NRDS therapy. KRT-14 and ET-1 levels may have potential as diagnostic indicators of therapy.
- Citation: Shi ZN, Zhang X, Du CY, Zhao B, Liu SG. Effects of pulmonary surfactant combined with noninvasive positive pressure ventilation in neonates with respiratory distress syndrome. World J Clin Cases 2024; 12(23): 5366-5373
- URL: https://www.wjgnet.com/2307-8960/full/v12/i23/5366.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i23.5366
Neonatal respiratory distress syndrome (NRDS) is one of the most common conditions in neonatal intensive care units. The incidence is about 7% and it is one of the main causes of neonatal death in hospitals in China[1,2]. The main mechanism of the disease is hypoxemia and hypercapnia caused by lack of surfactant. The diffusion efficiency of oxygen through the alveolar-capillary exchange barrier is disturbed owing to various factors, and lung injury in neonates leads to asthma, septicemia, pneumonia, and other complex symptoms[3]. Premature delivery of pregnant women and pregnancy disease may lead to morbidity. Only early diagnosis and treatment can improve the quality of life of neonates[4,5].
As the pathogenesis of NRDS is the lack of pulmonary surfactant, exogenous pulmonary surfactant (PS) replacement therapy has been found to be an effective treatment[6]. When neonates suffer from respiratory failure, respiratory support can also improve their condition. However, invasive ventilation tends to cause a series of complications such as lung infection, ventilator-associated lung injury, etc that draws attention to its widespread use[7]. It has been found in many studies that noninvasive positive pressure ventilation significantly improves NRDS neonates and has good safety[8,9]. At the same time, some studies have found that PS combined with non-invasive positive pressure ventilation can further improve clinical efficacy[10].
Keratin-14 (KRT-14) is a cytoskeleton protein that has good diagnostic value for lung tissue injury[11]. A study by Confalonieri[12] reported that KRT-14 was a viable biomarker for activation and repair/regeneration of lung cells. It is involved in the repair and regeneration of alveoli when the alveoli collapse, and lung cells are severely damaged in neonates with NRDS. Therefore, KRT14 may be used as an indicator of the improvement of the condition of neonates with RDS. Endothelin-1 (ET-1) is a vasoactive substance, which is mainly produced in lung tissue. It promotes the gradual change of pulmonary vascular reactivity through angiogenesis. It participates in vascular regulation, bronchoconstriction, and inflammatory reactions in the respiratory system. Endothelial and epithelial dysfunction in RDS patients can be induced of pro-inflammatory mechanisms. The use of endothelin receptor antagonists can regulate lung injury[13,14]. El Shemi et al team[15] examined the plasma ET-1 concentration of 69 premature neonates from 28 to 34 wk of age and diagnosed with NRDS. They found that the ET-1 concentration increased significantly 3 d after birth, and was predictive of the development of bronchopulmonary dysplasia. At present, there are few study results on the correlation of KRT-14 and ET-1 with the curative effect of NRDS neonates. Therefore, this study aimed to provide a basis and direction for clinical research on PS combined with noninvasive positive pressure ventilation to treat NRDS in neonates and observe the KRT-14 and ET-1 levels in peripheral blood.
A total of 137 neonates with NRDS were treated in our hospital between April 2019 and July 2021 and were included in the study. The neonates had been admitted to the hospital and received basic treatment to ensure smooth breathing, protect against infection, and maintain water and electrolyte balance. Sixty-four infants, 37 males and 27 females, were included in a control group that received noninvasive positive pressure ventilation and basic treatment. The other 73 infants, 38 males and 35 females, were included in an observation group treated with PS combined with noninvasive positive pressure ventilation in addition to basic treatment. The study was approved by the Medical Ethics Committee, and the parents of all infants signed an informed consent form.
Inclusion criteria: All neonates included in the study had complete clinical data, were diagnosed NRDS by imaging using the diagnostic criteria established by the 2016 update of the European Consensus Guidelines on the management of respiratory distress syndrome[16]. All families agreed with the treatment and follow-up. Exclusion criteria: Neonates with congenital immune defects, status complicated by other respiratory diseases, acute infectious disease, liver and kidney insufficiency, or allergic to therapeutic drugs or methods were excluded.
After admission, the two groups of neonates were treated with 21%-80% oxygen at a 6-8 L/min gas flow rate, and 4-7 cmH2O pressure. If the continuous positive airway pressure decreased to 2-3 cmH2O and the oxygen concentration decreased to 25%, and dyspnea was significantly relieved or had disappeared, the treatment was terminated. If the oxygen concentration was > 80%, the pressure was > 6-7 cmH2O, and the oxygen saturation was < 85% after 6-8 h of treatment, or if type II respiratory failure occurred, the treatment was changed to mechanical ventilation. Based on the above, neonates in the observation group were given exogenous PS[17] by tracheal administration as soon as possible.
After hospital admission and at 7:00 am the morning after treatment began, 5 mL of sterile venous blood was collected into a coagulation tube, serum was separated by centrifugation at 3000 g at 4 °C for 10 min and stored −80 °C. KRT-14 and ET-1 levels were determined by ELISA. The assay wells included a blank with 0 µL standard; a standard with 50 µL of standard substance at different concentrations, and a sample-to-be tested with 10 µL of sample and 40 µL diluent. Nothing was added to the blank well. In addition to the blank wells, 100 µL of horseradish peroxidase-labeled detection antibody was added to each of the standard wells and the sample wells. The reaction wells were sealed and incubated in a water bath at 37 °C for 65 min. The liquid was discarded, and the absorbent paper was patted dry. Each well was filled with washing liquid and allowed to stand for 2 min. The washing liquid was discarded, and the absorbent paper was patted dry. This procedure was repeated six times before 50 µL of substrate A and B solution was added to each well and incubated at 37 °C in the dark for 10 min. The optical density (OD) of each well was measured at 450 nm within 15 min after adding 50 µL of stop solution to each well, and the concentration was calculated.
After effective treatment, clinical symptoms resolved or improved, abnormal shadows in the lungs disappeared or improved on X-ray films, and blood gas indexes normalized or improved. After Ineffective treatment, the clinical symptoms worsened or did not improve, or the neonate died, X-ray films showed enlarged shadow areas or lack of improvement. Blood gas indexes worsened or did not improve.
Main the outcome measures were KRT-14 and ET-1 expression in the observation and control groups. The therapeutic effect, death, and complications in the two groups were compared. The secondary outcomes were the clinical data of the two groups of neonates The blood gas indexes PaO2, PaO2, and PaO2/FiO2 of the two groups of neonates were compared and the value of KRT-14 and ET-1 for predicting the therapeutic effect in NRDS neonates was evaluated by ROC curve analysis.
SPSS 20.0 (IBM Corp., Armonk, NY, United States) was used to perform the statistical analysis. GraphPad Prism 7 (GraphPad, La Jolla, CA, United States) was used to graph the collected data. Enumeration data were reported as numbers and percentages (%), and compared by chi-square tests. Measurement data were reported as means ± SD and independent sample t-tests were used to compare measurement data that were normally distributed. ROC was used to evaluate the diagnostic value of KRT-14 and ET-1 in the therapeutic effect of NRDS neonates. P < 0.05 was regarded as statistically significant.
Comparison of the clinical data (Table 1) found no significant differences between the two groups in sex, gestational age, body mass, Apgar score, age at pregnancy, hypertension during pregnancy, maternal diabetes, premature rupture of membranes, delivery mode, premature delivery, and delivery history.
| Parameter | Observation group, n = 73 | Control group, n = 64 | X2/t | P value |
| Sex | 0.456 | 0.499 | ||
| Male | 38 (52.05) | 37 (57.81) | ||
| Female | 35 (47.95) | 27 (42.19) | ||
| Gestational age in wk | 33.37 ± 2.12 | 32.87 ± 2.05 | 1.399 | 0.164 |
| Body mass in kg | 2.82 ± 0.69 | 2.71 ± 0.58 | 1.002 | 0.318 |
| Apgar score | 8.47 ± 1.31 | 8.28 ± 1.26 | 0.862 | 0.390 |
| Age of pregnant woman in yr | 27.3 ± 4.8 | 26.5 ± 4.1 | 1.041 | 0.230 |
| Hypertension of Pregnant Women | 9 (12.33) | 5 (7.81) | 0.758 | 0.384 |
| Diabetes of pregnant women | 11 (15.07) | 7 (10.94) | 0.510 | 0.475 |
| Premature rupture of membranes | 9 (12.33) | 10 (15.63) | 0.310 | 0.578 |
| Delivery mode | 0.566 | 0.452 | ||
| Eutocia | 41 (56.16) | 40 (62.50) | ||
| Cesarean section | 32 (43.84) | 24 (37.50) | ||
| Premature delivery | 0.804 | 0.370 | ||
| Yes | 52 (71.23) | 41 (64.06) | ||
| No | 21 (28.77) | 23 (35.94) | ||
| Delivery history | 0.027 | 0.870 | ||
| Primiparity | 50 (68.49) | 43 (67.19) | ||
| Multiparity | 23 (31.51) | 21 (32.81) |
We observed and compared the therapeutic effects of the two groups of neonates after treatment. The effectiveness of treatment was significantly better in the observation group than in the control group. As shown in Table 2 between-group differences in death, bronchial dysplasia, cyanosis, and shortness of breath were not significant.
| Outcome | Observation group, n = 73 | Control group, n = 64 | t | P value |
| Effective curative effect | 67 (91.78) | 51 (79.69) | 4.175 | 0.041 |
| Ineffective curative effect | 6 (8.22) | 13 (20.31) | ||
| Death | 3 (4.11) | 6 (9.38) | 1.540 | 0.215 |
| Bronchial dysplasia | 6 (8.22) | 11 (17.19) | 2.514 | 0.112 |
| Cyanopathy | 8 (10.96) | 10 (15.63) | 0.651 | 0.420 |
| Shortness of breath | 9 (12.33) | 14 (21.88) | 2.225 | 0.136 |
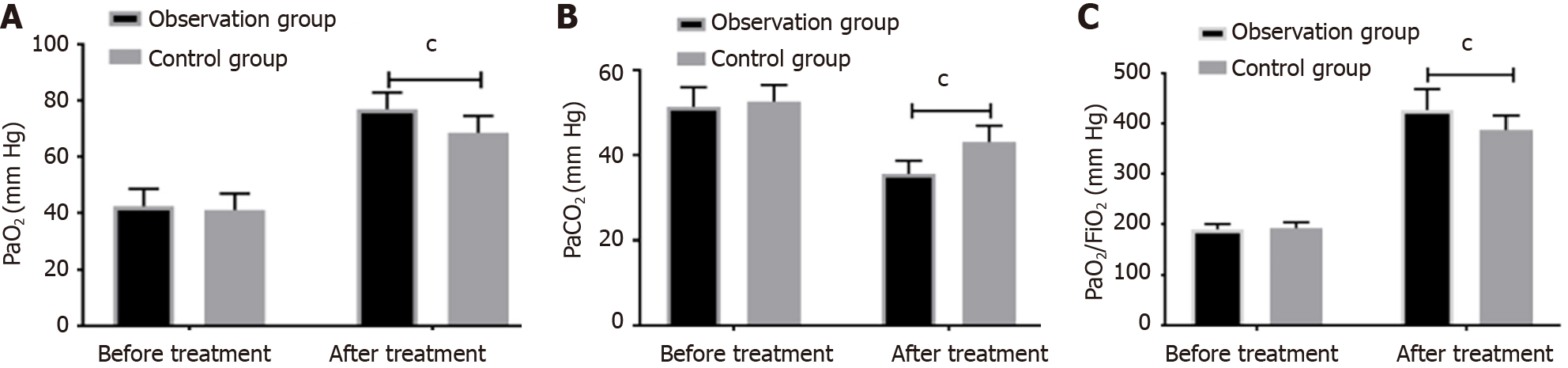
Comparing the blood gas indexes PaO2, PaCO2, and PaO2/FiO2 before and after treatment, found no significant difference between the control and observation groups before treatment. After treatment, PaO2, and PaO2/FiO2 in both groups were significantly higher than before treatment, and PaCO2 was notably lower than before treatment. The levels of PaO2, PaO2/FiO2 in the observation group were considerably higher than the control group, while PaCO2 was considerably lower than the control group, as shown in Figure 1.
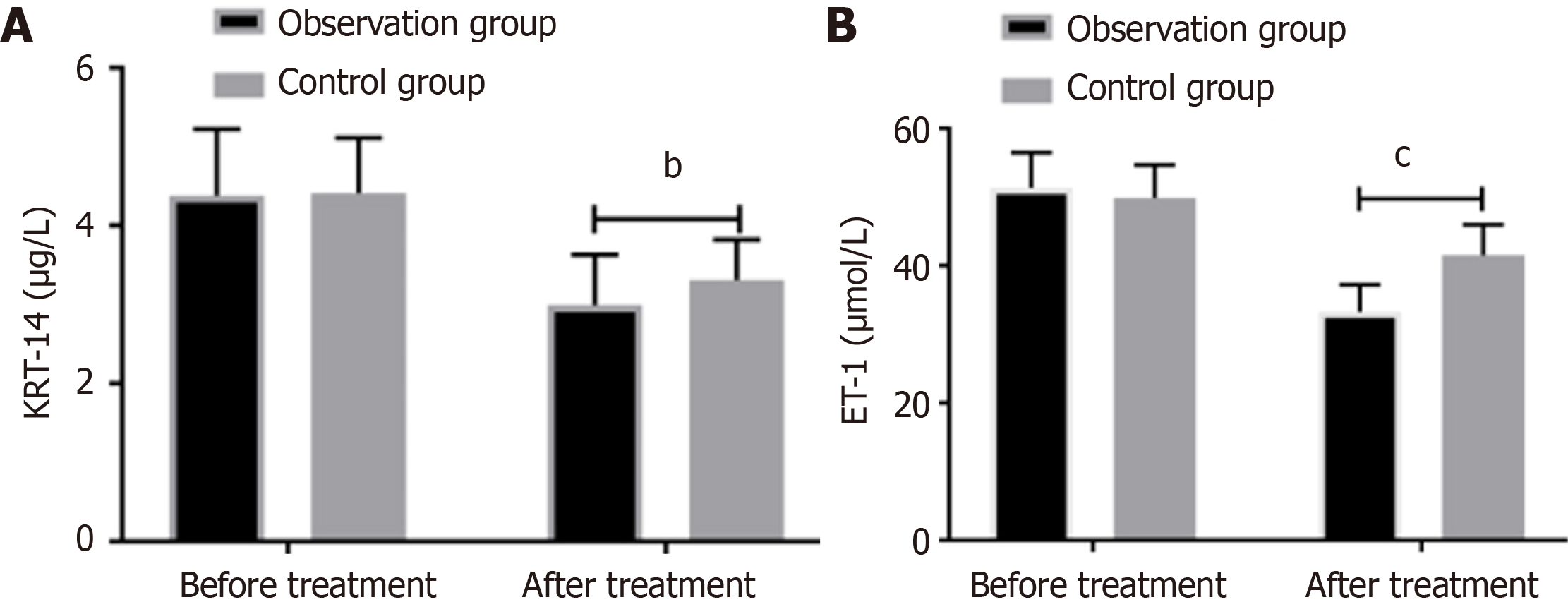
A comparison of the levels of KRT-14 and ET-1 before and after treatment in the two groups revealed that there is no difference between the two groups before the treatment. After treatment, the levels of KRT-14 and ET-1 were significantly reduced in both groups when compared to their pre-treatment levels. Moreover, the levels in the observation group were significantly lower than those in the control group, as shown in Figure 2.
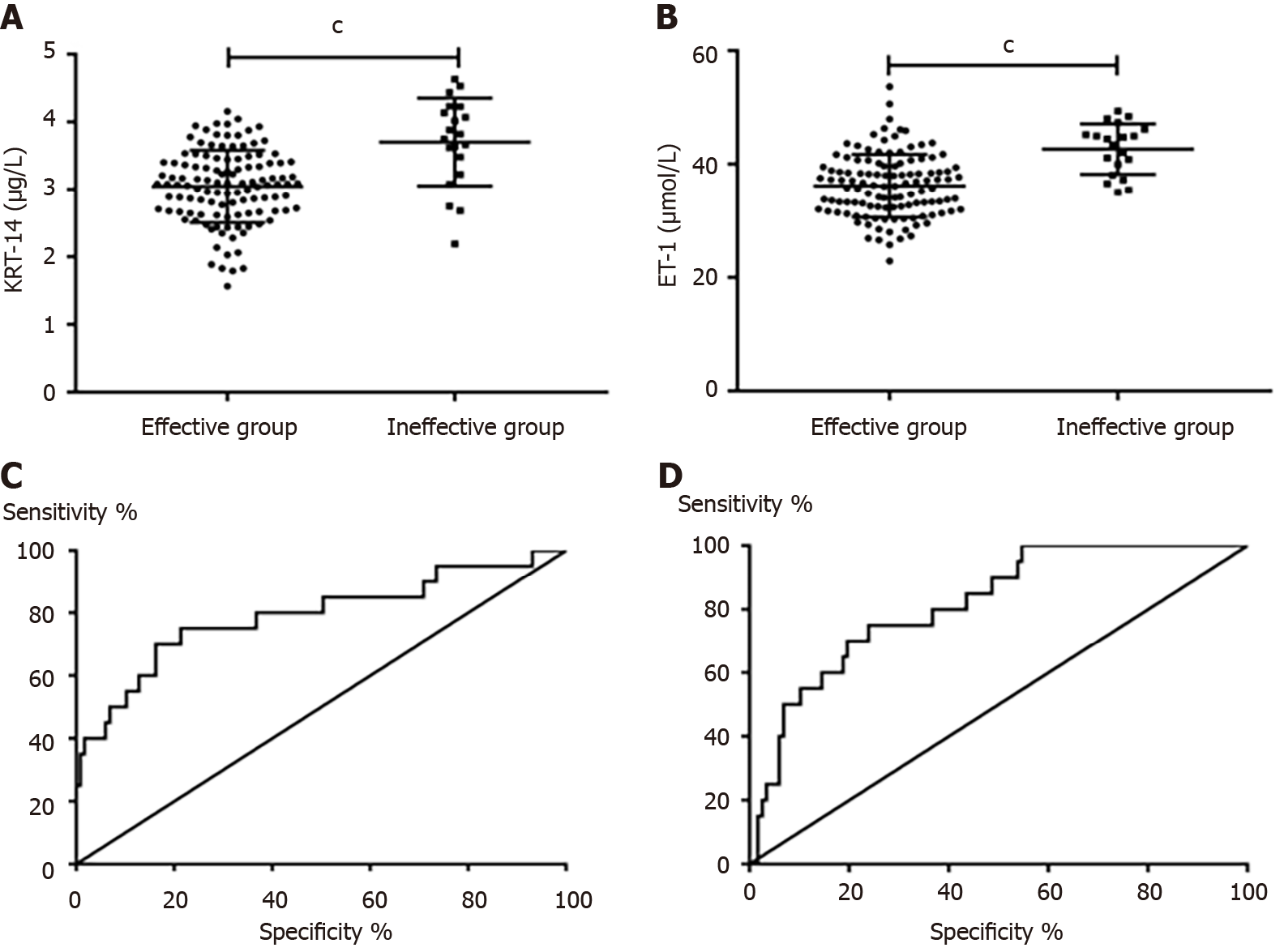
After comparing the levels of KRT-14 and ET-1 in neonates with effective and ineffective curative effects, we found that neonates with ineffective curative effects had significantly higher levels of KRT-14 and ET-1 than neonates with effective curative effects (P < 0.05). ROC curve analysis of the diagnostic value of KRT-14 and ET-1 in the curative effectiveness of NRDS neonates found that the area under the curve (AUC) of KRT-14 was 0.791, and the AUC of ET-1 was 0.816, as shown in Table 3 and Figure 3.
| Index | AUC | 95%CI | Specificity, % | Sensitivity, % | Youden index, % | Cut-off |
| KRT-14 | 0.791 | 0.665-0.917 | 85.47 | 60.00 | 45.47 | > 3.645 |
| ET-1 | 0.816 | 0.726-0.907 | 76.07 | 70.00 | 46.07 | > 40.060 |
The pathogenesis of NRDS involves acute diffuse alveolar-capillary injury, which leads to increased pulmonary capillary permeability, and alveolar and interstitial edema, and ultimately gives rise to type II alveolar cell damage. This kind of damage reduces PS, leading to an increase in alveolar surface tension, contraction of alveolar groups, and abnormal pulmonary ventilation/blood flow ratio, eventually triggering severe hypoxemia[18,19]. Noninvasive positive pressure ventilation can relax the alveoli of neonates, improve the compliance of neonate lungs, maintain the pressure in alveoli, and maintain smooth breathing. Exogenous PS can supplement the lack of PS in neonates, thus reducing the tension of alveoli in neonates, preventing alveoli atrophy, improving lung respiratory function and lung compliance, and increasing blood oxygen saturation in NRDS, thus reducing the mechanical ventilation time of neonates[20,21].
We compared the therapeutic effect and adverse reactions of the two groups after treatment and found that the combination of PS and noninvasive positive pressure ventilation had a significantly better therapeutic effect than noninvasive positive pressure ventilation alone. However, the difference of the mortality rates in the two groups was not significant. We also compared the blood gas indexes PaO2, PaO2, and PaO2/FiO2 of the two groups before and after treatment. After treatment, PaO2, PaO2/FiO2 increased in both groups, and PaCO2 decreased significantly. After treatment, the PaO2, and PaO2/FiO2 levels were significantly higher and PaO2 was significantly lower in the observation group than in the control group. The blood gas levels of neonates with NRDS are significantly worse than those of healthy newborns as a result of differences in lung oxygenation and respiratory function. After the symptoms are controlled and pulmonary function improves, the blood gas index of the neonates returns to normal[22]. The study results shows that the combined treatment was more effective.
We measured the levels of KRT-14 and ET-1 in both groups of neonates. KRT-14 and ET-1 are lung tissue factors[23,24] and KRT14 increases rapidly in response to lung injury. Previous studies reported a negative correlation between KRT14 and PaO2/FiO2, and KRT14 is elevated in RDS. We found that KRT-14 and ET-1 levels in both were significantly lower after treatment than they were before treatment, and they were significantly lower in the observation group than in the control group after treatment. Additionally, our findings indicate that KRT-14 and ET-1 levels were significantly elevated in neonates who did not respond effectively to therapy compared with those who did. This suggests that KRT-14 and ET-1 could potentially be valuable diagnostic indicators for neonates with NRDS who are undergoing treatment. Therefore, we the value of KRT-14 and ET-1 for diagnosing therapeutic effectiveness by ROC curve analysis, and the AUC of each factor indicated that both were specific and sensitive. Both factors have diagnostic value in assessing the effectiveness of NRDS treatment in neonates and could potentially serve as diagnostic indices of effective treatment. Thus, KRT-14 can serve as an indicator of improvement in RDS patients, decreasing as their condition improves.
The study has some weaknesses. The subjects included in our study were all sick neonates and healthy newborns were not included for comparison. We did not explore differences between the measures tested in this study and the indexes measured in healthy newborns. Secondly, various types of PS are available for treatment[25], and therefore it is expected that further research can be conducted to assess the differences of the therapeutic effectiveness of different PS preparations. Finally, we found that some complications occurred during the treatment of the neonates, but we did not explore the risk factors of these complications. We hope to add to this discussion after completing our follow-up study.
In conclusion, PS combined with noninvasive positive pressure ventilation significantly improved the effectiveness of NRDS therapy. KRT-14 and ET-1 levels are potential therapeutic diagnostic indicators.
| 1. | Zhang B, Dai Y, Chen H, Yang C. Neonatal Mortality in Hospitalized Chinese Population: A Meta-Analysis. Biomed Res Int. 2019;2019:7919501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Luo J, Chen J, Li Q, Feng Z. Differences in Clinical Characteristics and Therapy of Neonatal Acute Respiratory Distress Syndrome (ARDS) and Respiratory Distress Syndrome (RDS): A Retrospective Analysis of 925 Cases. Med Sci Monit. 2019;25:4992-4998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 3. | Mei H, Zhang Y, Liu C, Zhang Y, Liu C, Song D, Xin C, Wang J, Josephs-Spaulding J, Zhu Y, Tang F. Messenger RNA sequencing reveals similar mechanisms between neonatal and acute respiratory distress syndrome. Mol Med Rep. 2018;17:59-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Ye W, Zhang T, Shu Y, Fang C, Xie L, Peng K, Liu C. The influence factors of neonatal respiratory distress syndrome in Southern China: a case-control study. J Matern Fetal Neonatal Med. 2020;33:1678-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Lu CQ, Lin J, Yuan L, Zhou JG, Liang K, Zhong QH, Huang JH, Xu LP, Wu H, Zheng Z, Ping LL, Sun Y, Li ZK, Liu L, Lyu Q, Chen C. Pregnancy induced hypertension and outcomes in early and moderate preterm infants. Pregnancy Hypertens. 2018;14:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Bae CW, Kim CY, Chung SH, Choi YS. History of Pulmonary Surfactant Replacement Therapy for Neonatal Respiratory Distress Syndrome in Korea. J Korean Med Sci. 2019;34:e175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Zhu XW, Shi Y, Shi LP, Liu L, Xue J, Ramanathan R; NHFOV Study Group. Non-invasive high-frequency oscillatory ventilation vs nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome: Study protocol for a multi-center prospective randomized controlled trial. Trials. 2018;19:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Clément G, Reschke MF. Relationship between motion sickness susceptibility and vestibulo-ocular reflex gain and phase. J Vestib Res. 2018;28:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Anwaar O, Hussain M, Shakeel M, Ahsan Baig MM. Outcome Of Use Of Nasal Continuous Positive Airway Pressure Through Infant Flow Drivers In Neonates With Respiratory Distress In A Tertiary Care Hospital In Pakistan. J Ayub Med Coll Abbottabad. 2018;30:511-555. [PubMed] |
| 10. | Soll RF, Barkhuff W. Noninvasive Ventilation in the Age of Surfactant Administration. Clin Perinatol. 2019;46:493-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Da Silva Melo AR, Barroso H, Uchôa De Araújo D, Ruidomar Pereira F, De Oliveira NF. The influence of sun exposure on the DNA methylation status of MMP9, miR-137, KRT14 and KRT19 genes in human skin. Eur J Dermatol. 2015;25:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Confalonieri M, Buratti E, Grassi G, Bussani R, Chilosi M, Farra R, Abrami M, Stuani C, Salton F, Ficial M, Confalonieri P, Zandonà L, Romano M. Keratin14 mRNA expression in human pneumocytes during quiescence, repair and disease. PLoS One. 2017;12:e0172130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Mei M, Cheng G, Sun B, Yang L, Wang H, Sun J, Zhou W. EDN1 Gene Variant is Associated with Neonatal Persistent Pulmonary Hypertension. Sci Rep. 2016;6:29877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Jiang Y, Zeng Y, Huang X, Qin Y, Luo W, Xiang S, Sooranna SR, Pinhu L. Nur77 attenuates endothelin-1 expression via downregulation of NF-κB and p38 MAPK in A549 cells and in an ARDS rat model. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1023-L1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | El Shemi MS, Tawfik S, Khafagy SM, Hamza MT, Youssef AM. Endothelin 1 as a predictor marker for bronchopulmonary dysplasia in preterm neonates with respiratory distress syndrome. J Neonatal Perinatal Med. 2017;10:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GH, Halliday HL. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology. 2017;111:107-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (2)] |
| 17. | Zhang C, Zhu X. Clinical effects of pulmonary surfactant in combination with nasal continuous positive airway pressure therapy on neonatal respiratory distress syndrome. Pak J Med Sci. 2017;33:621-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Dimache G, Stoean C, Durbacă S, Croitoru M, Ionescu M, Nedelcu IN, Corbu I. Study of specific immune response to unadsorbed concentrated tetanus vaccine administered by intradermal route to non-immunized persons in the last ten years. Arch Roum Pathol Exp Microbiol. 1990;49:51-62. [PubMed] |
| 19. | Speer CP. Neonatal respiratory distress syndrome: an inflammatory disease? Neonatology. 2011;99:316-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Fan YZ, Wen ZL. [Efficacy of different dosages of ambroxol hydrochloride in the prevention of neonatal respiratory distress syndrome]. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11:771-772. [PubMed] |
| 21. | Wu X, Li S, Zhang J, Zhang Y, Han L, Deng Q, Wan X. Meta-analysis of high doses of ambroxol treatment for acute lung injury/acute respiratory distress syndrome based on randomized controlled trials. J Clin Pharmacol. 2014;54:1199-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Xiang J, Wang P. Efficacy of pulmonary surfactant combined with high-dose ambroxol hydrochloride in the treatment of neonatal respiratory distress syndrome. Exp Ther Med. 2019;18:654-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Gorąca A, Kleniewska P, Skibska B. ET-1 mediates the release of reactive oxygen species and TNF-α in lung tissue by protein kinase C α and β1. Pharmacol Rep. 2016;68:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, Runkle CM, Reynolds SD. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol. 2011;45:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Chen C, Tian T, Liu L, Zhang J, Fu H. Gender-related efficacy of pulmonary surfactant in infants with respiratory distress syndrome: A STROBE compliant study. Medicine (Baltimore). 2018;97:e0425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (1)] |