Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.5263
Revised: May 31, 2024
Accepted: June 20, 2024
Published online: August 6, 2024
Processing time: 64 Days and 0.1 Hours
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and clinically aggressive hematologic malignancy originating from the precursors of plasma
In this paper, we retrospectively analyzed 2 cases of BPDCN. Both patients were elderly males. The lesions manifested as skin masses. Morphological manifestations included diffuse and dense tumor cell infiltration of the dermis and subcu
In this paper, we retrospectively analyzed 2 cases of BPDCN. Both patients were elderly males. The lesions manifested as skin masses. Morphological manifestations included diffuse and dense tumor cell infiltration of the dermis and subcutaneous tissues. Immunohistochemistry staining showed that cluster of differentiation CD4, CD56, CD43, and CD123 were positive.
Core Tip: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a highly malignant tumor of the hematopoietic and lymphoid tissue, which is extremely rare. BPDCN is highly aggressive and often involves the skin, lymph nodes and bone marrow, with rapid clinical progression and a poor prognosis. In this paper, we retrospectively analyzed 2 cases of BPDCN and reviewed their pathological features, immunophenotype, differential diagnosis, treatment and prognosis, with the aim of improving pathologists’understanding of this entity.
- Citation: Cai JW, Li MY, Wang WH, Shi HQ, Yang YH, Chen JJ. Blastic plasmacytoid dendritic cell neoplasm in Jinhua, China: Two case reports. World J Clin Cases 2024; 12(22): 5263-5270
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/5263.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.5263
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a highly malignant and rare tumor of the hematopoietic and lymphoid tissues. This tumor constitutes 1% of blood cancers, primarily affecting the elderly population around 60 years old. Since its initial report by Patel et al[1] in 1994[2], the condition has undergone several name changes and was classified by the WHO (2008) as BPDCN, under the category of acute myeloid leukemia (AML) and related precursor cell tumors[3,4]. BPDCN is highly aggressive[5,6], often involving the skin, lymph nodes, and bone marrow, with rapid clinical progression and a poor prognosis[7]. In this paper, we retrospectively analyzed 2 cases of BPDCN and reviewed their pathological features, immunophenotype, differential diagnosis, treatment, and prognosis, aiming to improve pat
All biopsy specimens were fixed in 10% neutral formalin, dehydrated, and embedded in paraffin for sectioning, followed by hematoxylin-eosin staining and microscopic examination. Immunohistochemical labeling was performed using the EnVision two-step method. The antibodies used included cluster of differentiation markers such as CD4, CD56, CD43, CD123, CD20, CD79α, CD3, CD5, CD34, myeloperoxidase (MPO), CD117, terminal deoxynucleotidyl transferase (TdT), CD68, Ki-67, and S100. Antibodies and Epstein-Barr virus (EBV) in situ hybridization kits were obtained from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. Immunohistochemistry and in situ hybridization staining were performed according to the kit instructions, with positive and negative controls included.
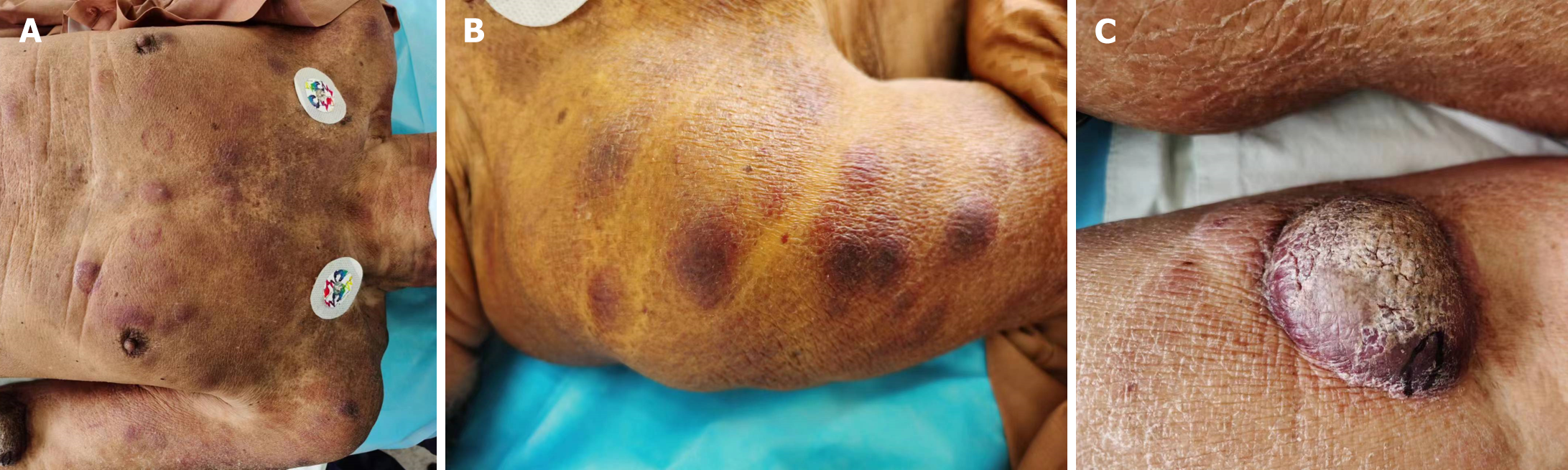
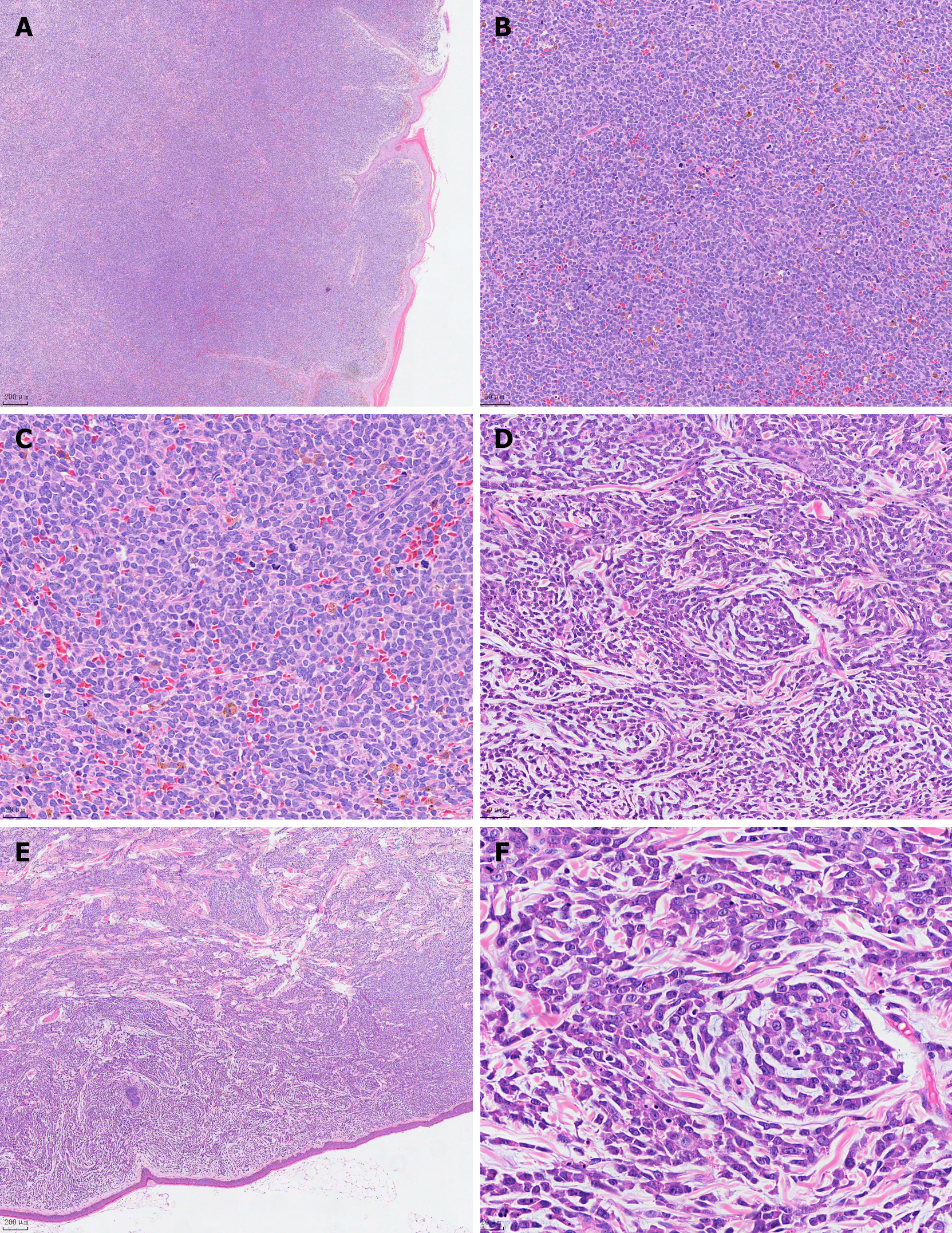
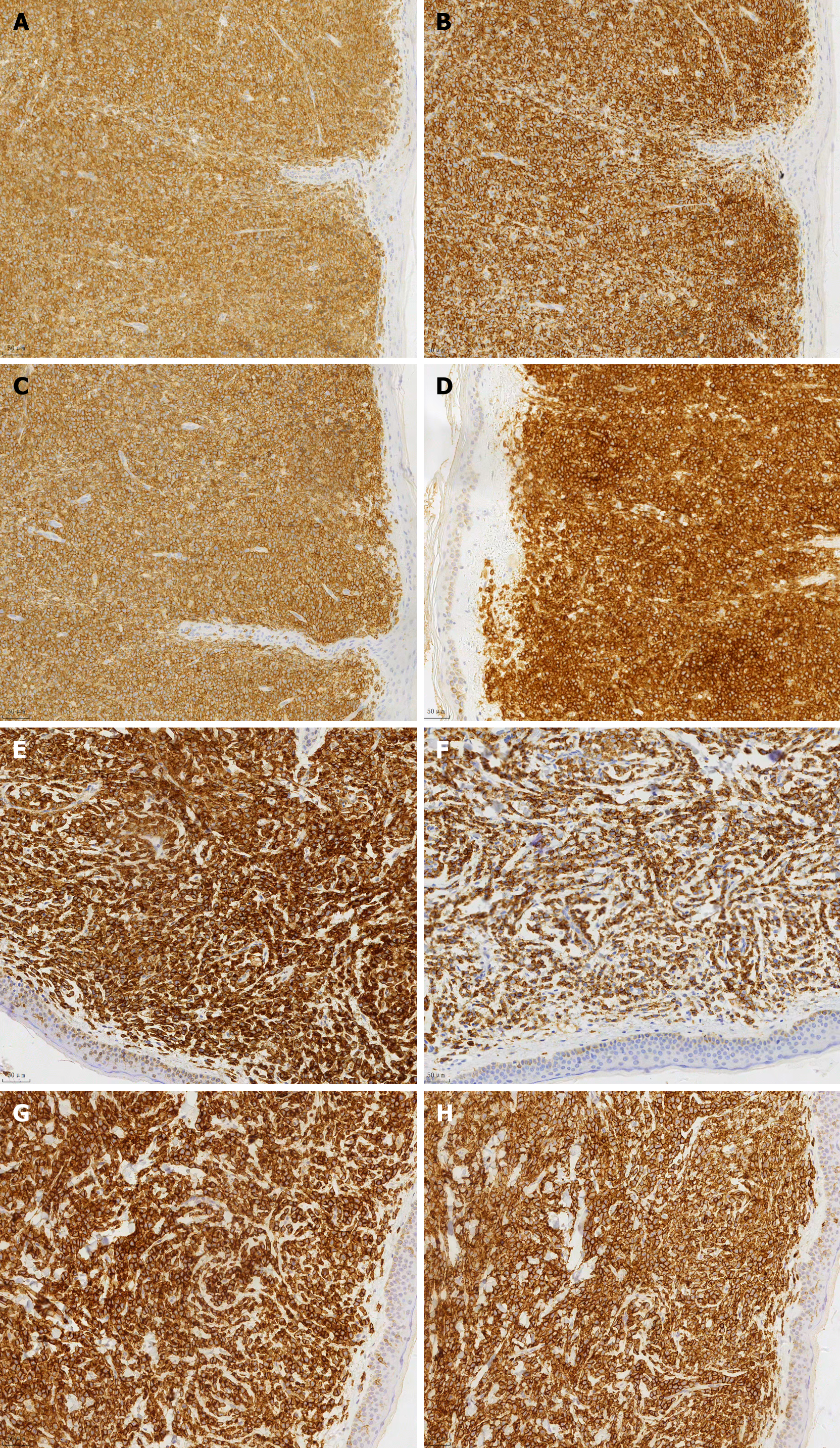
Case 1: The patient is a 76-year-old male who presented with multiple rashes or skin nodules without an apparent cause (Figure 1). The nodules ranged in diameter from 0.2 to 7.5 cm, with unknown bone marrow involvement. Routine blood tests and lactate dehydrogenase levels were normal, and abdominal ultrasound showed no significant abnormalities. A skin lesion biopsy revealed a tumor tissue measuring 0.5 cm × 0.7 cm × 1.3 cm macroscopically, with a gray-white cut surface and soft texture. Microscopic examination showed no eosinophils or apoptotic bodies, no evidence of vascular infiltration, but nine mitotic figures per high-power field. The tumor cells were located in the dermis and subcutaneous tissue, sparing the epidermis, and infiltrating (Figure 2A). The tumor cells were of moderate size, uniform in morphology, with round or oval nuclei and finely dispersed chromatin (Figure 2B). Additionally, individual or multiple small nucleoli were observed within the tumor cells, with sparse cytoplasm and active mitosis (Figure 2C). Immunohistochemical staining showed positive staining for CD4, CD56, CD43, and CD123 in tumor cells, partially positive staining for TdT and S-100, and negative staining for CD20, CD3, CD5, CD34, CD117, MPO, and EBV (Figure 3). Due to the patient's poor economic status, a bone marrow aspiration biopsy was not performed, and the patient did not receive subsequent treatment. (Since the patient was an outpatient and did not follow up, the patient was lost to follow-up and therefore could not be modified as required by the format.)
Case 2: The patient was diagnosed with AML over a year ago and has had a worsening rash for over 10 days.
Case 2: Over a year ago, the patient was admitted due to "fatigue lasting over a month." Blood tests revealed the following: White blood cell count 10.8109/L, hemoglobin 63 g/L, platelets 63109/L. Flow cytometry on 2018-08-26 showed 41.8% immature monocytes expressing CD56 and 21.3% mature monocytes, consistent with AML-M5. The diagnosis of " AML M5" was confirmed. Chemotherapy began on 2018-08-30 with azacitidine (25 mg d1-3) plus idarubicin (5 mg d4-6) and cytarabine (50 mg d4-9). Supportive therapy included red blood cell and platelet transfusions during cytopenic periods, granulocyte colony-stimulating factor injections, and infection prophylaxis with imipenem-cilastatin and vancomycin. On 2019-09-01, the patient presented with a "rash for a week." A skin biopsy on 2019-09-04 showed extensive infiltration of lymphocyte-like cells in the dermis, with eosinophilic cytoplasm, visible nucleoli, and frequent mitoses, consistent with granulocytic sarcoma. Further review and immunohistochemistry suggested a diagnosis of BPDCN, recommending additional immunohistochemical staining, including CD123 and 0D7 for further clarification. The patient refused further treatment and was discharged. Over the past 10 days, the patient's skin lesions have worsened, accompanied by fatigue and poor appetite, without cough, sputum, fever, chills, chest tightness, dyspnea, headache, dizziness, abdominal pain, diarrhea, nausea, or vomiting.
Case 2: The patient's general health condition was fair. Diagnosed with AML over a year ago.
Case 2: There is no family history of diabetes, hemophilia, infectious diseases, tumors, or other hereditary conditions.
Case 2: The patient was alert and oriented but appeared anemic. Prominent erythematous nodules were observed on the face and eyes. There was no tenderness over the sternum, and superficial lymph nodes were not palpable. Bilateral lung breath sounds were equal, with no rales or wheezes. The heart rhythm was regular, with no murmurs. The abdomen was soft, with no tenderness or rebound tenderness, and the liver and spleen were not palpable below the costal margin. A scattered rash was present throughout the body, particularly prominent on the abdomen. The lower limbs showed no edema, and localized purpura was observed on the upper limbs.
Case 2: (1) Blood routine: Leukocytes 1800/μL, hemoglobin 56 g/L, platelets 44000/μL. Lactate dehydrogenase: 461.3 IU/L; (2) AML 20 fusion gene analysis: Positive for ASXL1, NPM1, TET2; (3) Bone marrow aspiration: AML (AML-M5 subtype), with 67% immature monocytes, positive expression of CD33, CD13, CD14, CD64, CD56, and CD38; (4) Chromosome analysis: three copies of chromosomes 5 and 8 in four cells with normal karyotypes; and (5) Skin biopsy: Tumor tissue sizes were 0.6 cm × 0.7 cm × 1.5 cm and 0.6 cm × 0.8 cm × 1.1 cm, gray and gray-red on cut surfaces, with moderate consistency. Microscopic examination showed no eosinophils or apoptotic bodies, no vascular infiltration, and six mitoses per high-power field. The tumor cells were located in the dermis and subcutaneous tissue, preserving the epidermis, and infiltrating growth (Figure 2D). The tumor cells were of moderate size, with uniform morphology, exhibiting round or oval nuclei and finely dispersed chromatin (Figure 2E). Additionally, individual or multiple small nucleoli were visible within the tumor cells, with sparse cytoplasm and active mitotic activity (Figure 2F). Immunohistochemical staining of the tumor tissue showed positive staining for CD4, CD56, CD43, and CD123 in tumor cells, while CD20, CD3, CD5, CD34, CD117, MPO, and EBV were negative (Figure 3).
Case 2: Abdominal ultrasound showed no significant abnormalities.
AML M5; BPDCN.
Combination chemotherapy with IA regimen.
After discharge, the patient was regularly followed up in the outpatient department. At the last visit, the erythematous nodules on the skin had progressively increased. The patient and family refused further treatment, and the patient unfortunately passed away on 2019-10-2.
BPDCN is an extremely rare and clinically aggressive hematologic malignancy, with more than 200 cases reported to date since it was first identified by Patel et al[1] in 1994. It originates from abnormal plasmacytoid dendritic cells (PDC), and the WHO (2008) classification of hematopoietic and lymphoid tissues categorizes this tumor under the acute myeloid lineage and its associated precursor cell tumors. BPDCN predominantly affects elderly men, with the age at onset ranging from 53 to 70 years[8-10]. The two cases in our study involved elderly males with a me[an age of 77 years, which is older than the age range reported in the literature, likely due to the small number of cases. Notably, BPDCN has also been reported in children[11,12], indicating that it can occur in both older and younger patients. When suspected cases are encountered in clinical diagnosis, immunohistochemistry should be utilized to assist in the diagnosis. The tumor is characterized by a clonal proliferation of immature or precursor PDCs, typically presenting initially as a skin lesion, followed by rapid involvement of other hematopoietic and lymphoid tissues such as bone marrow and lymph nodes. Some patients present with only leukemic manifestations without identifiable extramedullary disease at the time of presentation[13]. Therefore, bone marrow biopsy and peripheral blood laboratory examinations are crucial for the ancillary diagnosis of BPDCN.
BPDCN is usually characterized by diffuse and dense tumor cell infiltration in the dermis and subcutaneous tissues without epidermal involvement. There is a cell-free zone between the infiltrated area and the epidermis, but sub
The tumor cells of BPDCN exhibit a characteristic immunophenotype[14,15], expressing CD4, CD56, CD43, and CD123, with varying degrees of positivity for CD68, TdT, Bcl-2, and MUM1. They do not express CD20, CD3, CD5, CD34, CD117, and MPO. In our study, both cases expressed CD4, CD56, CD43, CD123, CD68, and Bcl-2. One case expressed TdT and S-100, while the other case expressed MUM1, consistent with the literature[15]. Both cases showed negative results for EBV-EBER in situ hybridization, suggesting no association with EBV infection. S-100 protein is expressed in 25%-30% of all cases[16], and possibly more frequently.
Approximately 60% to 70% of BPDCN patients exhibit genetic abnormalities. Common chromosomal deletions in BPDCN patients include 9p21.3 (CDKN2A/CDKN2B), 12p13.2-p13.1 (CDKN1B), 13q13.1-q14.3 (RB1), 13q11-q12 (LATS2), and 7p12.2 (IKZF1), with 9p21.3 deletions being the most common and potentially associated with poor prognosis[17-20]. Research has identified frequently mutated genes such as tet2 (most common), NPM1, ASXL1, NRAS, IDH2, APC, ATM, ZRSR2, SRSF2, SF3B1, U2AF1, SF3A2, SF3B4, TP53, GNB1, ETV6, DNMT3A, RUNX1, CRIPAK, NEFH, HNF1A, PAX3, and SSC5D[21]. However, BPDCN-specific genetic changes have not yet been identified.
The morphology of BPDCN overlaps with several tumors, necessitating differentiation: (1) Extranodal NK/T-cell lymphoma (nasal type): Tumor cells of varying sizes often invade the vascular wall with necrosis, lacking a clear cell-free zone between the epidermis. The typical immunophenotype is positive for CD2, CD3, CD56, and cytotoxic molecules, but negative for CD4, CD5, CD123, and TdT. Additionally, NK/T-cell lymphoma development is associated with EBV infection, with positive EBV and EBER; (2) Lymphoblastic lymphoma: This typically affects adolescents, while BPDCN primarily occurs in elderly patients. Lymphoblastic lymphoma tumor cells express various T or B cell-associated antigens but not CD56, CD123, or CD4; (3) Myeloid sarcoma or leukemia: Like BPDCN, it can present with skin, lymph node, and bone marrow involvement. Myeloid sarcoma expresses CD56, CD123, and CD4 but often weakly. Additionally, myeloid sarcoma usually expresses a range of myeloid markers such as MPO, CD117, and CD34, which are commonly not expressed in BPDCN; and (4) Peripheral T-cell lymphoma: Tumor cells vary in size, with irregular nuclei and significant epidermotropism. The typical immunophenotype is positive for T-cell markers and monoclonal TCR gene rearrangement but negative for CD123 and CD56.
Treatment options reported in the literature include local radiotherapy, glucocorticoids alone, COP-based chemo
In conclusion, BPDCN is a rare and highly malignant tumor with rapid progression and a very poor prognosis, often confused with other lymphohematopoietic disorders. Therefore, raising pathologists' awareness of its pathological features is necessary to avoid missed diagnoses or misdiagnoses. Additionally, supplemental systemic chemotherapy and hematopoietic stem cell transplantation can effectively improve patient survival.
| 1. | Patel JL, Shetty S, Salama ME. An unusual case of cutaneous blastic plasmacytoid dendritic cell neoplasm with concomitant B-cell lymphoproliferative disorder. Am J Dermatopathol. 2011;33:e31-e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Wang H, Cao J, Hong X. Blastic plasmacytoid dendritic cell neoplasm without cutaneous lesion at presentation: case report and literature review. Acta Haematol. 2012;127:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102:83-87. [PubMed] |
| 4. | Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2981] [Cited by in RCA: 3182] [Article Influence: 198.9] [Reference Citation Analysis (0)] |
| 5. | Trottier AM, Cerquozzi S, Owen CJ. Blastic plasmacytoid dendritic cell neoplasm: challenges and future prospects. Blood Lymphat Cancer. 2017;7:85-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703-1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 2142] [Article Influence: 714.0] [Reference Citation Analysis (0)] |
| 7. | Sweet K. Blastic plasmacytoid dendritic cell neoplasm: diagnosis, manifestations, and treatment. Curr Opin Hematol. 2020;27:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Pemmaraju N, Lane AA, Sweet KL, Stein AS, Vasu S, Blum W, Rizzieri DA, Wang ES, Duvic M, Sloan JM, Spence S, Shemesh S, Brooks CL, Balser J, Bergstein I, Lancet JE, Kantarjian HM, Konopleva M. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N Engl J Med. 2019;380:1628-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 9. | Deconinck E. Blastic Plasmacytoid Dendritic Cell Neoplasm: The European Perspective. Hematol Oncol Clin North Am. 2020;34:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Venugopal S, Zhou S, El Jamal SM, Lane AA, Mascarenhas J. Blastic Plasmacytoid Dendritic Cell Neoplasm-Current Insights. Clin Lymphoma Myeloma Leuk. 2019;19:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Li Y, Sun V, Sun W, Pawlowska A. Blastic Plasmacytoid Dendritic Cell Neoplasm in Children. Hematol Oncol Clin North Am. 2020;34:601-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Cuglievan B, Connors J, He J, Khazal S, Yedururi S, Dai J, Garces S, Quesada AE, Roth M, Garcia M, McCall D, Gibson A, Ragoonanan D, Petropoulos D, Tewari P, Nunez C, Mahadeo KM, Tasian SK, Lamble AJ, Pawlowska A, Hammond D, Maiti A, Haddad FG, Senapati J, Daver N, Gangat N, Konopleva M, Meshinchi S, Pemmaraju N. Blastic plasmacytoid dendritic cell neoplasm: a comprehensive review in pediatrics, adolescents, and young adults (AYA) and an update of novel therapies. Leukemia. 2023;37:1767-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Pagano L, Valentini CG, Grammatico S, Pulsoni A. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016;174:188-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Sakamoto K, Baba S, Okumura Y, Momose S, Ono S, Tonooka A, Ichinohasama R, Takakuwa E, Nakasone H, Ohshima K, Takeuchi K. Comparison and Development of Immunohistochemical Diagnostic Criteria for Blastic Plasmacytoid Dendritic Cell Neoplasm. Mod Pathol. 2023;36:100253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Julia F, Dalle S, Duru G, Balme B, Vergier B, Ortonne N, Vignon-Pennamen MD, Costes-Martineau V, Lamant L, Dalac S, Delattre C, Déchelotte P, Courville P, Carlotti A, De Muret A, Fraitag S, Levy A, Mitchell A, Petrella T. Blastic plasmacytoid dendritic cell neoplasms: clinico-immunohistochemical correlations in a series of 91 patients. Am J Surg Pathol. 2014;38:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Lucioni M, Novara F, Fiandrino G, Riboni R, Fanoni D, Arra M, Venegoni L, Nicola M, Dallera E, Arcaini L, Onida F, Vezzoli P, Travaglino E, Boveri E, Zuffardi O, Paulli M, Berti E. Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood. 2011;118:4591-4594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Ruhangaza D, Mugabe MC, Kigonya CN, Lane AA, Morgan EA. Blastic Plasmacytoid Dendritic Cell Neoplasm: First Case Report From Rwanda and Review of the Literature. J Glob Oncol. 2019;5:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Yin CC, Pemmaraju N, You MJ, Li S, Xu J, Wang W, Tang Z, Alswailmi O, Bhalla KN, Qazilbash MH, Konopleva M, Khoury JD. Integrated Clinical Genotype-Phenotype Characteristics of Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Sullivan JM, Rizzieri DA. Treatment of blastic plasmacytoid dendritic cell neoplasm. Hematology Am Soc Hematol Educ Program. 2016;2016:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Petrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M, Meijer CJ, Courville P, Joly P, Grange F, De Muret A, Machet L, Dompmartin A, Bosq J, Durlach A, Bernard P, Dalac S, Dechelotte P, D'Incan M, Wechsler J, Teitell MA. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123:662-675. [PubMed] [DOI] [Full Text] |
| 21. | Menezes J, Acquadro F, Wiseman M, Gómez-López G, Salgado RN, Talavera-Casañas JG, Buño I, Cervera JV, Montes-Moreno S, Hernández-Rivas JM, Ayala R, Calasanz MJ, Larrayoz MJ, Brichs LF, Gonzalez-Vicent M, Pisano DG, Piris MA, Álvarez S, Cigudosa JC. Exome sequencing reveals novel and recurrent mutations with clinical impact in blastic plasmacytoid dendritic cell neoplasm. Leukemia. 2014;28:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Ohgami RS, Aung PP, Gru AA, Hussaini M, Singh K, Querfeld C, Yao K, Small C, Gollapudi S, Jaye D, Wang SA, Pullarkat S, George TI. An Analysis of the Pathologic Features of Blastic Plasmacytoid Dendritic Cell Neoplasm Based on a Comprehensive Literature Database of Cases. Arch Pathol Lab Med. 2023;147:837-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |