Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.5177
Revised: May 28, 2024
Accepted: June 13, 2024
Published online: August 6, 2024
Processing time: 110 Days and 7.2 Hours
Atezolizumab/bevacizumab is emerging as the new standard for advanced hepatocellular carcinoma (HCC), with ongoing real-world implementation to study its effectiveness. As the use of atezolizumab/bevacizumab increases, various side effects have been reported in clinical practice, most notably increased bleeding caused by bevacizumab.
In this case report, we present a rare and fatal case of intratumoral hemorrhage in a patient with advanced HCC following successful treatment with atezolizumab/bevacizumab. A 63-year-old male diagnosed with HCC initially underwent four cycles of intra-arterial chemotherapy. However, follow-up abdominal computed tomography (CT) revealed disease progression. Subse
Atezolizumab/bevacizumab for advanced HCC suggests that intratumoral hemorrhage may be crucial despite good tumor response after immunotherapy, emphasizing the continuous monitoring of this side effect.
Core Tip: Our study delves into the emerging therapy of atezolizumab/bevacizumab for unresectable hepatocellular carcinoma (HCC), contrasting its efficacy with traditional treatments like sorafenib. We highlight the real-world challenges of bleeding complications, particularly intratumoral bleeding, associated with bevacizumab. Presenting a rare case, we emphasize the need for proactive measures, such as baseline examinations, to mitigate bleeding risks before initiating atezo
- Citation: Park KH, Yoo JJ, Kim SG, Kim YS. Fatal intratumoral hemorrhage in a patient with hepatocellular carcinoma following successful treatment with atezolizumab/bevacizumab: A case report. World J Clin Cases 2024; 12(22): 5177-5183
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/5177.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.5177
Atezolizumab/bevacizumab, a combination therapy, is emerging as a promising treatment option for unresectable hepatocellular carcinoma (HCC). Until recently, sorafenib and lenvatinib were the sole systemic therapy options for patients with advanced HCC[1]. However, the IMbrave150 study revealed that atezolizumab/bevacizumab demon
Bleeding is a well-known side effect of bevacizumab, and the IMbrave150 study proposed that patients with untreated esophagogastric varices should be carefully treated before initiating atezolizumab/bevacizumab. The incidence of upper gastrointestinal bleeding in IMbrave150 was reported to be 7%, with documented cases of esophageal variceal bleeding[2]. In real-world settings, the incidence of bleeding events is reported to be 30.3%, significantly higher than in clinical trials[6]. The challenge lies in the absence of a standardized examination or guideline for proactively mitigating the risk of bleeding before initiating atezolizumab/bevacizumab. Regarding variceal bleeding, there is a growing consensus advocating for the implementation of baseline esophagogastroduodenoscopy (EGD) examinations prior to initiating atezolizumab/bevacizumab for all patients with HCC[7]. However, the incidence, prevention, and treatment of bleeding other than variceal bleeding are not well known. In particular, most patients with HCC have underlying liver cirrhosis and a high tendency to bleed, necessitating clinical attention. Here, we present a rare case of intratumoral bleeding attributed to atezolizumab/bevacizumab in a patient with HCC.
A patient undergoing treatment with atezolizumab/bevacizumab for hepatocellular carcinoma caused by chronic hepatitis C presented to the emergency room with sudden abdominal pain.
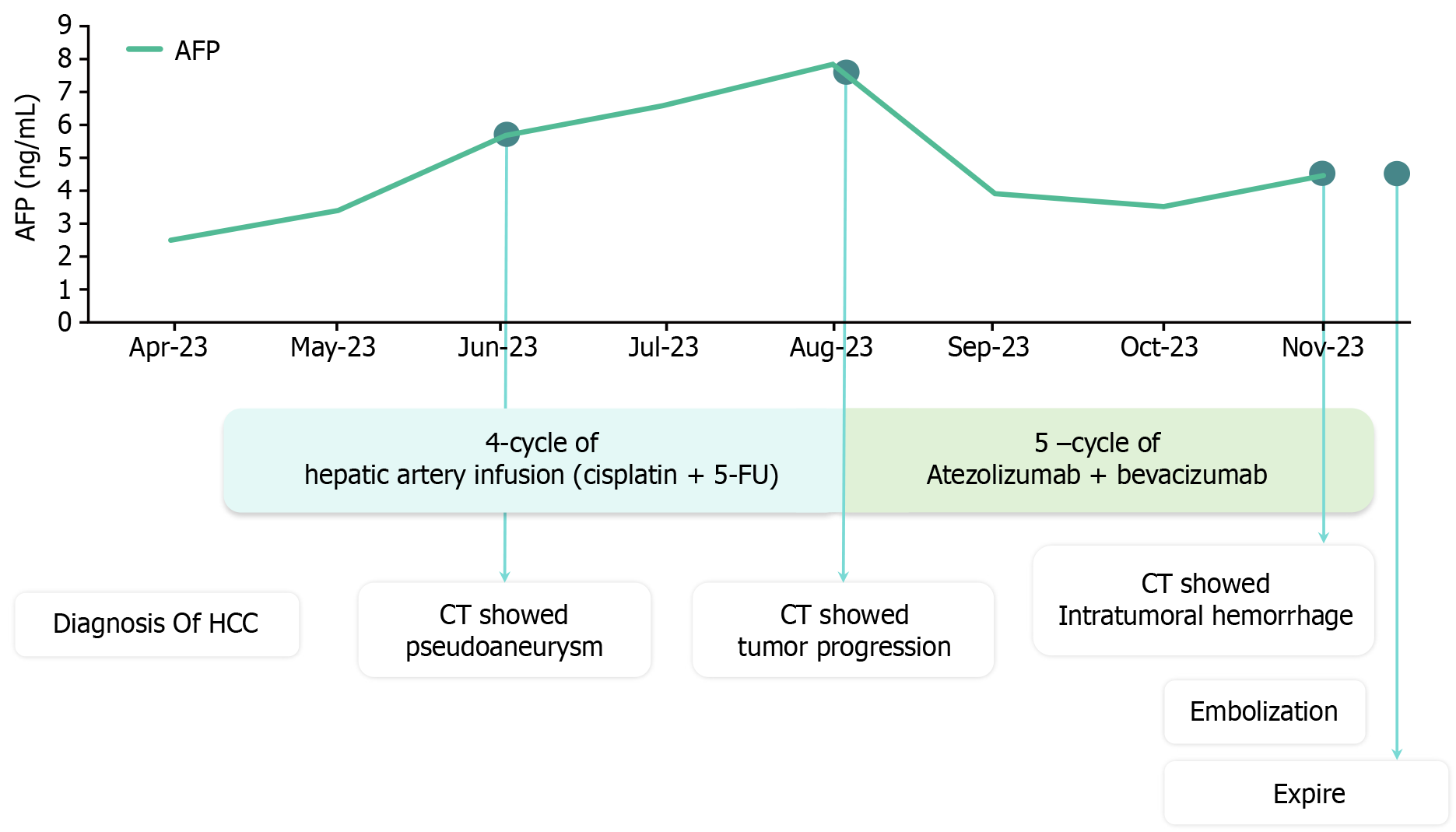
The patient's overall diagnosis and treatment status are summarized in Figure 1. A 63-year-old man diagnosed with hepatitis C, genotype 1b, at a hospital in China in 2017 underwent treatment with a direct-acting antiviral and had since achieved a sustained virologic response. However, the patient did not visit the hospital after 2017, and surveillance tests for HCC were not performed.
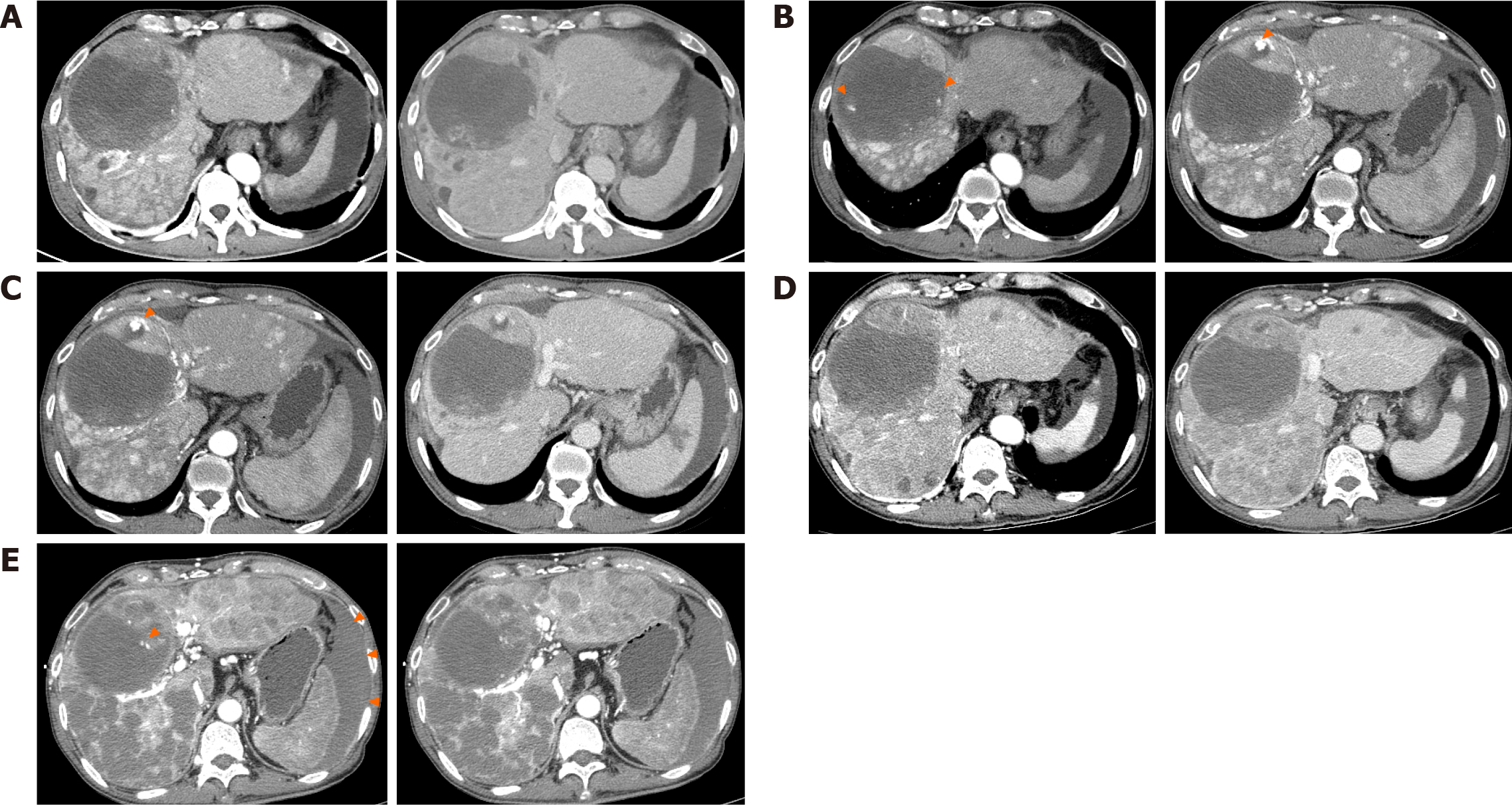
In April 2023, he visited a hospital in South Korea with abdominal pain and distension, and was diagnosed with advanced HCC and cirrhosis. The 3-phase computed tomography (CT) revealed a hepatic mass, with the largest lesion located in S4/8 measuring up to 11.7 cm (Figure 2A) and involving metastasis to the T12 spine. The cancer stage was modified Union for International Cancer Control stage IVB, T4N0M1, and Barcelona Clinic Liver Cancer stage D. The baseline alpha-fetoprotein (AFP) serum level was 2.5 ng/mL. The underlying liver was accompanied by cirrhosis and a moderate amount of ascites. Liver function had a Child-Turcotte-Pugh score of 8, and the Model for End-Stage Liver Disease score was 14. In EGD, minimal esophageal varices without red color sign were observed, and gastric varices were not present. The Eastern Cooperative Oncology Group performance status score was 1.
Due to the patient's reduced liver function, categorized as Child-Pugh class B (8), sorafenib, lenvatinib, or atezoli
However, after the fourth cycle of hepatic arterial infusion chemotherapy, the CT scan revealed progression of the viable tumor at the anterior aspect of the HCC and other extensive HCCs involving both lobes of the liver (Figure 2C), along with increasing AFP levels.
After the failure of hepatic arterial infusion chemotherapy, the patient was started on atezolizumab/bevacizumab. Atezolizumab (Tecentriq® 1200 mg per dose; Roche International LLC., Basel, Switzerland) and bevacizumab (Avastin® 15 mg/kg per dose; Genentech, South San Francisco, CA, United States) were administered every 3 wk. Following the third cycle of immunotherapy, the treatment response was formally evaluated using a CT scan. We observed a decrease in arterial enhancement in extensive HCCs involving both hemilivers, and the tumor response was evaluated as a partial response according to the modified Response Evaluation Criteria in Solid Tumors criteria (Figure 2D).
Seven days after the fifth cycle of atezolizumab/bevacizumab, he presented to the emergency room due to abrupt abdominal pain, rated 8 on the numerical rating scale.
Apart from chronic hepatitis C, the patient had no significant medical history.
There was no family history of any diseases or cancer.
His vital signs were generally normal: Blood pressure 128/60 mmHg, body temperature 36.8 ℃, heart rate 58 bpm, and breathing rate 24 breaths per minute.
Laboratory findings were as follows: White blood cell count of 17.78 × 103/μL, hemoglobin of 8.5 g/dL, platelet count 101 × 103/μL, and prothrombin time/international normalized ratio of 4.18.
A contrast-enhanced abdominal CT scan revealed a focal rupture of HCC in the medial segment inferior portion (previous intratumoral pseudoaneurysm) with active bleeding and a large amount of hemoperitoneum. Additionally, more necrosis of HCCs was observed in both lobes (Figure 2E).
The patient was diagnosed with an intratumoral rupture that occurred during atezolizumab/bevacizumab treatment for HCC.
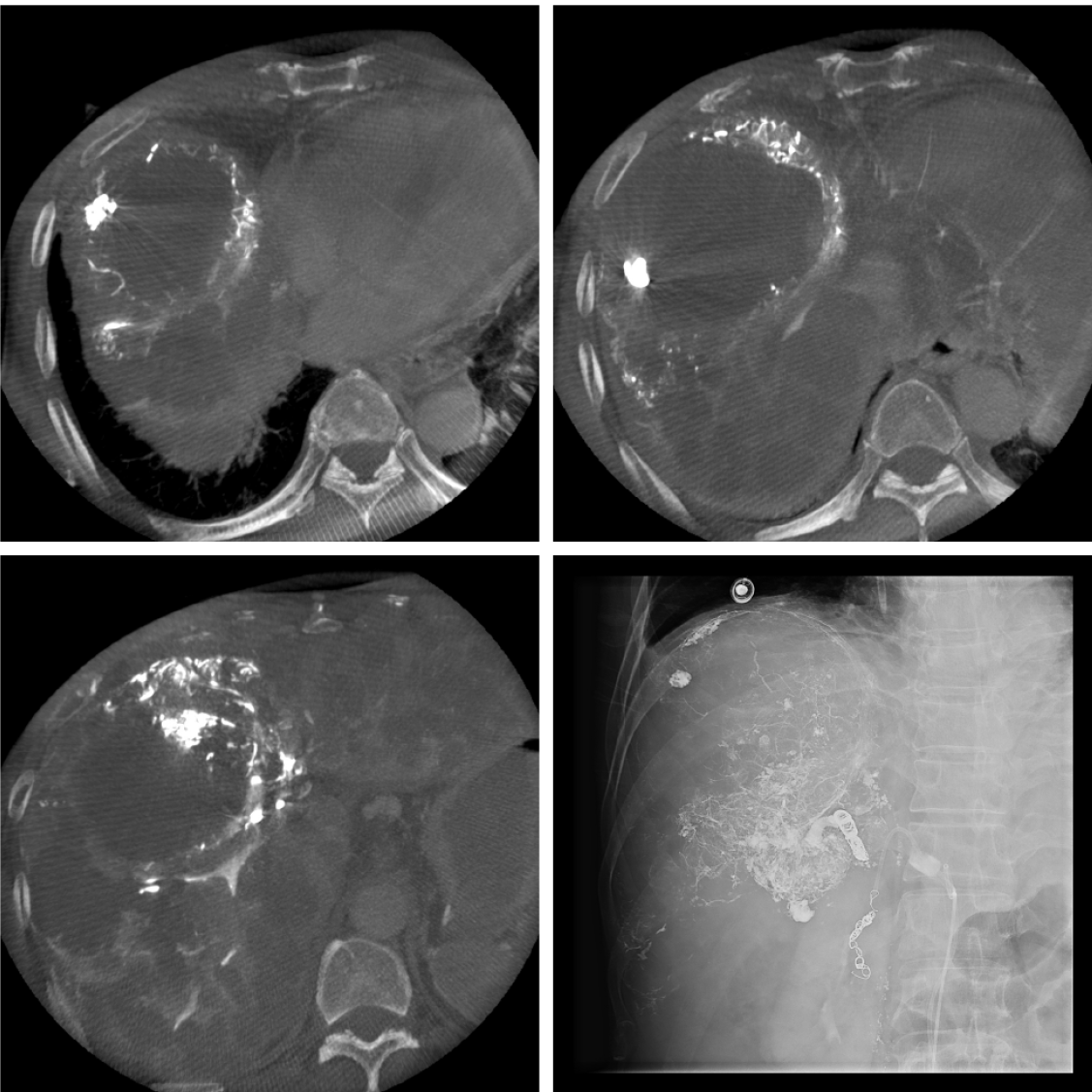
Emergency transcatheter hemostasis was performed due to the identified intratumor bleeding. Angiography revealed active contrast extravasation from the S4 tumor. Selective embolization of these vessels using a gelatin sponge and lipiodol was performed. After embolization, dynamic contrast-enhanced CT confirmed the complete hemostasis of the intratumoral hemorrhage (Figure 3).
Despite the successful embolization, the patient’s liver function gradually deteriorated, and hepatorenal syndrome developed on the 10th day after embolization. The patient died 12 d after embolization due to the worsening conditions of liver and kidney function.
In this case report, we present a rare occurrence where a patient with HCC experienced effective tumor regression following the administration of atezolizumab/bevacizumab but ultimately succumbed to intratumoral hemorrhage. We posit a close association between intratumoral hemorrhage and atezolizumab/bevacizumab.
Bevacizumab, the pioneering angiogenesis inhibitor, is a monoclonal antibody targeting vascular endothelial growth factor A (VEGF-A). It has been employed in various solid cancers, including colorectal cancer, non-small cell lung cancer (NSCLC), breast cancer, glioblastoma, and ovarian cancer[8]. The mechanism responsible for bevacizumab-related bleeding is not entirely elucidated, but one of the key contributing factors is the inhibition of VEGF, impacting the integrity of blood vessels and elevating the risk of bleeding events. Furthermore, bevacizumab-induced disruption of normal angiogenesis and vascular function can contribute to the occurrence of bleeding[8].
The most frequent side effects of bevacizumab include hypertension, proteinuria, epistaxis, gastrointestinal symptoms, and impaired wound healing. While less common, severe adverse events encompass gastrointestinal perforation, arterial thrombotic events, and hemorrhage. The bleeding risk may be dose-dependent and varies with the tumor type, but the incorporation of bevacizumab in cancer chemotherapy significantly heightens the risk[9]. Serious tumor-associated hemorrhage events were observed in specific indications, such as pulmonary hemorrhage/hemoptysis in NSCLC, or more rarely, GI bleeding in metastatic colorectal cancer patients, and central nervous system (CNS) bleeding in patients with CNS metastases[10,11].
We conducted a literature review on cases of intratumoral hemorrhage following atezolizumab/bevacizumab treatment in patients with HCC, similar to our case, and identified two comparable instances (Table 1)[12,13]. In the first case[6], intratumoral hemorrhage in rib metastasis occurred 1 mo after atezolizumab/bevacizumab. The patient presented with fatigue symptoms, leading to hospital admission, where a CT examination revealed bleeding. Emergency transcatheter arterial embolization successfully halted the bleeding, and atezolizumab/bevacizumab treatment was resumed. There was no recurrence of bleeding during the continuation of treatment for more than 6 mo.
| Ref. | Year | Country | Sex | Age | Etiology of liver disease | Onset | Treatment | Prognosis |
| Mitsuyama et al[12] | 2023 | Japan | Male | 73 | NA | 1 mo after atezolizumab/bevacizumab | Embolization | Survive |
| Matsumoto et al[13] | 2023 | Japan | Male | 70 | HBV | After 4th administration of atezolizumab/bevacizumab | Supportive care | Expire |
| The case reported in this study | 2023 | South Korea | Male | 63 | HCV | After 5th administration of atezolizumab/bevacizumab | Embolization | Expire |
In the second case[7], atezolizumab/bevacizumab was administered as the first-line treatment for a large, unresectable HCC in a patient positive for hepatitis B virus antigen. The patient initiated a combination therapy of atezolizumab (1200 mg) and bevacizumab (700 mg), but various interventions were attempted due to complications related to intratumoral bleeding. These interventions included transarterial chemoembolization, switching to atezolizumab monotherapy, and resuming bevacizumab in addition to atezolizumab due to further progression of HCC. However, the treatment was ultimately discontinued due to complications associated with intratumoral bleeding, manifesting as abdominal pain, severe inflammation, impaired liver function, loss of appetite, and fatigue. Eventually, the patient in the second case succumbed to liver failure, mirroring the outcome in our patient.
The primary challenge at present is the absence of identified prevention or treatment strategies for this uncommon intratumoral hemorrhage. Following the second hepatic arterial infusion chemotherapy, we conducted angiography for pseudoaneurysm; however, embolization was not performed as there was no evidence of bleeding. In hindsight, even in the absence of apparent bleeding, preventive embolization could have been considered to potentially avert fatal bleeding in the future. Yet, the effectiveness or safety of prophylactic embolization for pseudoaneurysm in patients without evidence of intratumoral bleeding remains undisclosed. In summary, this case underscores the varied and sometimes fatal bleeding complications that can arise after atezolizumab/bevacizumab treatment in patients with hepatocellular carcinoma.
Patients undergoing atezolizumab/bevacizumab therapy for HCC should undergo vigilant monitoring for bleeding complications. Physicians need to remain attentive to unusual or atypical bleeding manifestations. In cases of severe abdominal pain during atezolizumab/bevacizumab chemotherapy, a prompt diagnostic imaging test such as CT or angiography should be considered. If intratumoral hemorrhage is identified, early angiography and intervention become imperative. Additionally, careful consideration should be given to the possibility of discontinuing bevacizumab.
| 1. | Lee MMP, Chan LL, Chan SL. The role of lenvatinib in the era of immunotherapy of hepatocellular carcinoma. J Liver Cancer. 2023;23:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4682] [Article Influence: 936.4] [Reference Citation Analysis (2)] |
| 3. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 574] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 4. | Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer. 2023;23:1-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 85] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 5. | Lee YR. A multidisciplinary approach with immunotherapies for advanced hepatocellular carcinoma. J Liver Cancer. 2023;23:316-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Himmelsbach V, Pinter M, Scheiner B, Venerito M, Sinner F, Zimpel C, Marquardt JU, Trojan J, Waidmann O, Finkelmeier F. Efficacy and Safety of Atezolizumab and Bevacizumab in the Real-World Treatment of Advanced Hepatocellular Carcinoma: Experience from Four Tertiary Centers. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Ha Y, Kim JH, Cheon J, Jeon GS, Kim C, Chon HJ. Risk of Variceal Bleeding in Patients With Advanced Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab. Clin Gastroenterol Hepatol. 2023;21:2421-2423.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 725] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 9. | Hang XF, Xu WS, Wang JX, Wang L, Xin HG, Zhang RQ, Ni W. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2011;67:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Cao D, Guo CH, Liu JW, Yang X, Li Q. Bleeding after bevacizumab treatment in patients with metastatic colorectal cancer. Tumori. 2015;101:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Letarte N, Bressler LR, Villano JL. Bevacizumab and central nervous system (CNS) hemorrhage. Cancer Chemother Pharmacol. 2013;71:1561-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Mitsuyama Y, Kageyama K, Shinkawa H, Yamamoto A, Jogo A, Sohgawa E, Tanaka S, Takemura S, Kubo S, Ishizawa T, Miki Y. Atezolizumab plus bevacizumab-induced intratumoral hemorrhage in a patient with rib metastasis from unresectable hepatocellular carcinoma. Radiol Case Rep. 2023;18:3037-3040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Matsumoto M, Noritake H, Yamashita M, Hanaoka T, Umemura M, Kitsugi K, Takatori S, Ohta K, Ito J, Chida T, Kawata K. A case of hepatitis B virus-infected patient with bevacizumab-related severe intratumor hemorrhage of large hepatocellular carcinoma (HCC). Acta hepatologica Japonica. 2023;64:382-392. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |