Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.4992
Revised: May 29, 2024
Accepted: June 14, 2024
Published online: August 6, 2024
Processing time: 62 Days and 21.8 Hours
Perimenopausal syndrome (PMS) is a chronic disease associated with estrogen deficiency. Because of the unsatisfactory outcomes of current conventional treatments for this condition, its treatment must be continuously explored and optimized.
To assess the clinical effectiveness of γ-oryzanol in combination with Femoston for PMS.
A total of 119 patients with PMS were selected from June 2023 to December 2023, which included 59 and 60 patients in the control and observation group, respectively. The control and observation groups were treated with Femoston and γ-oryzanol + Femoston, respectively. Comparative analyses were performed in terms of clinical effectiveness, safety (dizziness and headache, nausea and vomi
Compared with the control group, the observation group had statistically higher total effective rates of treatment; lower overall incidence of adverse events; higher post-treatment E2 levels and L1–4 and bilateral femoral BMD; and lower LH and FSH levels, sleeping time, and frequency of awakenings from sleep after trea
Therefore, for the treatment of PMS, γ-oryzanol combined with Femoston is significantly better than Femoston alone in terms of clinical effectiveness, exhibiting more pronounced clinical advantages in improving safety, sex hormone levels, BMD, and sleep quality.
Core Tip: Perimenopausal syndrome (PMS) is a chronic condition associated with estrogen deficiency. Hormone replacement therapy is the conventional treatment for PMS; however, because of possible adverse events such as stroke, breast cancer, endometrial cancer, glucose intolerance, and thromboembolic disease, its treatment alternatives must be continuously explored and optimized. Compared with Femoston monotherapy, proposed combination therapy of γ-oryzanol and Femoston has certain clinical advantages, including significantly better clinical efficacy, for treating patients with PMS. This treatment can prevent adverse reactions, actively regulate sex hormone levels, increase patient bone mineral density, and actively help improve sleep quality.
- Citation: Kuang YY, Xiong MQ, Cai JX. Clinical efficacy of gamma-oryzanol combined with Femoston for perimenopausal syndrome. World J Clin Cases 2024; 12(22): 4992-4998
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/4992.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.4992
Perimenopausal syndrome (PMS) is a chronic disease related to estrogen deficiency, which compromises patients’ sleep and quality of life while imposing more burden on their families and society[1,2]. In addition, decreased estrogen secretion inhibits the ability of the small intestines to absorb calcium, thereby reducing bone mineral density (BMD) and increasing the risk of osteoporosis[3]. Previous studies have revealed that age, employment status, personality traits, and constipation are risk factors for PMS, and menstrual irregularities, constipation, severe PMS, and family disharmony also contribute to an increased risk of depression in patients with PMS[4]. According to statistics, PMS risk and severity are higher in rural women than in urban women, and fatigue, muscle and joint pain, and vertigo are the three most common symptoms[5]. The pathological mechanism of PMS is closely associated with the hypothalamus–pituitary–ovary axis dysfunction and imbalances in neurotransmitters or hormones[6,7]. PMS is usually treated by hormone replacement therapy; however, some patients are hesitant to receive it because of possible adverse events such as stroke, breast and endometrial cancer, glucose intolerance, and thromboembolic disease[8]. Therefore, the treatment strategy for PMS requires further exploration and optimization, which is of great value in enhancing patient efficacy and reducing related adverse reactions.
Femoston, a new hormone replacement drug, is a compound preparation of estradiol (E2) tablets and dydrogesterone (DG) tablets, with E2 and DG as its active ingredients[9]. Among them, E2, a natural estrogen, helps regulate bone metabolism and the nervous system[10]. As a progestational hormone, DG has a molecular structure similar to that of natural progesterone and relatively high activity, which can help regulate sex hormone balance in women and relieve clinical symptoms[11,12]. In a rat model experiment, enhanced E2 production effectively alleviated PMS in rats[13]. Studies have indicated that E2 and DG, when used in combination, can effectively relieve menopausal symptoms while exerting a good preventive effect on osteoporosis, with a favorable safety profile[14].
γ-oryzanol is one of the rice bran oil compound that has anti-inflammatory, antitumor, antioxidant, and antidiabetic pharmacological actions and relieves menopausal symptoms[15]. In a mouse experiment, γ-oryzanol alleviated the depressive behavior of ovariectomized mice by modulating the estrogen receptor β–neuronal nitric oxide synthase– extracellular signal-regulated kinase–cyclic adenosine monophosphate response element-binding protein-brain-derived neurotrophic factor (BDNF) molecular network, suggesting some therapeutic potential of γ-oryzanol in PMS[16].
In this study, we included 119 patients with PMS to comparatively analyze the clinical effectiveness of γ-oryzanol plus Femoston for PMS and validate the clinical advantages of this combined therapy.
This retrospective study selected 119 patients with PMS treated between June 2023 and December 2023 in the Jiujiang Maternal and Child Health Hospital. Of these patients, 59 (control group) were given Femoston, and 60 (observation group) received γ-oryzanol + Femoston. The intergroup comparison of the patients’ general information revealed no notable differences (P > 0.05).
Inclusion criteria: All patients diagnosed with PMS after examination; met the Kupperman index[17]; had symptoms (such as paresthesia, hot flashes, sweating, insomnia, dizziness, anxiety, depression, joint and muscle pain, fatigue, palpitations), complete case data, normal communication and cognitive abilities as well as no history of treatment for PMS.
Exclusion criteria: Ovarian dysfunction caused by organic ovarian lesions and space-occupying lesions; pregnant or suspected of pregnancy; diagnosed or suspected malignancy related to sex hormones; other endocrine system diseases; unexplained irregular vaginal bleeding; allergies to the drugs used in this study; long-term use of sedative and analgesic drugs; and psychiatric disorders.
The control group was treated with Femoston. Femoston was administered 1 tablet/time, once a day. Over the 28-day course, one red tablet containing E2 (2 mg) and one yellow tablet containing E2 (2 mg) and DG (10 mg) were administered to the participants in the first 14 and the last 14 days, respectively.
The observation group received oral administration of γ-oryzanol (10 mg/tablet), 1 tablet/time, 3 times a day, in addition to the above treatment. The total treatment duration was 12 weeks. Both groups were advised to maintain a good mood and eat a healthy diet.
The clinical effectiveness, safety (dizziness and headache, nausea and vomiting, and breast tenderness), sex hormones [E2, luteinizing hormone (LH), and follicle-stimulating hormone (FSH)], lumbar spine (L1–4) and bilateral femoral BMD, and sleep quality (sleeping time and frequency of awakenings from sleep) were compared and analyzed.
Clinical effectiveness: The efficacy evaluation followed the criteria in the modified Kupperman index, i.e., a reduction of > 80% in the total score after treatment, a reduction of 50%–80%, and a reduction of < 50% or even a worsening of symptoms are considered markedly effective, effective, and ineffective, respectively. Total effective rate = (significant effective and effective cases)/total case number.
Safety: The evaluation was mainly based on observing and recording the number of adverse reactions such as dizziness, headache, nausea and vomiting, and breast tenderness.
Sex hormones: Venous blood (3 mL) was collected from each patient before and after treatment, and the supernatant was collected after centrifugation to quantify the levels of sex hormones (E2, LH, and FSH) by radioimmunoassay.
BMD: Lumbar spine (L1–4) and bilateral femoral BMD values were measured by X-ray absorptiometry before and after treatment.
Sleep quality: The sleeping time and frequency of awakenings from sleep in the two groups were counted.
Measurement data, presented as mean ± SEM, were analyzed by independent sample t-tests (comparisons between groups) and paired t-tests (comparisons before and after treatment). Count data were expressed as rates (percentage), and the comparison between the two groups of count data was made using the chi-square test. Data were analyzed using IBM SPSS Statistics for Windows version 22.0. Statistical significance was present when the P value was < 0.05.
No significant intergroup difference was observed in terms of age, disease course, body mass, Kupperman index, and family history (P > 0.05) (Table 1).
| Factors | Control group (n = 59) | Observation group (n = 60) | χ2/t | P value |
| Age (years) | 46.29 ± 6.47 | 45.97 ± 5.33 | 0.295 | 0.769 |
| Disease course (months) | 5.98 ± 0.94 | 6.17 ± 1.45 | 0.847 | 0.399 |
| Body mass (kg) | 60.83 ± 7.05 | 60.05 ± 7.71 | 0.576 | 0.566 |
| Kupperman index (points) | 28.51 ± 10.37 | 30.25 ± 11.89 | 0.850 | 0.397 |
| Family medical history | 1.245 | 0.265 | ||
| With, n (%) | 9 (15.25) | 14 (23.33) | ||
| Without, n (%) | 50 (84.75) | 46 (76.67) |
The total effective rates were 71.19% and 93.33% in the control and observation groups, respectively, with a significant intergroup difference (P < 0.05) (Table 2).
| Efficacy assessment | Control group (n = 59) | Observation group (n = 60) | χ2 | P value |
| Markedly effective | 18 (30.51) | 35 (58.33) | ||
| Effective | 24 (40.68) | 21 (35.00) | ||
| Ineffective | 17 (28.81) | 4 (6.67) | ||
| Total effective rate | 42 (71.19) | 56 (93.33) | 10.040 | 0.002 |
The number and percentage of cases of dizziness and headache, nausea and vomiting, and breast tenderness in the two groups were counted. The total incidence in the control group (15.25%) was higher than that in the observation group (3.33%) (P < 0.05) (Table 3).
| Adverse reactions | Control group (n = 59) | Observation group (n = 60) | χ2 | P value |
| Dizziness and headache | 2 (3.39) | 0 (0.00) | ||
| Nausea and vomiting | 3 (5.08) | 0 (0.00) | ||
| Breast tenderness | 4 (6.78) | 2 (3.33) | ||
| Total | 9 (15.25) | 2 (3.33) | 5.039 | 0.025 |
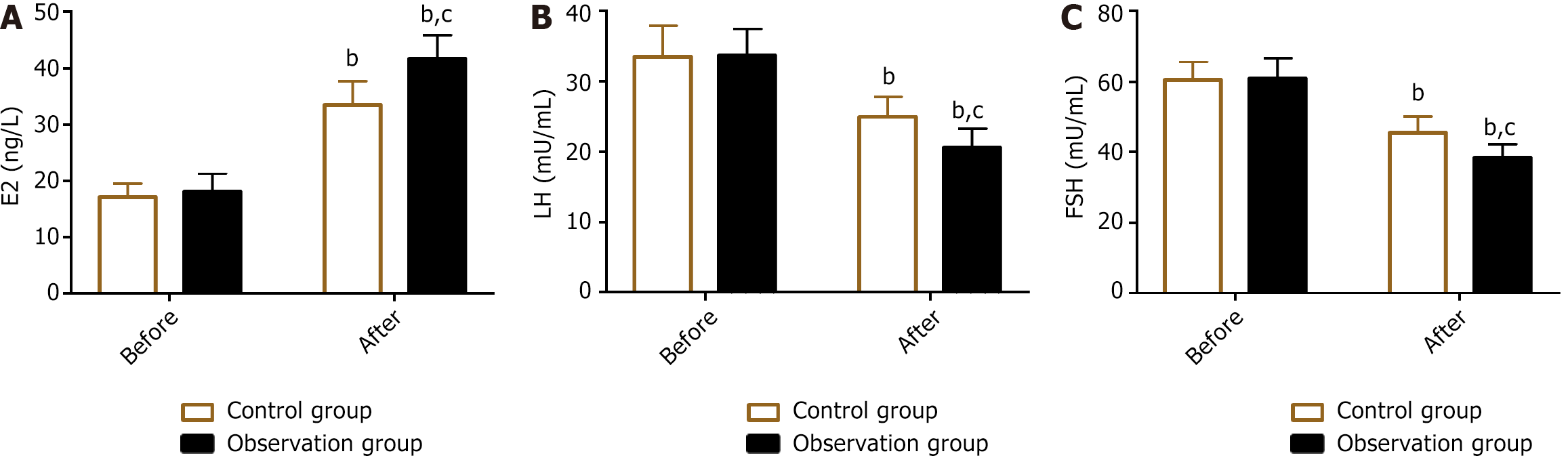
The sex hormones detected were E2, LH, and FSH. Their levels were not significantly different between the groups before treatment (P > 0.05). The levels of E2 increased in both groups, whereas LH and FSH levels were inhibited after treatment (P < 0.05). In comparison with the control group, the observation group had E2 levels but lower LH and FSH levels (P < 0.05) (Figure 1).
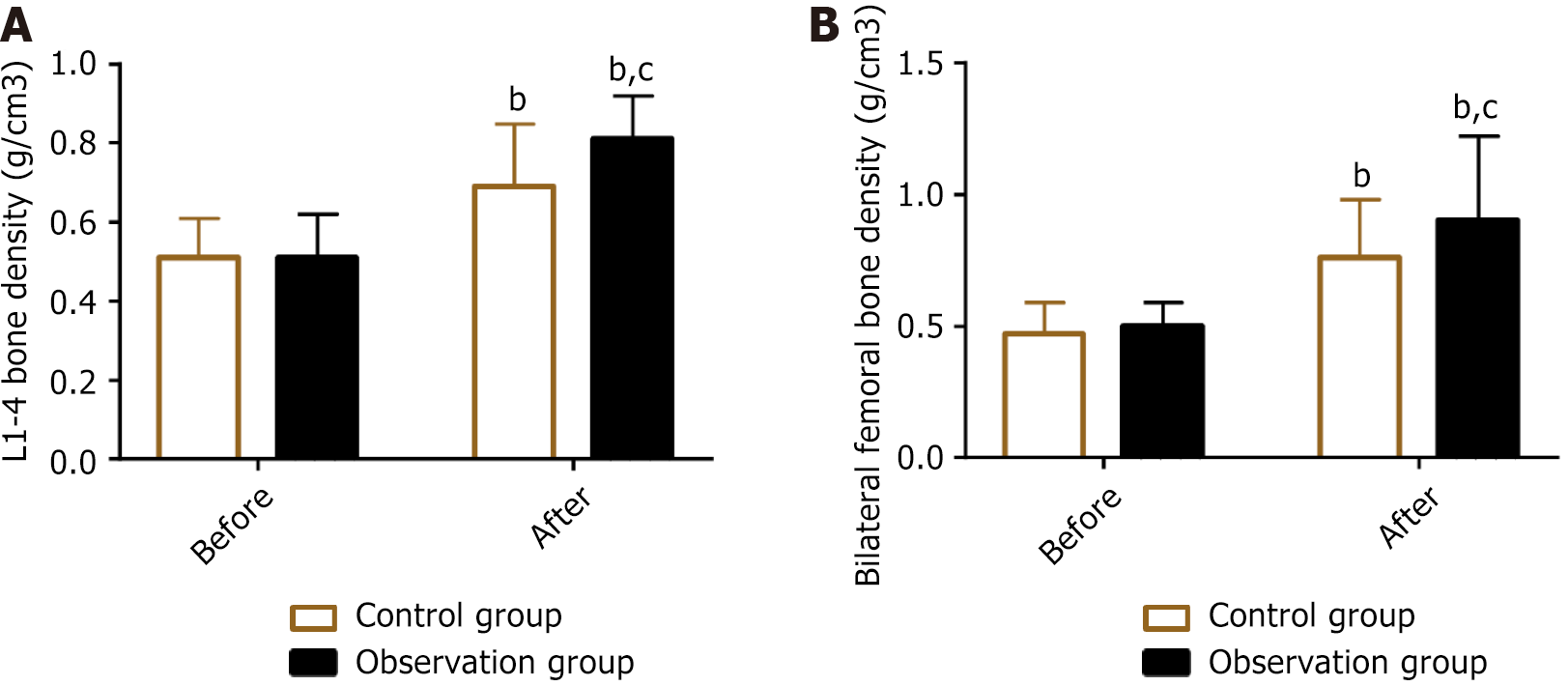
The pre- and post-treatment lumbar spine (L1–4) and bilateral femoral BMD values were measured in both groups. The results showed no significant intergroup difference in BMD before treatment (P > 0.05). The BMD of the lumbar spine (L1–4) and bilateral femurs in both groups increased significantly after treatment, with a more significant improvement in the observation group (P < 0.05) (Figure 2).
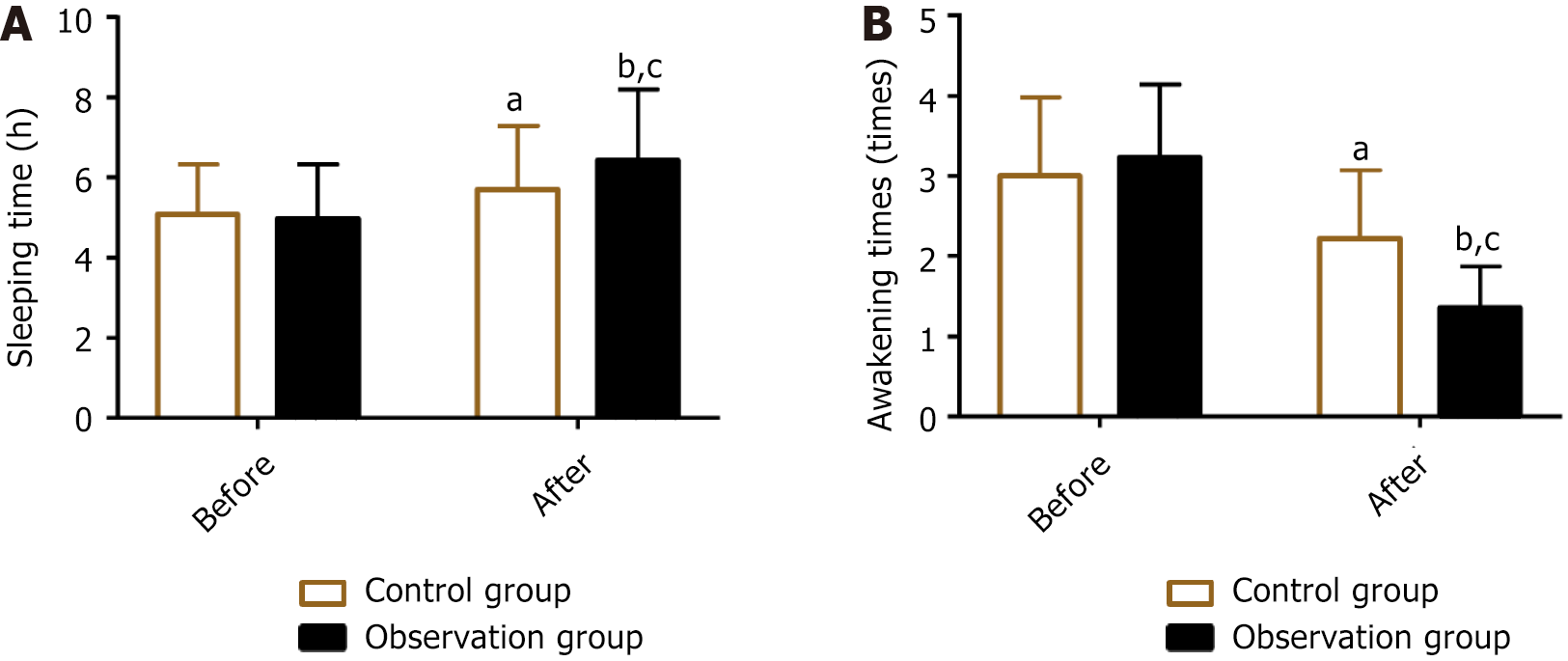
The sleep quality of both groups was analyzed by evaluating the sleeping time and frequency of awakenings from sleep before and after treatment. No significant difference in pretreatment sleeping time and frequency of awakenings from sleep was found between the two groups (P > 0.05). After treatment, both groups had increased sleeping time and reduced frequency of awakenings from sleep (P < 0.05), with the observation group demonstrating better performance than the control group (P < 0.05) (Figure 3).
Perimenopause is a stage of physiological changes experienced by women in middle age, where ovarian function gradually declines and menstrual cycles are irregular, with approximately 40%–60% of perimenopausal women experiencing PMS[18,19]. Women with PMS are also at increased risk of adverse events such as intracerebral hemorrhage, depression, hypertension, myocardial infarction, osteoporosis, and atherosclerosis[20,21]. In this study, we proposed a combination of γ-oryzanol and Femoston to confirm its potential advantage in the prevention and treatment of PMS, rendering more useful clinical evidence for PMS management.
γ-oryzanol, a steryl ferulate, can be extracted from the rice bran layer, which can repair metabolic disorders by improving insulin activity, cholesterol metabolism, and mitigating chronic inflammation[22]. In an animal study, long-term γ-oryzanol intervention in an obese mouse model improved lipid metabolism[23]. In this study, the total effective rate was higher in the observation group than in the control group, suggesting that the treatment scheme of γ-oryzanol combined with Femoston used in the observation group is significantly effective in enhancing curative effects. Previous evidence suggests that γ-oryzanol has anxiolytic effects on long-term stressed mice, and the mechanism may be related to its promotion of 5-hydroxyindoleacetic acid and norepinephrine levels in the brain[24]. Evidence also shows that γ-oryzanol exerts anti-anxiety, neurogenic, and anti-neuroinflammatory properties to certain extent and inhibits alcohol-related anxiety-like behavior in mice by upregulating central monoamines related to BDNF and interleukin-1β signaling[25]. This evidence may partially explain the underlying mechanisms by which γ-oryzanol plays a therapeutic role in patients with PMS through neurotransmitter regulation. Moreover, the overall incidence of adverse reactions was lower in the observation group than in the control group, indicating that the treatment of PMS by γ-oryzanol combined with Femoston helps prevent the occurrence of adverse events, such as dizziness and headache, nausea and vomiting, and breast tenderness to some extent. The results of sex hormone tests showed significantly reduced LH and FSH levels (lower than that in the control group) and increased E2 levels (higher than that in the control group) in the observation group after treatment, suggesting that γ-oryzanol plus Femoston is more conducive to achieving positive regulation of sex hormones in PMS. The BMD values of the lumbar spine (L1–4) and bilateral femurs were notably higher in the observation group than those before treatment and in the control group, suggesting that the treatment of PMS with γ-oryzanol + Femoston helps significantly increase the BMD of patients. In terms of sleep quality, the observation group also showed longer sleeping time and lower frequency of awakenings from sleep than the pretreatment level and the control group after treatment, indicating that γ-oryzanol plus Femoston helps improve the sleep quality of patients with PMS. Similar to our findings, Um et al[26] revealed that γ-oryzanol helped improve sleep efficiency and sleeping time in adults with sleep disorders. After in-depth analysis, the therapeutic mechanism of γ-oryzanol against PMS is as follows: First, γ-oryzanol enhances the release of neurotransmitters by influencing the endocrine center and autonomic nervous system, thereby stabilizing endocrine disorders and neurological dysfunction and alleviating PMS symptoms[27]. Second, γ-oryzanol can stimulate osteoblast generation by upregulating bone formation genes, which increases BMD and prevents osteoporosis[28]. Third, γ-oryzanol can effectively improve sleep quality by inhibiting the histamine H1 receptor, thereby reducing sleeping time and promoting nonrapid eye movement sleep[29].
In summary, γ-oryzanol combined with Femoston for treating PMS has evident clinical advantages, which can enhance efficacy, prevent the occurrence of adverse reactions, actively regulate the levels of sex hormones, increase BMD, and effectively improve sleep quality, with clinical popularization value. The results are also applicable to a wider range of patients with PMS, providing more reliable medication options and clinical references as well as clinical data and cognition for the drug treatment of PMS.
| 1. | Sourouni M, Zangger M, Honermann L, Foth D, Stute P. Assessment of the climacteric syndrome: a narrative review. Arch Gynecol Obstet. 2021;304:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Chen W, Chen M, Tang H, Wei W, Shao P, Dou S, Wu J, Lu B, Shi R, Chen J. Advances in diagnosis and treatment of perimenopausal syndrome. Open Life Sci. 2023;18:20220754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Mei Z, Hu H, Zou Y, Li D. The role of vitamin D in menopausal women's health. Front Physiol. 2023;14:1211896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 4. | Li RX, Ma M, Xiao XR, Xu Y, Chen XY, Li B. Perimenopausal syndrome and mood disorders in perimenopause: prevalence, severity, relationships, and risk factors. Medicine (Baltimore). 2016;95:e4466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | An J, Li L. Urban-rural differences in epidemiology and risk factors of menopause syndrome in middle-aged Chinese women. Menopause. 2023;30:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Amiel Castro RT, Ehlert U, Fischer S. Variation in genes and hormones of the hypothalamic-pituitary-ovarian axis in female mood disorders - A systematic review and meta-analysis. Front Neuroendocrinol. 2021;62:100929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Naik SS, Nidhi Y, Kumar K, Grover S. Diagnostic validity of premenstrual dysphoric disorder: revisited. Front Glob Womens Health. 2023;4:1181583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Inayama Y, Mizuno K, Yamaguchi K, Hamanishi J, Takeuchi M, Egawa M, Mandai M, Kawakami K. Hormone replacement therapy and cancer risks in perimenopausal women: A retrospective cohort study using a Japanese claims database. J Obstet Gynaecol Res. 2023;49:1805-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Schneider C, Jick SS, Meier CR. Risk of gynecological cancers in users of estradiol/dydrogesterone or other HRT preparations. Climacteric. 2009;12:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Hammes SR, Levin ER. Impact of estrogens in males and androgens in females. J Clin Invest. 2019;129:1818-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Crandall CJ, Mehta JM, Manson JE. Management of Menopausal Symptoms: A Review. JAMA. 2023;329:405-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 12. | Glintborg D, T'Sjoen G, Ravn P, Andersen MS. MANAGEMENT OF ENDOCRINE DISEASE: Optimal feminizing hormone treatment in transgender people. Eur J Endocrinol. 2021;185:R49-R63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Tang BX, Meng QY, Xie C, Zhao SS, Wu KL, Wang F, Du LY. Ziyin Bushen Decoction Alleviates Perimenopausal Syndrome in Rats by Enhancing Estradiol Production. Evid Based Complement Alternat Med. 2020;2020:8895809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Stevenson JC, Panay N, Pexman-Fieth C. Oral estradiol and dydrogesterone combination therapy in postmenopausal women: review of efficacy and safety. Maturitas. 2013;76:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Ramazani E, Akaberi M, Emami SA, Tayarani-Najaran Z. Biological and Pharmacological Effects of Gamma-oryzanol: An Updated Review of the Molecular Mechanisms. Curr Pharm Des. 2021;27:2299-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Kim M, Yoon M, Cho S, Lee C, Um MY. γ-Oryzanol Ameliorates Depressive Behavior in Ovariectomized Mice by Regulating Hippocampal Nitric Oxide Synthase: A Potential Therapy for Menopausal Depression. Mol Nutr Food Res. 2024;68:e2300253. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Ding L, Xie LL, Zhang H, Ding J, Li J, Wang S, Mao J, Zhou Q. Present situation and analysis of factors affecting perimenopausal syndrome among clinical nurses-a cross-sectional survey. Ann Palliat Med. 2022;11:2432-2442. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Li B, Wang L, Liu Y, Chen Y, Zhang Z, Zhang J. Jujube promotes learning and memory in a rat model by increasing estrogen levels in the blood and nitric oxide and acetylcholine levels in the brain. Exp Ther Med. 2013;5:1755-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Xu HW, Du W, He L, Kuang X. Effectiveness and safety of warm needle acupuncture on insomnia in climacteric women: Protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e15637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Lan J, Wu C, Liang W, Shen J, Zhuo Z, Hu L, Ruan L, Zhang P, Ye X, Xu L, Li C, Lin S, Yang C, Wu S, Dong Y, Ren H, Huang H, Gao B, Yao H, Lin T, Chen X, Li C. An Effective Treatment of Perimenopausal Syndrome by Combining Two Traditional Prescriptions of Chinese Botanical Drugs. Front Pharmacol. 2021;12:744409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Nelson HD. Menopause. Lancet. 2008;371:760-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 522] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 22. | Minatel IO, Francisqueti FV, Corrêa CR, Lima GP. Antioxidant Activity of γ-Oryzanol: A Complex Network of Interactions. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Kobayashi E, Ito J, Shimizu N, Kokumai T, Kato S, Sawada K, Hashimoto H, Eitsuka T, Miyazawa T, Nakagawa K. Evaluation of γ-oryzanol Accumulation and Lipid Metabolism in the Body of Mice Following Long-Term Administration of γ-oryzanol. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Akter S, Sasaki H, Uddin KR, Ikeda Y, Miyakawa H, Shibata S. Anxiolytic effects of γ-oryzanol in chronically- stressed mice are related to monoamine levels in the brain. Life Sci. 2019;216:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Akter S, Uddin KR, Sasaki H, Lyu Y, Shibata S. Gamma oryzanol impairs alcohol-induced anxiety-like behavior in mice via upregulation of central monoamines associated with Bdnf and Il-1β signaling. Sci Rep. 2020;10:10677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Um MY, Yang H, Han JK, Kim JY, Kang SW, Yoon M, Kwon S, Cho S. Rice bran extract supplement improves sleep efficiency and sleep onset in adults with sleep disturbance: A randomized, double-blind, placebo-controlled, polysomnographic study. Sci Rep. 2019;9:12339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Akter S, Uddin KR, Sasaki H, Shibata S. Gamma Oryzanol Alleviates High-Fat Diet-Induced Anxiety-Like Behaviors Through Downregulation of Dopamine and Inflammation in the Amygdala of Mice. Front Pharmacol. 2020;11:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Muhammad SI, Maznah I, Mahmud R, Zuki AB, Imam MU. Upregulation of genes related to bone formation by γ-amino butyric acid and γ-oryzanol in germinated brown rice is via the activation of GABAB-receptors and reduction of serum IL-6 in rats. Clin Interv Aging. 2013;8:1259-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Um MY, Kim S, Jin YH, Yoon M, Yang H, Lee J, Jung J, Urade Y, Huang ZL, Kwon S, Cho S. A novel neurological function of rice bran: a standardized rice bran supplement promotes non-rapid eye movement sleep in mice through histamine H(1) receptors. Mol Nutr Food Res. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |