Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4742
Revised: May 16, 2024
Accepted: June 7, 2024
Published online: July 26, 2024
Processing time: 128 Days and 2.1 Hours
Primary hepatic lymphoma (PHL) is a lymphoproliferative disorder confined to the liver without peripheral lymph node involvement and bone marrow invasion. PHL is extremely rare in clinical practice. The etiology and pathogenesis of PHL are largely unknown. There are no common standard protocols or guidelines for the treatment of PHL.
We report the case of a 66-year-old man who presented with fever and abdominal pain for three weeks. Computed tomography and magnetic resonance imaging scans showed a pyogenic liver abscess. The patient underwent a right posterior hepatectomy. The surgical pathology revealed aggressive B-cell lymphoma, with a primary consideration of diffuse large B-cell lymphoma of non-germinal center origin.
This article reviews the characteristics, mechanism and treatment of PHL and provides insight into the diagnosis of PHL.
Core Tip: We report a patient with liver abscess as the main manifestation. Repeated anti-infection treatment was not effective, and the patient was finally diagnosed with primary hepatic lymphoma (PHL) after surgical laparotomy. This report provides further insights into the diagnosis and treatment of PHL.
- Citation: Xu ZY, Pan Y, Ye WJ, Liu JL, Wu XJ, Tang CL. Primary hepatic lymphoma presenting as pyogenic liver abscess: A case report. World J Clin Cases 2024; 12(21): 4742-4747
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4742.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4742
Primary hepatic lymphoma (PHL) was first described by Lei[1]. It is a rare subtype of diffuse large B-cell lymphoma (DLBCL) with an extremely low incidence, accounting for 0.016% of non-Hodgkin’s lymphoma (NHL) and 0.4% of extranodal lymphomas[2].
Due to the non-specific clinical manifestations and examination results associated with this disease, the diagnosis of primary hepatic DLBCL is challenging[3]. Typically, confirmation is achieved through puncture biopsy or postoperative pathology. The definitive diagnosis of primary hepatic DLBCL can only be made after excluding liver infiltration caused by primary hematologic lymphoid system malignant tumors.
This report details the treatment process of a patient with PHL admitted to our department.
The patient had fever and abdominal pain for three weeks.
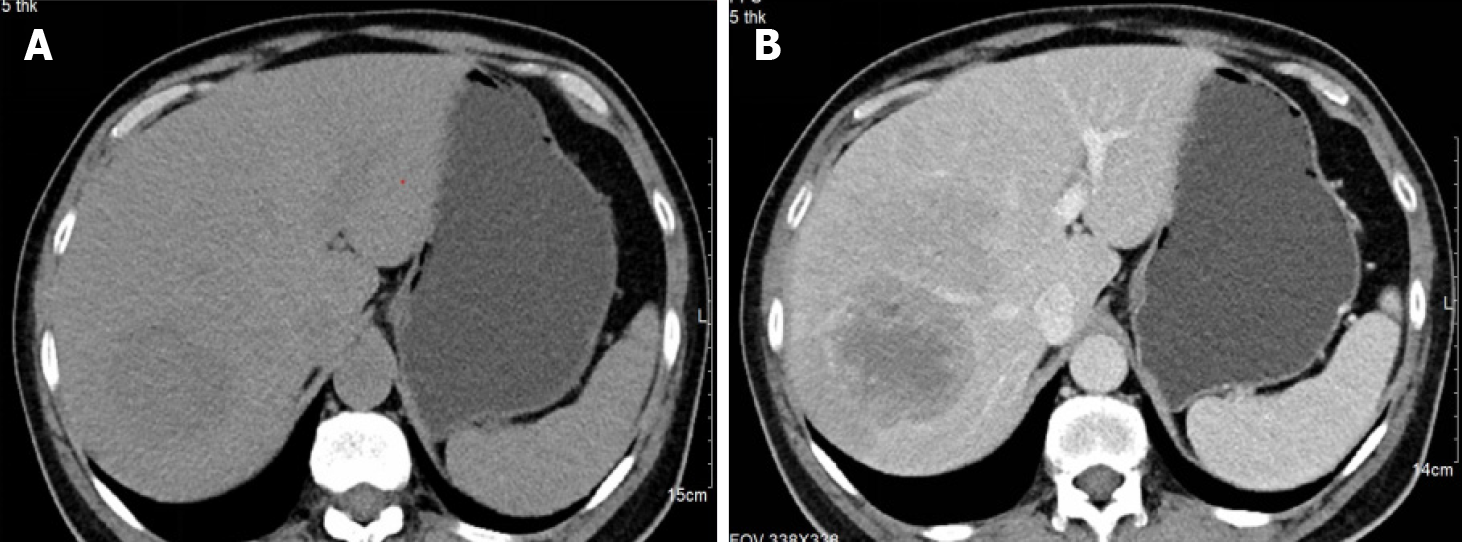
A 66-year-old man with a three-week history of fever and abdominal pain sought examination at the infection clinic. Abdominal computed tomography (CT) revealed a 75 mm × 65 mm clumpy hypoattenuating lesion in segment VII of the liver with blurred edges. The lesion exhibited slight enhancement after contrast administration, and the internal enhancement was uneven. He was diagnosed with pyogenic liver abscess and received anti-infection therapy.
The patient had a history of hypertension and prostatic hyperplasia.
The patient had no specific personal or family history.
His vital signs were normal on physical examination.
Routine blood analysis indicated normal white blood cell counts with a predominance of neutrophils. C-reactive protein levels were elevated at 198.6 mg/L, and lactate dehydrogenase (LDH) level was 779 U/L. The patient tested negative for human immunodeficiency virus (HIV), hepatitis B and C, cytomegalovirus, and Epstein-Barr virus (EBV). Serum tumor marker levels, including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9, and alpha-fetoprotein (AFP), were within normal ranges. However, Protein Induced by Vitamin K Absence or Antagonist II (PIVKA-II) and neuron-specific enolase (NSE) were found to be elevated.
Abdominal CT revealed a 75 mm × 65 mm clumpy hypoattenuating lesion in segment VII of the liver with blurred edges. The lesion exhibited slight enhancement following contrast administration, and internal enhancement was uneven (Figure 1).
PHL.
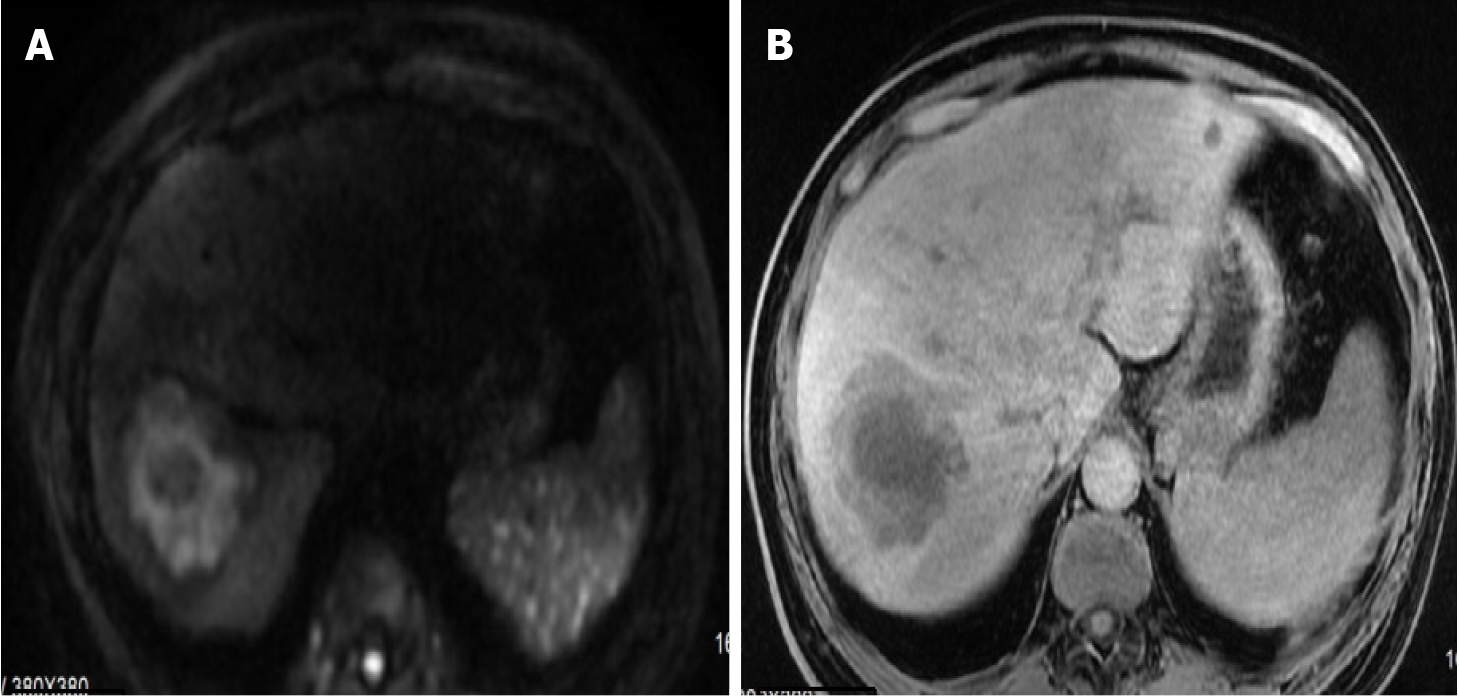
Following admission, the patient received one week of anti-infection therapy with Imipenem and Cilastatin Sodium. Subsequent review of enhanced magnetic resonance imaging (MRI) of the liver revealed a mass shadow measuring 7.1 cm × 6.5 cm in segment VII. The lesion exhibited unclear edges, low signal on T1, layered high signal on T2, and a thick wall around the tumor with stratified and uneven internal enhancement after contrast administration (Figure 2).
Ultrasound-guided percutaneous hepatic puncture drainage was conducted, and revealed approximately 150 mL of red liquid. Drainage culture yielded negative results. Imipenem and Cilastatin Sodium were administered again for an additional two weeks. Throughout this period, the patient continued to experience high fever with no significant fluctuations in inflammatory indicators, and there was a persistent decline in hemoglobin and platelet levels.
After a multidisciplinary team discussion, a decision was made to proceed with surgical exploration. The procedure involved removal of the gallbladder, followed by dissection of the first hilum of the liver and the right posterior hepatic pedicle outside the sheath. Prefabrication and blocking of the hilum of the liver and inferior vena cava were performed, followed by severing the liver parenchyma along the ischemic line and complete removal of the right posterior lobe of the liver. Intraoperative pathology revealed a mass in the posterior lobe of the right liver, characterized by scattered large and deeply stained heterotypic cell nests, indicating a malignant tumor. In addition, scattered large hyperchromatic cells were identified in the liver tissue at the incised margin of the severed end.
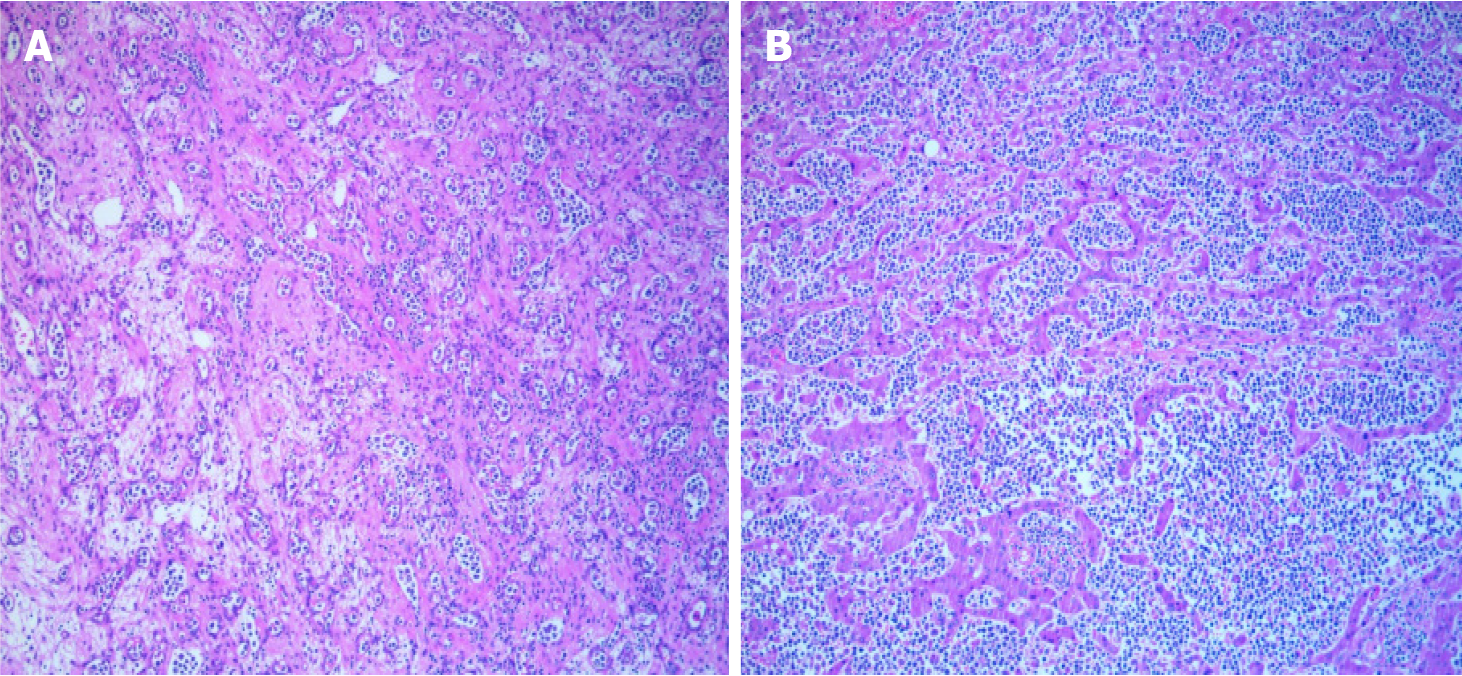
The postoperative biopsy revealed aggressive B-cell lymphoma, with a primary consideration of diffuse large B-cell lymphoma of non-germinal center origin (Figure 3). Immunohistochemical staining indicated positive reactions for CD20, CD79a, CD19, Bcl-2, Bcl-6, MUM-1, PAX-5, and Ki-67 in the tumor cells. Examination of the resected gallbladder exhibited features consistent with chronic cholecystitis, including cholesterol deposition and cholesterol polyp formation. MYC gene break rearrangement and EBV-encoded small RNA in situ hybridization yielded negative results.
The patient requested a transfer to the hematology department of another hospital for further treatment. Positron emission tomography-CT revealed a lymphoma diagnosis following partial resection of the right liver, with no abnormal increase in 18F fluorodeoxyglucose metabolism detected in the remaining liver. Further examinations, including bone marrow morphology, flow cytometry, and bone marrow biopsy, showed no abnormalities. The patient is currently undergoing treatment with the rituximab combined with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) regimen for a total of 4 cycles.
PHL is a lymphoproliferative disorder confined to the liver without peripheral lymph node involvement and bone marrow invasion[4,5]. PHL is uncommon and typically remains localized to the liver at an early stage without infiltration of other sites, accounting for 0.4% of extranodal lymphomas and 0.016% of all NHLs[6]. PHL-DLBCL is even rarer[7]. PHL predominantly affects individuals aged between 50 and 62 years, with a male-to-female ratio of 2.3:1[8].
The etiology and pathogenesis of PHL are largely unknown. The development of malignant lymphocytes during immune activation initiated by chronic liver inflammation may be related to the occurrence of PHL. Tumor cells might originate from hepatic macrophages and transformed lymphocytes. The pathogenesis of PHL is currently thought to be primarily related to infectious factors, especially hepatitis C virus (HCV) infection[9]. Although viral infections such as HBV, HCV, EBV, human T-lymphotropic virus, liver cirrhosis, primary biliary cirrhosis, immunosuppressive therapy, and autoimmune diseases have been implicated[10,11], chronic antigenic stimulation by HBV could potentially lead to the development of lymphoproliferative disease[12]. Some reports support the theory that HCV can infect reactive and neoplastic B cells, with persistent HCV infection possibly inducing primary hepatic as well as other organ B cell lym
Clinical manifestations of PHL typically involve nonspecific symptoms, including high fever, upper abdominal pain, weight loss, fatigue, and hepatomegaly[20]. Unlike primary liver cancer, AFP and CEA levels are usually normal in PHL cases[21]. However, these tumor markers lack specificity and sensitivity[22]. In our patient, tumor markers were within normal levels, except for NSE and PIVKA-II.
PHL lacks specific imaging findings on conventional examinations, making it challenging to distinguish from other benign and malignant liver tumors. Apicella et al[23] proposed that the “vascular floating” sign, observed as enhanced scanning of the continuous blood vessel shadow within the tumor, is a characteristic manifestation of the disease. The cause of the “vascular floating sign” may be attributed to PHL originating from the stromal cells of the liver, with the tumor spanning or growing along the liver anatomy while preserving the original anatomical structure in the viscera[24]. Therefore, the diagnosis of PHL relies on histopathological examination, immunohistochemical staining, and gene rearrangement detection. The definitive diagnosis of primary hepatic DLBCL should be made only after excluding liver invasion caused by primary hematological malignancy.
Currently, there are no common standard protocols or guidelines for the treatment of PHL. Treatment options include surgery, radiation therapy, chemotherapy, or combinations thereof[25,26]. PHL is generally chemo-sensitive, and the most common chemotherapy regimen used is R-CHOP[27].
The prognosis of most PHL cases is complete remission, with a 5-year survival rate approaching 87.1%[26]. Prognostic factors include age, general condition, lesion size, pathological subtype, elevated LDH, and concurrent underlying diseases such as cirrhosis, chronic active hepatitis, HIV infection, and immunodeficiency[28].
PHL is extremely rare in clinical practice, and its clinical manifestations, laboratory findings, and imaging characteristics are nonspecific. The etiology of PHL remains largely unclear, and there is no universally recommended optimal treatment. Therefore, clinicians should deepen their understanding of the disease to facilitate early diagnosis and treatment.
| 1. | Lei KI. Primary non-Hodgkin's lymphoma of the liver. Leuk Lymphoma. 1998;29:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Zheng J, Hou Y, Zhou R, Zhong D. Clinicopathological features of primary hepatic diffuse large B-cell lymphoma: a report of seven cases and a literature review. Int J Clin Exp Pathol. 2015;8:12955-12960. [PubMed] |
| 3. | López-Guillermo A, Colomo L, Jiménez M, Bosch F, Villamor N, Arenillas L, Muntañola A, Montoto S, Giné E, Colomer D, Beà S, Campo E, Montserrat E. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005;23:2797-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Padhan RK, Das P. Primary hepatic lymphoma. Trop Gastroenterol. 2015;36:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Agmon-Levin N, Berger I, Shtalrid M, Schlanger H, Sthoeger ZM. Primary hepatic lymphoma: a case report and review of the literature. Age Ageing. 2004;33:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Daum S, Moos V, Loddenkemper C, Schürmann C, Märker-Hermann E, Stein H, Zeitz M, Schneider T. Immune reconstitution inflammatory syndrome (IRIS) of the small bowel in an immunocompromised patient suffering from systemic lupus erythematosus and non-tuberculous mycobacteriosis. Z Rheumatol. 2008;67:277-278, 280. [PubMed] [DOI] [Full Text] |
| 7. | Liu Y, Jiang J, Wu Q, Zhang Q, Xu Y, Qu Z, Ma G, Wang X, Wang X, Jin W, Fang B. A Case of Primary Hepatic Lymphoma and Related Literature Review. Case Reports Hepatol. 2016;2016:6764121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Peng Y, Qing AC, Cai J, Yue C, French SW, Qing X. Lymphoma of the liver: Clinicopathological features of 19 patients. Exp Mol Pathol. 2016;100:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Bronowicki JP, Bineau C, Feugier P, Hermine O, Brousse N, Oberti F, Rousselet MC, Dharancy S, Gaulard P, Flejou JF, Cazals-Hatem D, Labouyrie E. Primary lymphoma of the liver: clinical-pathological features and relationship with HCV infection in French patients. Hepatology. 2003;37:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Noronha V, Shafi NQ, Obando JA, Kummar S. Primary non-Hodgkin's lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Patel TS, Malvania R, Shah MC, Shah MJ, Gami AG. Primary hepatic lymphoma: A case report. J Cytol. 2015;32:36-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Mrabet S, Zaghouani H, Mestiri S, Akkari I, Ben Jazia E. Primary Hepatic Lymphoma in a Patient with Chronic Hepatitis B. Case Rep Gastroenterol. 2020;14:632-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Somaglino C, Pramaggiore P, Polastri R. Primary hepatic lymphoma in a patient with chronic hepatitis B and C infection: diagnostic pitfalls and therapeutic challenge. Updates Surg. 2014;66:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Masood A, Kairouz S, Hudhud KH, Hegazi AZ, Banu A, Gupta NC. Primary non-Hodgkin lymphoma of liver. Curr Oncol. 2009;16:74-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Oda Y, Kou T, Watanabe M, Sakuma Y, Taguchi N, Kato Y, Kudo Y, Yamauchi A, Sugiura Y, Ohashi S, Asada M, Fukunaga T, Kawaguchi K, Ito H, Nakamura T, Yazumi S. Regression of B-cell lymphoma of the liver with hepatitis C virus infection after treatment with pegylated interferon-alpha and ribavirin. Dig Dis Sci. 2010;55:1791-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Widjaja D, AlShelleh M, Daniel M, Skaradinskiy Y. Complete remission of primary hepatic lymphoma in a patient with human immunodeficiency virus. World J Clin Cases. 2015;3:186-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Muttillo EM, Dégot T, Canuet M, Riou M, Renaud-Picard B, Hirschi S, Guffroy B, Kessler R, Olland A, Falcoz PE, Pessaux P, Felli E. Primary Hepatic Lymphoma After Lung Transplantation: A Report of 2 Cases. Transplant Proc. 2021;53:692-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | El Nouwar R, El Murr T. Primary Hepatic Diffuse Large B-cell Lymphoma Mimicking Acute Fulminant Hepatitis: A Case Report and Review of the Literature. Eur J Case Rep Intern Med. 2018;5:000878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Ferluga D, Luzar B, Gadzijev EM. Follicular lymphoma of the gallbladder and extrahepatic bile ducts. Virchows Arch. 2003;442:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Ugurluer G, Miller RC, Li Y, Thariat J, Ghadjar P, Schick U, Ozsahin M. Primary Hepatic Lymphoma: A Retrospective, Multicenter Rare Cancer Network Study. Rare Tumors. 2016;8:6502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Qiu MJ, Fang XF, Huang ZZ, Li QT, Wang MM, Jiang X, Xiong ZF, Yang SL. Prognosis of primary hepatic lymphoma: A US population-based analysis. Transl Oncol. 2021;14:100931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Lohana AK, Tariq MA, Abid S. Hepatic Lymphoma. 2023 May 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. [PubMed] |
| 23. | Apicella PL, Mirowitz SA, Weinreb JC. Extension of vessels through hepatic neoplasms: MR and CT findings. Radiology. 1994;191:135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Steller EJ, van Leeuwen MS, van Hillegersberg R, Schipper ME, Rinkes IH, Molenaar IQ. Primary lymphoma of the liver - A complex diagnosis. World J Radiol. 2012;4:53-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Page RD, Romaguera JE, Osborne B, Medeiros LJ, Rodriguez J, North L, Sanz-Rodriguez C, Cabanillas F. Primary hepatic lymphoma: favorable outcome after combination chemotherapy. Cancer. 2001;92:2023-2029. [PubMed] [DOI] [Full Text] |
| 26. | Valladolid G, Adams LL, Weisenberg E, Maker VK, Maker AV. Primary hepatic lymphoma presenting as an isolated solitary hepatic cyst. J Clin Oncol. 2013;31:e21-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8:114-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Zhang SL, Chen C, Rao QW, Guo Z, Wang X, Wang ZM, Wang LS. Incidence, Prognostic Factors and Survival Outcome in Patients With Primary Hepatic Lymphoma. Front Oncol. 2020;10:750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |