Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4726
Revised: May 22, 2024
Accepted: June 7, 2024
Published online: July 26, 2024
Processing time: 94 Days and 8.7 Hours
Malignant tumors are one of the leading causes of death worldwide, imposing a substantial economic and social burden. Early detection is the key to improving cure rates and reducing mortality rates, which requires the development of sensitive early detection technologies. Signal amplification techniques play a crucial role in aptamer-based early detection of tumors and are increasingly garnering attention from researchers.
To investigate the current research status, developmental trajectories, and hotspots in signal amplification for aptamer-based tumor detection through bibliometric analysis.
English publications pertaining to signal amplification in aptamer-based tumor detection were retrieved from the Web of Science Core Collection database. VOSviewer and CiteSpace software were employed to analyze various informa
A total of 757 publications were included in this study. China accounted for 85.47% of all publications, with Nanjing University (China) emerging as the institution with the highest publication output. The most influential authors and journals were Hasanzadeh M. from Iran and "Biosensors and Bioelectronics", respectively. Exosomes and carcinoembryonic antigen (CEA) stood out as the most researched tumor-related molecules. Currently, the predominant signal amplification technique, nanomaterial, and signal transduction method were identified as hybridization chain reactions, gold nanoparticles, and electrochemical methods, respectively. Over the past 3 years, exosomes, CEA, electrochemical biosensors, and nanosheets have emerged as research hotspots, exhibiting a robust burst of intensity.
This study is the first bibliometric analysis of literature on signal amplification in aptamer-based tumor detection and elucidates the current status, hotspots, and prospective research directions within this realm. Additionally, it provides an important reference for researchers.
Core Tip: This study is the first bibliometric analysis of the literature pertaining to signal amplification in aptamer-based tumor detection. It unveils that China accounts for 85.47% of all publications, with Hasanzadeh M of Iran and the journal "Biosensors and Bioelectronics". Furthermore, it highlights exosomes and carcinoembryonic antigen as the most extensively studied tumor-related molecules.
- Citation: Cai D, Chen GL, Wang T, Zhang KH. Trends and frontiers in signal amplification for aptamer-based tumor detection: A bibliometric analysis. World J Clin Cases 2024; 12(21): 4726-4741
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4726.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4726
Malignant tumors are one of the leading causes of death worldwide, imposing a substantial economic and social burden[1]. Achieving early detection is key to improving cure rates and reducing mortality rates, which requires the deve
However, the performance of aptamer-based sensors in detecting low-abundance targets is poor. Unfortunately, tumor biomarkers often exhibit low abundance, significantly limiting the utility of aptamer-based sensors in early tumor detection. Therefore, many strategies have been devised to enhance signal amplification, such as hybridization chain reaction (HCR)[6,7], enzyme-based amplification[8], and nanomaterial amplification[9]. These approaches hold great promise for enabling early and highly sensitive tumor detection, thus poised to emerge as pivotal tools for tumor detection.
Bibliometric analysis is a novel statistical approach aimed at delineating the current state of research, trends, and frontiers within a specified research field by quantitatively analyzing the characteristics and structure of the literature in that field[10,11]. VOSviewer and CiteSpace software are widely used for performance analysis, elucidating the contributions of research components, as well as visualization mapping, delineating the interrelations among research components[12]. Consequently, these approaches provide insights and avenues for researchers. Currently, bibliometric analysis has made significant contributions in the fields of tumor etiology[13], tumor metastasis[10], and tumor treatment[14].
Nonetheless, no bibliometric research has been conducted on signal amplification in aptamer-based tumor detection. In this study, a bibliometric analysis was performed to delineate the history and current status of signal amplification strategies in aptamer-based tumor research. The analysis aimed to uncover research trends, identify hotspots, delineate future directions, and provide references for researchers interested in this field.
The Web of Science Core Collection (WoSCC) is renowned as one of the most comprehensive and authoritative academic databases, often employed for bibliometric analysis[15]. The research data for this study were acquired via a term search of this database, utilizing the following terms: TS = ("aptamer" OR "aptamers" OR "aptasensor" OR "aptasensors" OR "aptasensing" OR "aptazyme" OR "aptazymes") AND TS = ("signal amplification" OR "signal amplifications" OR "signal-amplified" OR "signal amplifier" OR "signal amplifiers" OR "signal amplifying" OR "signal amplifyings" OR "signal amplifiable" OR “amplified” OR “cycling” OR “chain reaction” OR “enzyme assisted”) AND TS = ("carcinoma" OR "carcinomas" OR "cancer" OR "cancers" OR "tumor" OR "tumors" OR "neoplasm" OR "neoplasms").
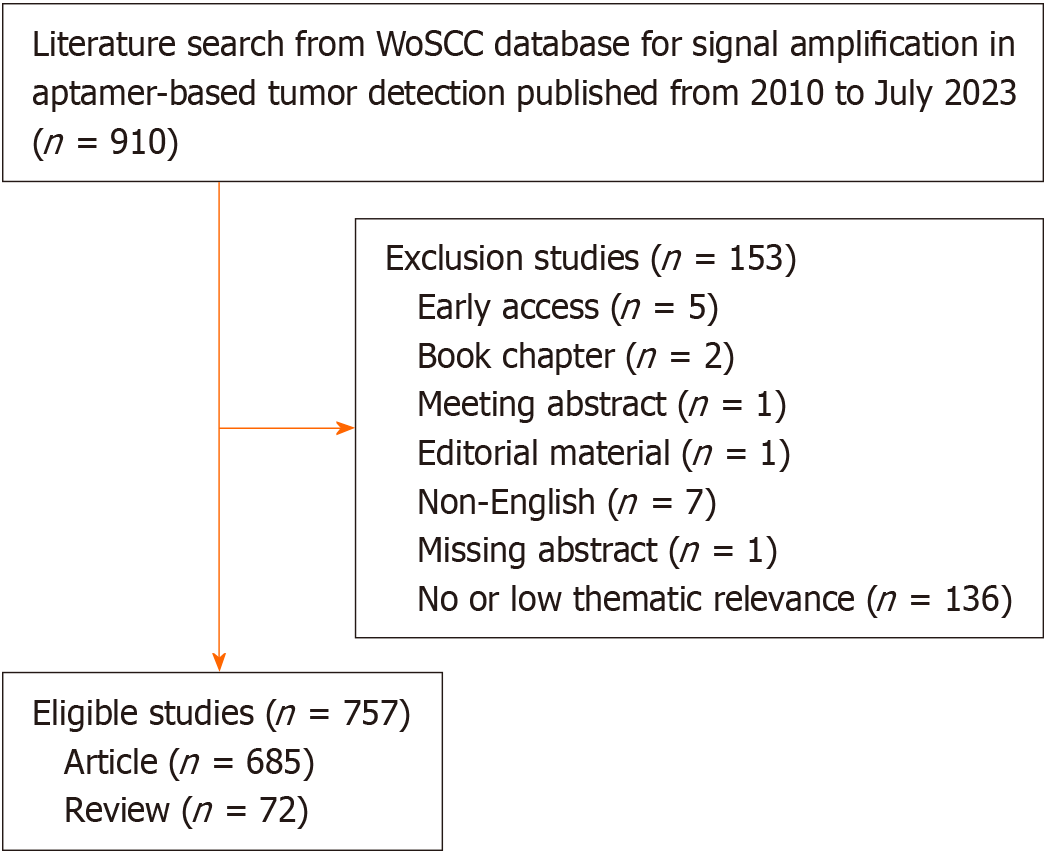
During the search, the publication period was set to January 2010 to July 2023, and the publication type was set to articles and reviews. All literature was exported and archived in plain text format, in the form of full records and referenced citations. The search process and subsequent screening were performed independently by two authors. Disagreements were resolved through discussion or, if necessary, the intervention of a third author. A total of 757 eligible articles were included in this study. Figure 1 illustrates the search and screening process.
The VOSviewer (V.1.6.19) and CiteSpace (V.6.2.4) software were employed for the analysis and visualization of various research components, including countries, institutions, authors and co-cited authors, journals and co-cited journals, co-cited references, and keywords. In addition, CiteSpace was employed to analyze dual-map overlays, co-cited reference clustering, burst citations, and keyword burst citations.
In data analysis visualization maps, each node represents an analyzed research component, with its size proportionate to the frequency of occurrence. The interconnections among these nodes illustrate the co-occurrence relationships between research components, with the thickness of connections contingent upon the frequency of such co-occurrences[12]. Total publications, total citations, average citations, and the H-index were employed to assess the influence of research elements. The H-index denotes the number of publications garnering at least h citations for h publications published by a specific research element. These metrics offer insights into both the overall and individual impact of research components[12,16].
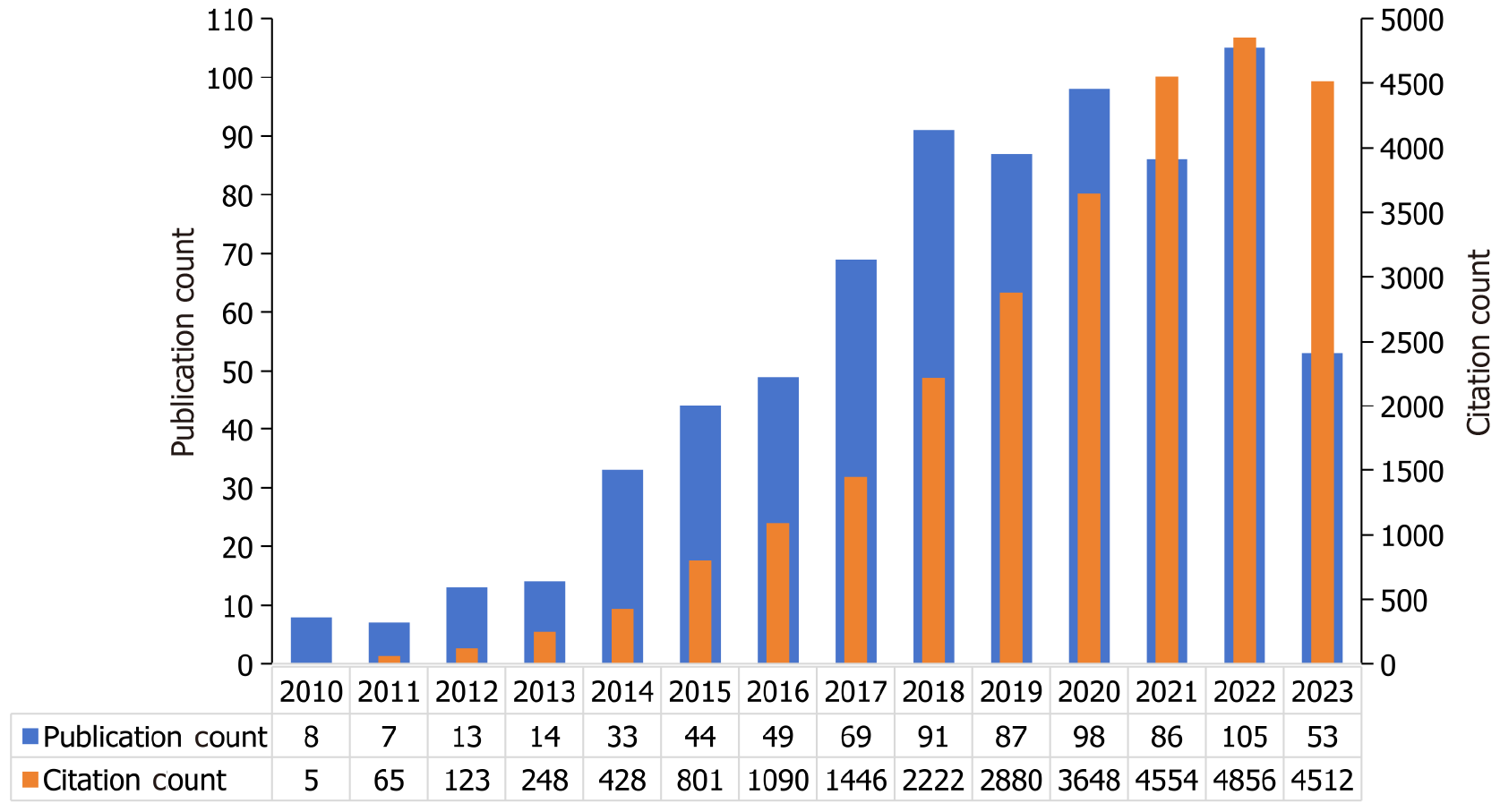
Annual publication and citation analyses offer insights into the developmental trajectory of a given research field[17]. Figure 2 illustrates the yearly trends in both publications and citations within the literature concerning signal amplification in aptamer-based tumor detection, spanning from January 2010 to July 2023. Signal amplification for aptamer-based tumor detection was first reported in 2010. Before 2013, this field was in its infancy, characterized by slow growth. From 2014 to 2018, a phase of rapid growth ensued, marked by an exponential rise in the number of annual publications, indicating that the significance of signal amplification for aptamer-based tumor detection was increasingly being recognized by researchers. Since 2018, the field seems to have stabilized, albeit with fluctuations in annual publication figures. Compared to the number of publications, the number of annual citations has steadily increased, suggesting that the field continues to be of interest among researchers.
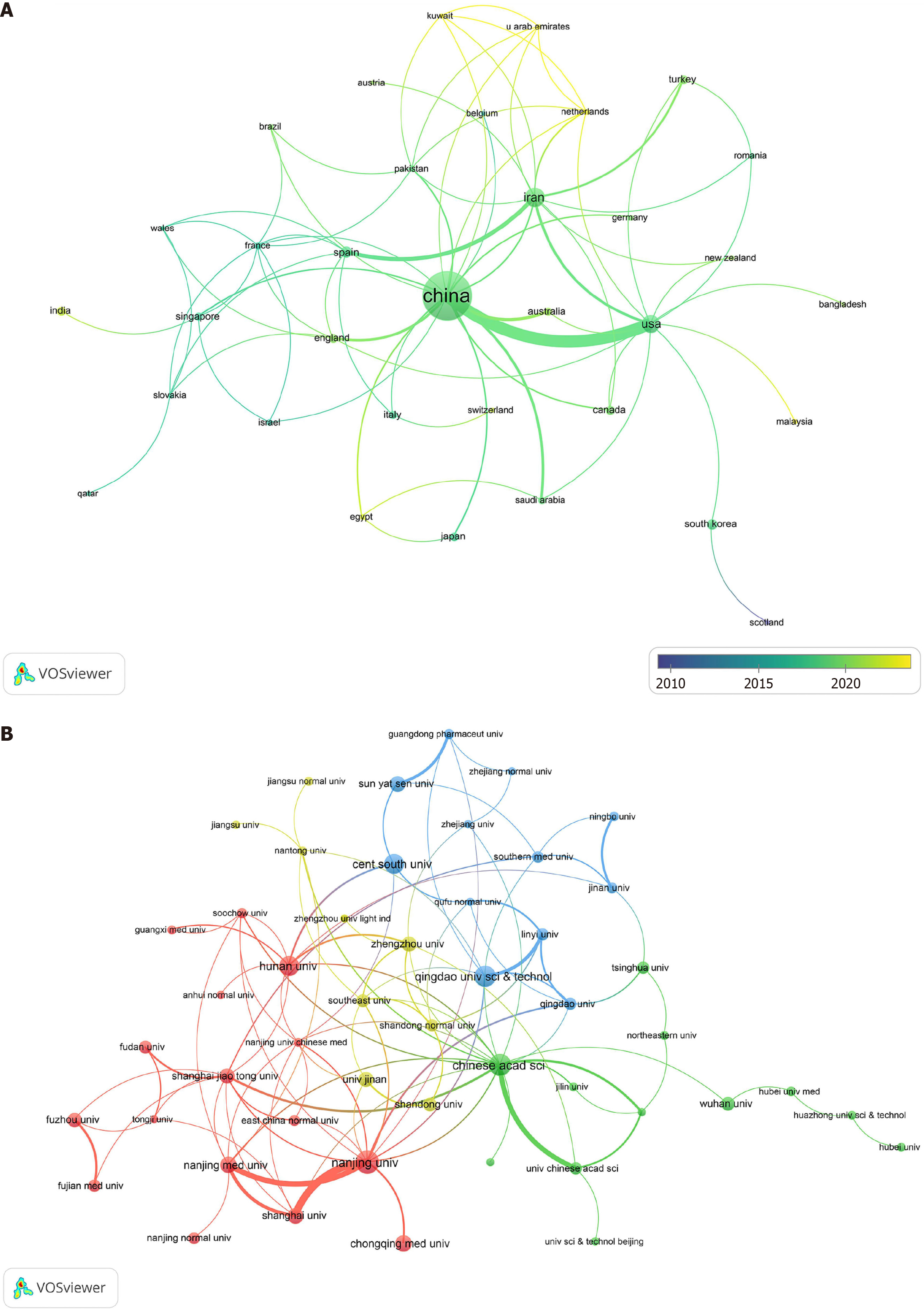
A total of 757 publications emanated from 594 institutions spanning 39 countries. The ranking of the top 10 countries and institutions based on publication count is shown in Table 1. China led with the highest number of publications, accounting for 85.47% of the total publications, followed by Iran (7.01%) and the United States (5.55%). Citations and H-index exhibited a similar trend. It is worth highlighting that although Singapore (rank 11) published only five articles, its influence was significant, with average citations of 219.2. Among institutions, the top 10 entities with the highest publication counts all hail from China, collectively contributing 40.4% (n = 306) of the total publications. Furthermore, these institutions boasted an H-index of ≥ 10, underscoring their substantial influence in this field.
| Rank | Country/institution | Publications | Citations | Average citations | H-index |
| Country | |||||
| 1 | China | 647 | 20896 | 32.3 | 77 |
| 2 | Iran | 53 | 2514 | 47.4 | 30 |
| 3 | United States | 42 | 1753 | 41.7 | 26 |
| 4 | Spain | 13 | 636 | 48.9 | 11 |
| 5 | South Korea | 11 | 437 | 39.7 | 6 |
| 6 | Japan | 7 | 142 | 20.3 | 5 |
| 7 | Canada | 6 | 274 | 45.7 | 6 |
| 8 | England | 6 | 256 | 42.7 | 4 |
| 9 | Turkey | 6 | 235 | 39.2 | 5 |
| 10 | India | 6 | 83 | 13.8 | 5 |
| Institution | |||||
| 1 | Nanjing University | 45 | 2290 | 50.9 | 27 |
| 2 | Chinese Academy of Sciences | 40 | 1605 | 40.1 | 21 |
| 3 | Southwest University | 38 | 1230 | 32.4 | 22 |
| 4 | Qingdao University Science and Technology | 36 | 1247 | 34.6 | 22 |
| 5 | Hunan University | 33 | 1373 | 41.6 | 20 |
| 6 | Central South University | 32 | 1089 | 34.0 | 24 |
| 7 | Nanjing Medical University | 22 | 589 | 26.8 | 12 |
| 8 | Chongqing Medical University | 21 | 548 | 26.1 | 11 |
| 9 | Shanghai University | 20 | 753 | 37.7 | 15 |
| 10 | Sun Yat-Sen University | 19 | 678 | 35.7 | 13 |
Collaboration serves as a pivotal means to promote efficient and high-quality research endeavors. The examination of collaboration among countries in the field of signal amplification for aptamer-based tumor detection is depicted in Figure 3A. The most pronounced collaborations emerged between China and the United States, as well as between Iran and Spain. With reference to the color bar of the timeline, it is evident that the bulk of collaborative papers (green nodes) were published around 2019, and a few collaborative articles (yellow nodes) were published in the past 2 years. This trend suggests a relatively recent commencement of collaboration among countries in this field. Figure 3B illustrates the collaboration network among institutions with more than five publications (n = 63). Notably, the collaboration among institutions was more extensive than the collaboration among countries. Moreover, close collaborations were observed between the Chinese Academy of Sciences and various institutions across different provinces of China.
When two publications co-occur as references in another publication, they are deemed to exhibit a co-citation relationship[18]. Analyzing the authors and their co-cited authors within a given field can provide a succinct overview of representative scholars in that domain. Co-cited authors are those whose works are referenced concurrently by another publication, and the frequency of such joint citations indicates their significance. This study encompassed 3282 authors who contributed to research concerning signal amplification for aptamer-based tumor detection. Table 2 presents the top 10 authors with the highest publication counts, among whom 9 hail from China, while the remaining individual hails from Iran. Yuan R emerged prominently with the highest publication count, substantially surpassing Xiang Y and Yang MH, who secured the second rank. The most frequently co-cited author was Hasanzadeh M, followed by Wang J and Zhang Y, with citation counts of 221, 121, and 113, respectively. Employing Price's Law and VOSviewer, we found that there existed 80 core authors (each with five or more publications) who collectively published 584 articles (77.1%). This underscores the establishment of a relatively stable group of core authors in this field.
| Rank | Author | Counts | Average citations | Country | Co-cited author | Citations |
| 1 | Yuan R | 33 | 32.3 | China | Hasanzadeh M | 221 |
| 2 | Xiang Y | 19 | 23.2 | China | Wang J | 121 |
| 3 | Yang MH | 17 | 33.7 | China | Zhang Y | 113 |
| 4 | Wang KM | 16 | 33.2 | China | Bi S | 102 |
| 5 | Li GX | 14 | 43.4 | China | Wang Y | 95 |
| 6 | Hasanzadeh M | 13 | 56.6 | Iran | Li J | 87 |
| 7 | Zhang SS | 11 | 51.5 | China | He Y | 86 |
| 8 | Yu JH | 10 | 28.3 | China | Ellington AD | 84 |
| 9 | Sun DP | 10 | 50.3 | China | Li Y | 84 |
| 10 | Ding SJ | 10 | 56.4 | China | Zhang J | 83 |
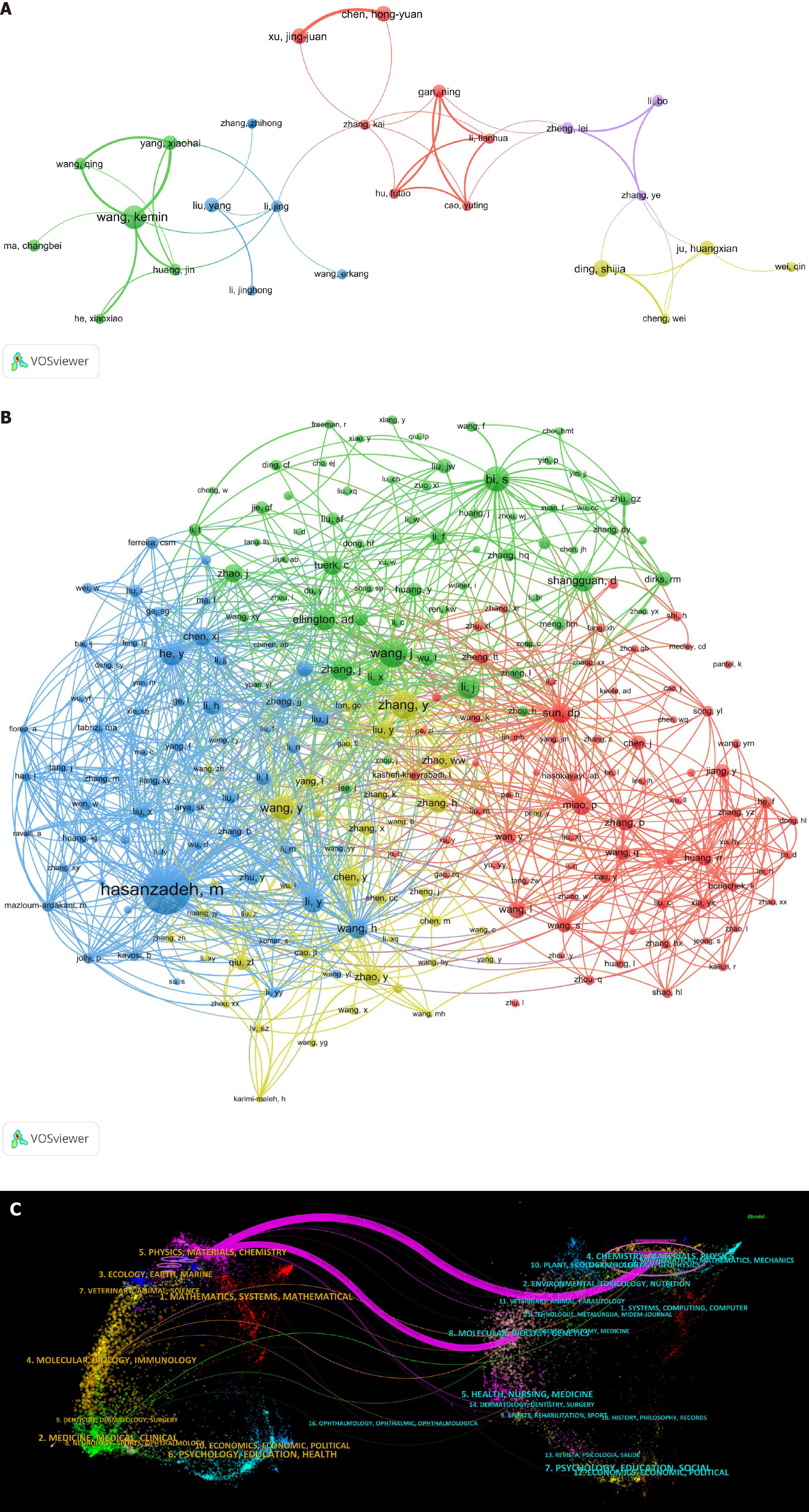
Figure 4A depicts the network map, illustrating collaboration among the core authors. It is evident that the connections among individual authors are significantly weaker than those between countries and institutions, indicating that current research is primarily focused on individuals and representative groups rather than large-scale academic collaboration. Therefore, enhancing communication and collaboration among teams is necessary to facilitate more expansive and profound research endeavors in this field.
Figure 4B depicts the network relationship among co-cited authors with more than 20 citations. The visualization illustrates these authors grouped into clusters, wherein the node representing Hasanzadeh M exhibits connections with most of the other nodes and intersects with the other three clusters. This indicates that this author may have established the research framework pivotal for the advancement of this field.
The 757 articles originated from 108 distinct journals. Co-cited journals are defined as articles stemming from two journals that were cited jointly by another article. Table 3 delineates the top 10 journals by publication count and co-citation frequency (all positioned in Q1 or Q2 of the journal citation reports). "Biosensors and Bioelectronics" and "Analytical Chemistry" emerged as the foremost journals in terms of both publications and citations, while "Sensors and Actuators. B, Chemical" and "Journal of the American Chemical Society" secured the third position. These journals are esteemed within the field of signal amplification for aptamer-based tumor detection. These findings hold potential utility for scholars in the field as they prepare their scholarly contributions.
| Rank | Journal | Article count | IF (2023) | Co-cited journal | Citation | IF (2023) |
| 1 | Biosensors and Bioelectronics | 105 | 12.6 | Biosensors and Bioelectronics | 4879 | 12.6 |
| 2 | Analytical Chemistry | 93 | 7.1 | Analytical Chemistry | 4725 | 7.1 |
| 3 | Sensors and Actuators. B, Chemical | 66 | 8.4 | Journal of the American Chemical Society | 1458 | 15.0 |
| 4 | Analytica Chimica Acta | 51 | 6.2 | Sensors and Actuators. B, Chemical | 1373 | 8.4 |
| 5 | Talanta | 45 | 6.1 | Chemical Communications | 1204 | 4.9 |
| 6 | Microchimica Acta | 43 | 5.7 | Angewandte Chemie- International Edition | 974 | 16.6 |
| 7 | Analyst | 29 | 4.2 | Analytica Chimica Acta | 909 | 6.2 |
| 8 | Chemical Communications | 26 | 4.9 | ACS Applied Materials and Interfaces | 853 | 9.5 |
| 9 | ACS Applied Materials and Interfaces | 19 | 9.5 | Talanta | 827 | 6.1 |
| 10 | Trends in analytical chemistry: TRAC | 13 | 13.1 | Analyst | 802 | 4.2 |
Figure 4C vividly illustrates the effective collaboration across various fields of publications and cited literature. The left panel displays the citing journals, whereas the right panel depicts the cited ones. Two important purple citation paths indicated that the journals Chemistry, Materials, Physics and the journals Molecular, Biology, Genetics were often cited by the journals Physics, Materials, Chemistry. Therefore, signal amplification for aptamer-based tumor detection is closely associated with materials science, chemistry, and medicine, and enhancing multidisciplinary management emerges as a pivotal imperative for future endeavors.
Co-cited references refer to two publications cited together by another publication[18,19]. Analyzing co-cited references can illuminate core documents in specific fields and evolutionary trends in research focus[20]. Table 4 presents the top 10 co-cited references, comprising predominantly original articles with the exception of the ninth entry. Ranks 1, 2, 4, and 8 were associated with aptamer selection, while ranks 3 and 9 pertained to HCR. The remaining references predominantly explored aptamer sensors based on nanomaterials for tumor research, which demonstrates that aptamer selection is the foundation of aptamer-based sensors for tumor research, the prominence of the HCR technique, and the burgeoning interest in aptamer sensors based on nanomaterials.
| Rank | Co-cited reference | Citations | DOI | IF (2023) |
| 1 | Ellington AD, 1990, Nature, v346, p818 | 77 | 10.1038/346818a0 | 64.1 |
| 2 | Tuerk C, 1990, Science, v249, p505 | 70 | 10.1126/science.2200121 | 56.9 |
| 3 | Dirks RM, 2004, Proc Natl Acad Sci USA, v101, p15275 | 50 | 10.1073/pnas.0407024101 | 11.1 |
| 4 | Shangguan D, 2006, Proc Natl Acad Sci USA, v103, p11838 | 49 | 10.1073/pnas.0602615103 | 11.1 |
| 5 | Wang S, 2017, Acs Nano, v11, p3943 | 35 | 10.1021/acsnano.7b00373 | 17.1 |
| 6 | He Y, 2012, Nanoscale, v4, p2054 | 33 | 10.1039/c2nr12061e | 6.7 |
| 7 | Zheng TT, 2014, J Am Chem Soc, v136, p2288 | 32 | 10.1021/ja500169y | 15.0 |
| 8 | Tan WH, 2013, Chem Rev, v113, p2842 | 32 | 10.1021/cr300468w | 62.1 |
| 9 | Bi S, 2017, Chem Soc Rev, v46, p4281 | 32 | 10.1039/c7cs00055c | 46.2 |
| 10 | Jiang Y, 2017, Angew Chem Int Edit, v56, p11916 | 32 | 10.1002/anie.201703807 | 16.6 |
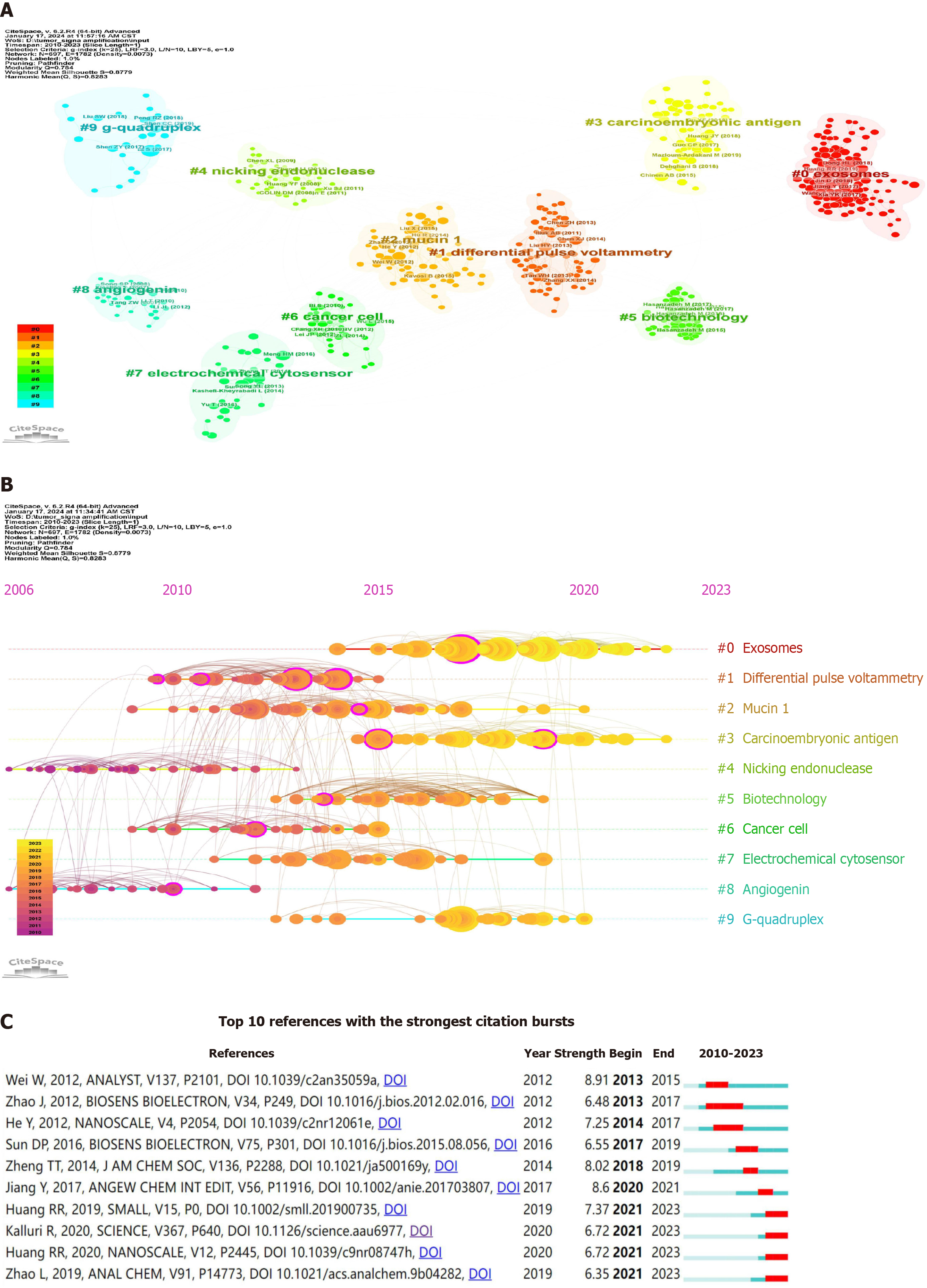
A cluster map of co-cited references was generated using CiteSpace, as shown in Figure 5A, revealing 10 significant clusters characterized by high Q-values (Q = 0.784) and silhouette scores (S = 0.8779), demonstrating good clustering patterns and high reliability. The largest cluster was "#0 exosomes" with most of the literature pertaining to the detection of tumor-derived exosomes[21-23]. Regarding temporal distribution (Figure 5B), clusters "#0 exosomes" and "#3 carcinoembryonic antigen" are positioned towards the far right end of the timeline, predominantly represented by yellow and orange nodes, indicating their current prominence as focal points of research within the field.
The high citation burst strength of references can reflect frontiers in a given field. Figure 5C illustrates the top 10 references exhibiting the highest citation burst strength, ranging from 6.35 to 8.91. Citations with high burst intensity (denoted by red squares) during 2013–2019 (first five) and 2020–2023 (last five) corresponded to tumor cell surface-associated proteins, particularly Mucin 1, and exosome detection, respectively. Among them, "Huang RR, 2019, SMALL"[22], "Huang RR, 2020, NANOSCALE"[24], and "Zhao L, 2019, ANAL CHEM"[25] were closely associated with signal amplification in exosome detection, while "Kalluri R, 2020, SCIENCE"[26] reviewed the application of exosomes in biomedical fields. This indicates a shift in the researchers' focus from detecting tumor cell surface-associated proteins to achieving precise detection of tumor-derived exosomes.
Keywords typically indicate the central theme of a publication. We analyzed the keywords, after merging synonymous terms, to identify the primary research direction in signal amplification for aptamer-based tumor detection.
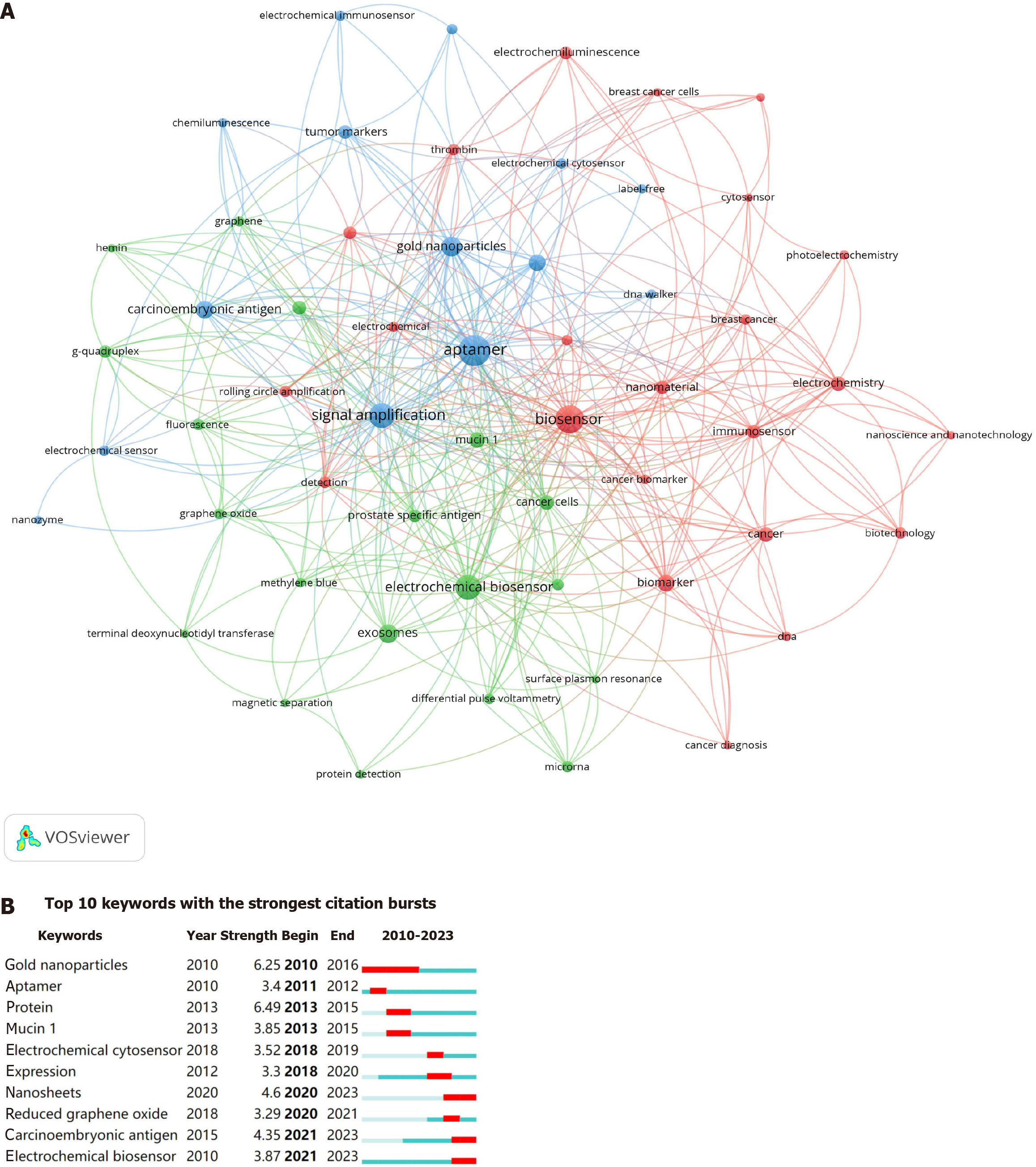
Using VOSviewer, we identified 20 high-frequency keywords (Table 5) and found that signal-amplified aptamer sensors have been used to detect various tumor-related biomarkers, such as tumor-derived exosomes, soluble tumor markers, cancer cells, and tumor surface-related proteins, with exosomes and carcinoembryonic antigen (CEA) emerging as the most frequently detected entities. Furthermore, we found that three keywords (ranked 2nd, 9th, and 19th) were associated with electrochemical methods. In addition, the keywords associated with signal amplification methods included "gold nanoparticles" (GNPs) (rank 6), "hybridization chain reaction" (rank 8), "nanomaterial" (rank 17), and "DNAzyme" (rank 18), indicating that the most frequently used signal amplification methods in this field were nanomaterials (especially GNPs), HCR, and DNAzyme-based amplification. Using CiteSpace software, we analyzed the corresponding years of the keywords associated with signal amplification techniques, yielding insightful results: DNAzyme-based amplification (2010), rolling circle amplification (2012), HCR (2014), and catalytic hairpin assembly (2015). On the basis of the aforementioned results, we found that, currently, nanomaterials, particularly GNPs, are most frequently employed in signal amplification in aptamer-based tumor detection research, with HCR emerging as the most frequently used technique for nucleic acid signal amplification.
| Rank | Keyword | Count | Rank | Keyword | Count |
| 1 | Aptamer | 133 | 11 | Mucin 1 | 27 |
| 2 | Electrochemical biosensor | 79 | 12 | Biomarker | 26 |
| 3 | Signal amplification | 64 | 13 | Cancer cells | 24 |
| 4 | Biosensor | 50 | 14 | Cancer | 17 |
| 5 | Aptasensor | 48 | 15 | Tumor markers | 17 |
| 6 | Gold nanoparticles | 42 | 16 | Circulating tumor cells | 16 |
| 7 | Exosomes | 37 | 17 | Nanomaterial | 16 |
| 8 | Hybridization chain reaction | 37 | 18 | DNAzyme | 15 |
| 9 | Electrochemistry | 35 | 19 | Electrochemiluminescence | 15 |
| 10 | Carcinoembryonic antigen | 29 | 20 | Fluorescence | 14 |
Figure 6A displays a network map of keywords that have appeared more than five times, encompassing 68 keywords and forming three clusters. These clusters, arranged in descending order of size, were "electrochemical biosensor", "aptamer", and "biomarker". Figure 6B shows the top 10 keywords with the strongest citation burst strength. GNPs emerged as the earliest burst keyword, maintaining its prominence from 2010 to 2016. Conversely, recent burst keywords over the past 3 years included "nanosheets", "carcinoembryonic antigen", and "electrochemical biosensors". This suggests a potential shift in researchers' focus within the field from GNPs to nanosheets, while concurrently intensifying attention towards CEA and electrochemical analysis.
The importance of early detection of tumors is undeniable; however, the scarcity of tumor markers poses a significant challenge to achieving this goal. Thus, the development of ultrasensitive, specific, and cost-effective approaches for detecting tumor-related molecules has emerged as a paramount concern in tumor research. Nucleic acid aptamers are innovative molecular diagnostic tools characterized by unique properties that facilitate the highly sensitive detection of targets with low abundance through various signal amplification techniques.
In this study, we conducted a bibliometric analysis of the literature pertaining to signal amplification for aptamer-based tumor research, spanning the period from 2010 to July 2023. A total of 757 articles from 108 journals were analyzed, and these articles were published by 594 institutions across 39 countries, involving a collective of 3282 authors and 24689 co-cited references.
The statistical analysis of annual publications and their citations consistently indicated an upward trend in both the number of publications and citations in this field, underscoring a sustained interest in the subject over the past years. China emerged as the frontrunner in terms of publication output (647 publications), followed by Iran (53 publications) and the United States (42 publications). The total citations and H-index of these publications. The top 10 institutions in terms of publication count were from China, with Nanjing University at the forefront. Yuan R holds the highest publication count (33), while Hasanzadeh M has garnered the most citations (221). While collaborations among various countries and institutions were observed, there remains untapped potential for further collaboration among researchers. The two journals with the most publications and citations in this field are "Biosensors and Bioelectronics" and "Analytical Chemistry". Ranking third in terms of the number of publications and citations are "Sensors and Actuators. B, Chemical" and "Journal of the American Chemical Society" respectively.
Three of the top four most cited references[27-29] pertain to aptamer selection strategy (Table 4), representing pioneering works in the field of aptamers. The capacity of aptamers to bind to their targets significantly impacts the sensitivity, specificity, and stability of assays. Consequently, since the emergence of aptamers in 1990, researchers have sustained a robust interest in refining the selection or modification of aptamers to enhance tumor detection efficacy[27,30,31].
The timeline map of cited references (Figure 5B) illustrates that, in recent years, highly cited references primarily pertain to the detection of exosomes and CEA, indicating a growing emphasis on the potential applications of exosomes and CEA in tumor research. Exosomes, which are small vesicles released by cells, including cancer cells, serve as carriers of proteins, nucleic acids, lipids, and other molecules, thereby facilitating the transmission of rich molecular information between cells. A comprehensive exploration of exosomes can unveil the mechanisms of cellular communication and the molecular processes underlying diseases[26]. Owing to their significance in early diagnosis, monitoring metastasis, assessing prognosis, and personalized therapies for tumors, as well as their stability and ease of access, exosomes represent ideal liquid biomarkers[32,33] and are rapidly gaining ground in tumor research[34]. Unfortunately, exosome concentration remains low during the initial phases of tumors, and conventional analytical methods, such as western blot[35] and enzyme-linked immunosorbent assay[36], are plagued by limitations such as low sensitivity, high sample volume requirements, and protracted analysis duration. Therefore, in recent years, researchers have endeavored to design and develop signal amplification techniques for the aptamer-based ultrasensitive detection of exosomes[22].
In terms of the most pronounced reference citation bursts (Figure 5C), in the field of aptamer-based tumor detection, earlier investigations (2013–2019) primarily focused on tumor cell surface-associated proteins. Conversely, recent studies (2020–2023) shifted their emphasis towards exosomes. Despite the importance of detecting tumor cell surface-associated proteins for understanding the mechanisms of tumorigenesis, progression, diagnosis, and treatment, its application in liquid biopsy is constrained by the scarcity and heterogeneous nature of circulating tumor cells[32]. In contrast, the biomedical significance of tumor-derived exosomes is becoming more prominent, with exosome detection based on the aptamer strategy poised to remain a pivotal trajectory in future tumor research. The precise detection approach of tumor-derived exosomes is expected to improve the accuracy of early diagnosis, monitoring of disease progression, and evaluation of treatment response, thus providing patients with a more tailored treatment regimen.
However, several challenges hinder the realization of this goal. Firstly, researchers must develop more sensitive, stable, and specific exosome assays to reduce background interference and improve detection sensitivity. Secondly, there is a pressing need to advance precise, time-efficient, and cost-effective techniques for exosome isolation and single-exosome analysis to further investigate the heterogeneity of exosomes and their roles in various disease states, as well as for accurately assessing biomarkers in exosomes. In addition, extensive clinical sample studies are imperative to validate the practical effectiveness of exosome-based precision assays in the diagnosis and treatment of tumors.
CEA, widely employed as a tumor marker, exhibits overexpression in colorectal, gastric, pancreatic, breast, ovarian, and lung cancers, rendering it instrumental in tumor screening, diagnosis, monitoring, treatment response assessment, and prognostic prediction[37]. During the early stages of tumors, CEA concentration in human serum remains relatively low, prompting a notable interest in the development of an ultrasensitive detection method for CEA based on an aptamer strategy. Huang et al[38] developed an ultrasensitive electrochemical aptasensor based on DNAzyme-assisted amplification, achieving a detection limit of 0.34 fg/mL for CEA. However, there exists a necessity for reinforcing clinical validation studies of aptamer-based CEA assays, particularly concerning real-time quantitative and portable assays[37].
In this study, the top 20 author keywords (Table 5) indicated that the electrochemical method was the most frequently used signal transduction method, with GNPs being the predominant nanomaterial-based signal amplification strategy, and HCR being the most frequently utilized nucleic acid signal amplification technique. However, given the rapid evolution of technologies, this conclusion may be time-sensitive. New signal amplification technologies may emerge in the future.
Electrochemical biosensing is a low-cost, easy-to-use, minimally affected by a low background signal, highly sensitive, and fast real-time system, thereby garnering considerable interest from researchers[39]. The utilization of aptamer recognition and signal amplification techniques augments the electrochemical signal, thereby bolstering detection sensitivity and specificity[40]. Thus, it exhibits broad application prospects in the field of tumor diagnosis and therapy. Since most medical samples (e.g., blood) are typically opaque, rendering optical detection less feasible, electrochemical biosensors have become increasingly important in the medical field. However, the implementation of electrochemical biosensors in real-world sample analyses mandates a comprehensive evaluation of the effects of various interfering factors on detection outcomes, alongside meticulous validation of the stability and reproducibility of the sensors. In addition, ongoing optimization of electrochemical biosensor performance, including enhancements to response time and reductions in detection thresholds, is imperative to provide more accurate and efficient avenues for tumor diagnosis and treatment.
Nanomaterial-based signal amplification strategies have garnered considerable attention. The utilization of nanomaterials significantly improves the sensitivity and detection capabilities of aptamer sensors, surpassing those devoid of nanomaterials[41]. GNPs, a typical nanomaterial, have emerged as excellent carriers for signal transduction and am
HCR, an enzyme-free signal amplification technique, relies on a DNA hybridization reaction that was first introduced to the aptamer field by Dirks RM and Pierce NA in 2004[44]. The HCR system comprises an input strand (initiator) and two sequence-specific nucleic acid hairpins (amplifiers). Upon introduction of the input strand, it triggers hybridization and the alternate strand replacement of the two hairpins, culminating in the formation of an elongated double strand that carries a multitude of detected signals, thereby achieving signal amplification[45]. This technique is highly specific, sensitive, and programmable, and has been widely employed for various bioanalyses, including tumor-related molecular analysis[45,46]. Therefore, HCR emerges as a promising method for nucleic acid signal amplification, and its integration with nanomaterials and/or nanotechnology holds the potential to further improve the detection performance of sensors.
The citation burst of keywords can serve as an indicator of prevailing research trajectories and hotspots. The keywords exhibiting the most robust bursts in the past 3 years were "carcinoembryonic antigen", "electrochemical biosensor", and "nanosheets" (Figure 6B), indicating that CEA and electrochemical biosensors will remain the focus of future research endeavors, alongside the burgeoning interest in nanosheets among researchers. Taken together with the burst in keywords, a trend emerges wherein researchers have shifted their focus from GNPs (2010-2016) to nanosheets (2020-2023). Nanosheets, defined as two-dimensional nanomaterials, have garnered considerable interest from researchers owing to their greater body surface area ratio and superior biomedical properties compared to nanoparticles[47]. In the domain of tumor detection, common types of nanosheets employed for the development of aptamer sensors include carbon-based nanosheets (e.g., graphene oxide)[48], and semiconductor nanosheets (e.g., black phosphorus)[49], as well as metal (e.g., MOF)[50] and transition metal nanosheets (e.g., Ti3C2 MXene)[51].
In signal amplification for aptamer-based tumor detection, the primary role of nanosheets is to serve as a building block for the sensing platform and as a medium for signal amplification. By loading aptamers, nanosheets can capture target molecules, thereby inducing significant signal changes. Such signal changes can be translated into specific electrochemical or optical signals, leading to highly sensitive and specific detection, with the potential for achieving early and accurate tumor detection. However, despite some progress in the preparation of nanosheets, their stability and consistency of structural/compositional parameters remain to be further optimized, and their biosafety and biocompatibility warrant evaluation to ensure their safety and efficacy for in vivo applications[47].
After an in-depth analysis of the keywords, we affirmed that among the signal amplification methods employed in aptamer-based tumor detection, nanomaterial-based signal amplification is the most widely utilized method, with GNPs being the most frequently employed. However, as technological advancements evolve, nanosheets are expected to gradually replace GNPs from their central position in this field. In addition to nanoparticles, other common signal amplification methods include HCR, DNAzyme-based signal amplification, rolling circle amplification, and catalytic hairpin assembly. HCR has emerged as the most popular nucleic acid signal amplification technique owing to its enzyme-free and isothermal characteristics. In practical implementation, each signal amplification technology has its advantages; therefore, the selection of appropriate signal amplification techniques for aptamer-based tumor detection, requires careful consideration of the specific application requirements and conditions.
In addition, the most effective signal amplification methods may encompass a synthesis of multiple advantages. For instance, the concurrent utilization of nanomaterials alongside signal amplification technologies has shown promise in enhancing the detection capabilities of aptasensors. Xie et al[52] developed an ultrasensitive aptamer sensor by coupling MOF signal tags with HCR, resulting in a lower detection limit of 1.34 fM for Mucin 1 by exploiting the unique properties of black phosphorus, GNPs, Co-MOF, and HCR. The combined use of biomaterials and biological techniques for signal amplification in aptamer-based sensor detection presents a viable strategy for augmenting the sensitivity, specificity, and stability of the assay. Moreover, it holds promise as a pivotal approach towards achieving early and precise tumor detection. Realizing this potential necessitates intensified interdisciplinary collaboration and the investment of resources in the future.
Our study exhibits several limitations. Firstly, we exclusively collected articles from the WoSCC database, potentially omitting some publications, even though this database exhibits good quality and comprehensive data commonly employed for bibliometric analyses. Secondly, our study excluded certain types of publications, such as early access and non-English articles, which may have led to oversight of some literature. However, this exclusionary approach aligns with prevailing methods in literature screening. Finally, given the time-dependent nature of publication impact analysis, there is a possibility that newly published high-quality papers were overlooked. However, these limitations exert a relatively minimal impact on the overarching findings and are unlikely to alter the primary trends delineated in this study. This assertion is substantiated by the study's focus on papers published in the last 7 years (80% of the analyzed publications). Furthermore, temporal metrics, including timeline graphs and citation burst analysis, were employed to discern emerging research priorities.
In summary, we performed a bibliometric analysis to elucidate the prevailing trends and frontier areas in signal amplification for aptamer-based tumor detection for the first time. In this field, China and Nanjing University (China) emerge as the leading countries and institutions, respectively, in terms of the number of publications. The most influential researcher and journal were identified as Hasanzadeh M (Iran) and "Biosensors and Bioelectronics", respectively. Currently, the most commonly employed signal transduction method, nanomaterial-based signal amplification strategy, and signal amplification technique are the electrochemical method, GNPs, and HCR, respectively, with exosomes and CEA being the most extensively studied tumor-related targets. Over the past 3 years, research hotspots have focused on exosomes, CEA, electrochemical biosensors, and nanosheets. We believe that two-dimensional nanosheets will be widely employed to develop signal amplification techniques for aptamer biosensors and that the integration of nanocomposites with biotechnology could be a prospective avenue for research exploration in this field.
This study represents the first bibliometric analysis of the literature concerning signal amplification in aptamer-based tumor detection. It elucidates the current status, research hotspots, and future research directions in this field, thereby furnishing a crucial reference for researchers.
We thank the members of the First Affiliated Hospital of Nanchang University for their contribution to the discussions. We apologize to the scientists whose work could not be cited owing to space limitations.
| 1. | Deo SVS, Sharma J, Kumar S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann Surg Oncol. 2022;29:6497-6500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 219] [Article Influence: 73.0] [Reference Citation Analysis (33)] |
| 2. | Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, Esener S, Fitzgerald RC, Gambhir SS, Kuhn P, Rebbeck TR, Balasubramanian S. Early detection of cancer. Science. 2022;375:eaay9040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 516] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 3. | Li L, Xu S, Yan H, Li X, Yazd HS, Li X, Huang T, Cui C, Jiang J, Tan W. Nucleic Acid Aptamers for Molecular Diagnostics and Therapeutics: Advances and Perspectives. Angew Chem Int Ed Engl. 2021;60:2221-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 4. | Yazdian-Robati R, Arab A, Ramezani M, Abnous K, Taghdisi SM. Application of aptamers in treatment and diagnosis of leukemia. Int J Pharm. 2017;529:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Zhao L, Wang H, Fu J, Wu X, Liang XY, Liu XY, Wu X, Cao LL, Xu ZY, Dong M. Microfluidic-based exosome isolation and highly sensitive aptamer exosome membrane protein detection for lung cancer diagnosis. Biosens Bioelectron. 2022;214:114487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 6. | Yuan B, Guo L, Yin K, Wang X, Liu Q, He M, Liu K, Zhao J. Highly sensitive and specific detection of tumor cells based on a split aptamer-triggered dual hybridization chain reaction. Analyst. 2020;145:2676-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Li P, Li W, Xie Z, Zhan H, Deng L, Huang J. A label-free and signal-amplifiable assay method for colorimetric detection of carcinoembryonic antigen. Biotechnol Bioeng. 2022;119:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Yan M, Bai W, Zhu C, Huang Y, Yan J, Chen A. Design of nuclease-based target recycling signal amplification in aptasensors. Biosens Bioelectron. 2016;77:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Cajigas S, Orozco J. Nanobioconjugates for Signal Amplification in Electrochemical Biosensing. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Shi Y, Wei W, Li L, Wei Q, Jiang F, Xia G, Yu H. The global status of research in breast cancer liver metastasis: a bibliometric and visualized analysis. Bioengineered. 2021;12:12246-12262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Wei N, Xu Y, Li Y, Shi J, Zhang X, You Y, Sun Q, Zhai H, Hu Y. A bibliometric analysis of T cell and atherosclerosis. Front Immunol. 2022;13:948314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 12. | Ahmad SJ, Ahmed AR, Kowalewski KF, Nickel F, Rostami K, Stocker CJ, Hakky SM, Archid R, McWhinnie D, Mohajer-Bastami A, Seimenis DS, Ahmad S, Mansour S, Ahmed MH, Mital D, Exadaktylos AK. Citation classics in general medical journals: assessing the quality of evidence; a systematic review. Gastroenterol Hepatol Bed Bench. 2020;13:101-114. [PubMed] |
| 13. | Wu W, Ouyang Y, Zheng P, Xu X, He C, Xie C, Hong J, Lu N, Zhu Y, Li N. Research trends on the relationship between gut microbiota and colorectal cancer: A bibliometric analysis. Front Cell Infect Microbiol. 2022;12:1027448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Shen J, Shen H, Ke L, Chen J, Dang X, Liu B, Hua Y. Knowledge Mapping of Immunotherapy for Hepatocellular Carcinoma: A Bibliometric Study. Front Immunol. 2022;13:815575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 15. | Aggarwal A, Lewison G, Idir S, Peters M, Aldige C, Boerckel W, Boyle P, Trimble EL, Roe P, Sethi T, Fox J, Sullivan R. The State of Lung Cancer Research: A Global Analysis. J Thorac Oncol. 2016;11:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 16. | Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5772] [Cited by in RCA: 4421] [Article Influence: 221.1] [Reference Citation Analysis (0)] |
| 17. | Yang Y, Luo D, Inam M, Hu J, Zhou Y, Xu C, Chen W. A scientometrics study of the nanomedicines assisted in respiratory diseases. Front Bioeng Biotechnol. 2022;10:1053653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: An overview and guidelines. Journal of Business Research. 2021;133:285-296. [DOI] [Full Text] |
| 19. | Wu F, Li C, Mao J, Zhu J, Wang Y, Wen C. Knowledge mapping of immune thrombocytopenia: a bibliometric study. Front Immunol. 2023;14:1160048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Tang C, Liu D, Fan Y, Yu J, Li C, Su J, Wang C. Visualization and bibliometric analysis of cAMP signaling system research trends and hotspots in cancer. J Cancer. 2021;12:358-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Dong H, Chen H, Jiang J, Zhang H, Cai C, Shen Q. Highly Sensitive Electrochemical Detection of Tumor Exosomes Based on Aptamer Recognition-Induced Multi-DNA Release and Cyclic Enzymatic Amplification. Anal Chem. 2018;90:4507-4513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 22. | Huang R, He L, Xia Y, Xu H, Liu C, Xie H, Wang S, Peng L, Liu Y, Liu Y, He N, Li Z. A Sensitive Aptasensor Based on a Hemin/G-Quadruplex-Assisted Signal Amplification Strategy for Electrochemical Detection of Gastric Cancer Exosomes. Small. 2019;15:e1900735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 23. | Wang S, Zhang L, Wan S, Cansiz S, Cui C, Liu Y, Cai R, Hong C, Teng IT, Shi M, Wu Y, Dong Y, Tan W. Aptasensor with Expanded Nucleotide Using DNA Nanotetrahedra for Electrochemical Detection of Cancerous Exosomes. ACS Nano. 2017;11:3943-3949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 327] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 24. | Hammond G, Johnston K, Huang K, Joynt Maddox KE. Social Determinants of Health Improve Predictive Accuracy of Clinical Risk Models for Cardiovascular Hospitalization, Annual Cost, and Death. Circ Cardiovasc Qual Outcomes. 2020;13:e006752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Zhao L, Sun R, He P, Zhang X. Ultrasensitive Detection of Exosomes by Target-Triggered Three-Dimensional DNA Walking Machine and Exonuclease III-Assisted Electrochemical Ratiometric Biosensing. Anal Chem. 2019;91:14773-14779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 26. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6573] [Article Influence: 1314.6] [Reference Citation Analysis (0)] |
| 27. | Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci U S A. 2006;103:11838-11843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1235] [Cited by in RCA: 1172] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 28. | Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7422] [Cited by in RCA: 7431] [Article Influence: 212.3] [Reference Citation Analysis (0)] |
| 29. | Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7090] [Cited by in RCA: 7045] [Article Influence: 201.3] [Reference Citation Analysis (0)] |
| 30. | Ji D, Feng H, Liew SW, Kwok CK. Modified nucleic acid aptamers: development, characterization, and biological applications. Trends Biotechnol. 2023;41:1360-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 31. | Oliveira R, Pinho E, Sousa AL, DeStefano JJ, Azevedo NF, Almeida C. Improving aptamer performance with nucleic acid mimics: de novo and post-SELEX approaches. Trends Biotechnol. 2022;40:549-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, Breakefield XO, Skog JK. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32:466-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 557] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 33. | Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, Jiang Y. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 792] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 34. | Rajagopal C, Harikumar KB. The Origin and Functions of Exosomes in Cancer. Front Oncol. 2018;8:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 35. | Zhou H, Yuen PS, Pisitkun T, Gonzales PA, Yasuda H, Dear JW, Gross P, Knepper MA, Star RA. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006;69:1471-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 487] [Cited by in RCA: 464] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 36. | Ueda K, Ishikawa N, Tatsuguchi A, Saichi N, Fujii R, Nakagawa H. Antibody-coupled monolithic silica microtips for highthroughput molecular profiling of circulating exosomes. Sci Rep. 2014;4:6232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 37. | Xiang W, Lv Q, Shi H, Xie B, Gao L. Aptamer-based biosensor for detecting carcinoembryonic antigen. Talanta. 2020;214:120716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 38. | Huang JY, Zhao L, Lei W, Wen W, Wang YJ, Bao T, Xiong HY, Zhang XH, Wang SF. A high-sensitivity electrochemical aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-nafion nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens Bioelectron. 2018;99:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 39. | Hu R, Wen W, Wang Q, Xiong H, Zhang X, Gu H, Wang S. Novel electrochemical aptamer biosensor based on an enzyme-gold nanoparticle dual label for the ultrasensitive detection of epithelial tumour marker MUC1. Biosens Bioelectron. 2014;53:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | Zhao Z, Yang S, Tang X, Feng L, Ding Z, Chen Z, Luo X, Deng R, Sheng J, Xie S, Chang K, Chen M. DNA four-way junction-driven dual-rolling circle amplification sandwich-type aptasensor for ultra-sensitive and specific detection of tumor-derived exosomes. Biosens Bioelectron. 2024;246:115841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 41. | Muzyka K, Saqib M, Liu Z, Zhang W, Xu G. Progress and challenges in electrochemiluminescent aptasensors. Biosens Bioelectron. 2017;92:241-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Ahmadzadeh-Raji M, Ghafar-Zadeh E, Amoabediny G. An Optically-Transparent Aptamer-Based Detection System for Colon Cancer Applications Using Gold Nanoparticles Electrodeposited on Indium Tin Oxide. Sensors (Basel). 2016;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Shafiei F, Saberi RS, Mehrgardi MA. A label-free electrochemical aptasensor for breast cancer cell detection based on a reduced graphene oxide-chitosan-gold nanoparticle composite. Bioelectrochemistry. 2021;140:107807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Dirks RM, Pierce NA. Triggered amplification by hybridization chain reaction. Proc Natl Acad Sci U S A. 2004;101:15275-15278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1311] [Cited by in RCA: 1455] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 45. | Zhu Y, Wu J, Zhou Q. Functional DNA sensors integrated with nucleic acid signal amplification strategies for non-nucleic acid targets detection. Biosens Bioelectron. 2023;230:115282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 46. | Ge S, Zhao J, Wang S, Lan F, Yan M, Yu J. Ultrasensitive electrochemiluminescence assay of tumor cells and evaluation of H(2)O(2) on a paper-based closed-bipolar electrode by in-situ hybridization chain reaction amplification. Biosens Bioelectron. 2018;102:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 47. | Hu T, Mei X, Wang Y, Weng X, Liang R, Wei M. Two-dimensional nanomaterials: fascinating materials in biomedical field. Sci Bull (Beijing). 2019;64:1707-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 48. | Mazloum-Ardakani M, Tavakolian-Ardakani Z, Sahraei N, Moshtaghioun SM. Fabrication of an ultrasensitive and selective electrochemical aptasensor to detect carcinoembryonic antigen by using a new nanocomposite. Biosens Bioelectron. 2019;129:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 49. | Liu S, Luo J, Jiang X, Li X, Yang M. Gold nanoparticle-modified black phosphorus nanosheets with improved stability for detection of circulating tumor cells. Mikrochim Acta. 2020;187:397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Ding Z, Lu Y, Wei Y, Song D, Xu Z, Fang J. DNA-Engineered iron-based metal-organic framework bio-interface for rapid visual determination of exosomes. J Colloid Interface Sci. 2022;612:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Noh S, Lee H, Kim J, Jang H, An J, Park C, Lee MH, Lee T. Rapid electrochemical dual-target biosensor composed of an Aptamer/MXene hybrid on Au microgap electrodes for cytokines detection. Biosens Bioelectron. 2022;207:114159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Xie FT, Li YL, Guan Y, Liu JW, Yang T, Mao GJ, Wu Y, Yang YH, Hu R. Ultrasensitive dual-signal electrochemical ratiometric aptasensor based on Co-MOFs with intrinsic self-calibration property for Mucin 1. Anal Chim Acta. 2022;1225:340219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |