Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4590
Revised: May 30, 2024
Accepted: June 13, 2024
Published online: July 26, 2024
Processing time: 68 Days and 2.9 Hours
Acute lower extremity deep venous thrombosis (LEDVT) is a common vascular emergency with significant morbidity risks, including post-thrombotic syndrome (PTS) and pulmonary embolism. Traditional treatments like catheter-directed thrombolysis (CDT) often result in variable success rates and complications.
To investigate the therapeutic efficacy of percutaneous mechanical thrombus removal in acute LEDVT.
A retrospective analysis was performed to examine 58 hospitalised patients with acute LEDVT between August 2019 and August 2022. The patients were categorised into the percutaneous mechanical thrombectomy (PMT) group (n = 24) and CDT group (n = 32). The follow-up, safety and treatment outcomes were compared between the two groups. The main observational indexes were venous patency score, thrombus removal effect, complications, hospitalisation duration and PTS.
The venous patency score was 9.04 ± 1.40 in the PMT group and 8.81 ± 1.60 in the CDT group, and the thrombus clearance rate was 100% in both groups. The complication rate was 8.33% in the PMT group and 34.84% in the CDT group, and the difference was statistically significant (P < 0.05). The average hospitalisation duration was 6.54 ± 2.48 days in the PMT group and 8.14 ± 3.56 days in the CDT group. The incidence of PTS was lower in the PMT group than in the CDT group; however, the difference was not statistically significant (P < 0.05).
Compared with CDT, treatment of LEDVT via PMT was associated with a better thrombus clearance rate, clinical therapeutic effect and PTS prevention function, but the difference was not statistically significant. Moreover, PMT was associated with a reduced urokinase dosage, shortened hospitalisation duration and reduced incidence of complications, such as infections and small haemorrhages. These results indicate that PMT has substantial beneficial effects in the treatment of LEDVT.
Core Tip: This retrospective study compares the efficacy of percutaneous mechanical thrombectomy (PMT) and catheter-directed thrombolysis (CDT) in treating acute lower extremity deep venous thrombosis (LEDVT). Results indicate that PMT achieves a comparable thrombus clearance rate to CDT, with significantly lower complication rates, shorter hospital stays, and potentially reduced incidence of post-thrombotic syndrome. The study suggests that PMT offers a safer, more efficient alternative for LEDVT treatment with a notable reduction in the use of urokinase, highlighting its advantages in clinical outcomes and patient care efficiency. Further research is recommended to validate these findings through prospective studies and to explore long-term treatment effects.
- Citation: Xue JQ, Yin P, He JP, Wei H, Geng CJ, Luo YX. Efficacy of percutaneous mechanical thrombus removal in acute lower extremity deep venous thrombosis. World J Clin Cases 2024; 12(21): 4590-4600
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4590.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4590
Lower extremity deep venous thrombosis (LEDVT) is a prevalent condition in the vascular surgery and cardiovascular field; it may be caused by trauma, major surgery, limb immobilisation, cancer and prolonged bed rest[1]. Studies have shown that 20%–30% of inadequately managed calf deep venous thrombosis (DVT) may spread to the proximal thigh and approximately 40% progress to pulmonary embolism (PE)[2-4]. Delayed or inappropriate treatment approaches may lead to thrombus organisation and subsequent development of post-thrombotic syndrome (PTS)[5-7]. PE and PTS may cause unmanageable oedema, leg ulcers and chronic leg discomfort, all of which pose a major risk to the patients’ quality of life and mortality. Currently, endovascular surgery and anticoagulant regimens are the mainstays of treatment for LEDVT[8]. According to Hekman et al[9] 30%–50% of patients with LEDVT may develop PTS even with standardised anticoagulation therapy. The European Society for Vascular Surgery has recommended that thrombus removal should be performed as early as possible in patients with acute proximal LEDVT, which immediately improves venous patency and reduces the risk of acute fatal PE and subsequent development of PTS[10,11]. With increased research related to LEDVT and advancement in endovascular treatment technology in recent years, treatment methods have been continuously optimised. Currently, percutaneous mechanical thrombectomy (PMT) is a commonly used treatment, and thrombus removal devices based on rotational mechanisms are the most widely used devices types[12]. To improve the safety and efficacy of PE treatment, reduce perioperative patient discomfort and enhance patient outcomes, this study assessed the issue of inadequate safety, tolerability and prognosis in patients with PE along with the performance of PMT.
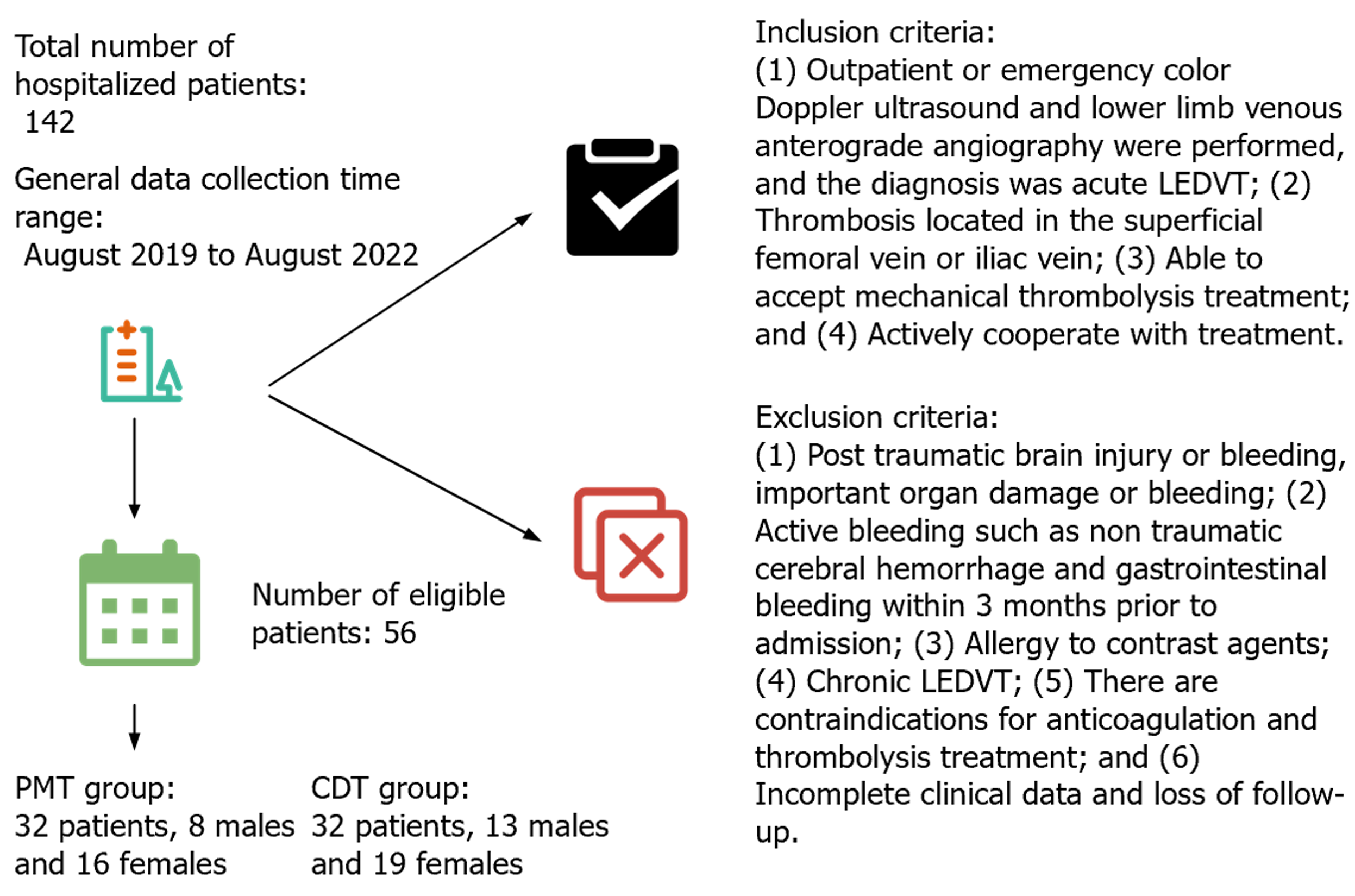
Figure 1 presents the study’s general information. The hospital’s Ethics Committee assessed and approved the current study.
Lower extremity venous anterograde angiography: The patient was made to lie flat. A tourniquet was first applied to tie the ankles bilaterally and 10 cm above the knees, and a 20- to 40-mL contrast agent was injected through a puncture needle inserted via the dorsal vein of the foot to visualise the anterior and posterior calf veins, peroneal vein, popliteal vein (Pop-V) and femoral vein (Fem-V). Once the tourniquet was released above the knee, the physician elevated the affected limb and pressed the calf under fluoroscopy to visualise the iliac and inferior vena cava veins. The tourniquet at the ankle was then released to visualise the superficial veins of the lower leg. To determine the location and loading of the thrombus, the veins were examined for patency, morphological abnormalities, compression signs and the presence of double tracks or filling defects[13].
Implantation and removal of inferior vena cava filters: After determining the location of the patient’s thrombus, the Fem-V was punctured, and the Seldinger technique was used to insert a 6F catheter sheath by performing LEDVT diagnostic venography. Femoral venography was used to precisely locate the renal vein opening while maintaining the patient under the influence of heparin, and an inferior vena cava filter was positioned in the space under the renal vein entrance. While removing the filter, the appropriate puncture path should be selected according to the filter type. For conical filters, access was established via the right internal jugular vein, and an 8F catheter sheath and retrieval kit were used to remove the filter. Shuttle filters can be removed via access through the Fem-V[14].
Catheter-directed thrombolysis (CDT): Patients in the CDT group received only CDT. Following vena cava filter implantation, the patient was turned onto their side, the Pop-V was perforated and a 5F catheter sheath was inserted under ultrasound guidance. To determine the location and extent of lower extremity venous thrombosis, imaging was performed. Access was established through a 0.035-inch guidewire, and a thrombolytic catheter was inserted along the venous system to ensure that the entire thrombus site was effectively perfused. The end of the catheter was closed, the exposed portion was immobilised and urokinase (1 × 104 U/kg/d) was delivered via a micropump. If the patient’s fibrinogen level was < 1.0 g/L, thrombolytic therapy was interrupted, and heparinised saline was infused continuously for 24 h to keep the catheter open. The criteria for discontinuing thrombolysis depended on whether there were bleeding signs, the thrombus was completely dissolved or the thrombus remained unchanged in the wall after 24 h of review[15].
PMT: Patients in the PMT group underwent thrombus-crushing aspiration performed using the AngioJet system. The patients received preoperative rehydration prophylaxis via intravenous infusion of 1000–2000 mL and a urinary catheter. The Fem-V was passed through the Pop-V or Fem-V via a healthy puncture to obstruct the vessel. After placing a 6F catheter sheath and performing venography to document the venous obstruction, a guidewire-assisted suction catheter was manoeuvred through the obstructed area into the inferior vena cava, where pharmacologic thrombolysis was performed first, followed by suction after a 20- to 30-min wait period. The catheter was aspirated retrogradely from the distal end of the thrombus toward the source at a controlled rate of 1 to 2 mL/s, with time adjustments based on the intraoperative thrombus size. The procedure can be repeated 3–5 times if imaging results indicate the presence of a large amount of residual thrombus. The puncture site was then compressed with an elastic bandage, the affected limb was elevated, adequate rehydration and regulation of urinary pH were performed and anticoagulation therapy was continued to monitor relevant blood, liver and kidney functions as well as coagulation indexes[16].
Percutaneous transluminal angioplasty (PTA) and stent implantation (SI): We performed PTA and SI in patients with iliofemoral vein stenosis or occlusion despite thrombus aspiration and thrombolysis. When the thrombus was effectively cleared and immediate imaging revealed > 50% stenosis of the iliofemoral vein, the vein was dilated with a balloon and a stent was placed while popping back[17].
Postoperative treatment: The patients’ postoperative temperature and cardiopulmonary function were closely monitored and managed accordingly. All patients were administered intraoperative and postoperative injections of low molecular weight heparin and long-term oral anticoagulation with rivaroxaban or warfarin for ≥ 3 months. During the treatment period, the coagulation function was assessed weekly, oral blood activators were administered and patients were asked to wear elastic stockings for secondary compression therapy.
Follow-up: A periodic ultrasound examination of the lower extremity veins and venous computed tomography venography or venography tests were performed during outpatient or telephone visits at 1, 3, 6 and 12 months postoperatively and every 6 months thereafter. The Villalta scoring system was applied to assess the PTS status of the affected limbs at the final follow-up visit.
Venous patency rate: The venous patency (VP) rate was defined as the difference between the pre- and post-treatment VP scores as a percentage of pre-treatment VP scores. The VP status was assessed via digital subtraction angiography or ultrasonography and categorised as incompetent, partially incompetent and completely incompetent, which were assigned VP scores of 2, 1 and 0, respectively, and the cumulative sum of each segment indicated the total score.
Thrombus clearance effect: The thrombus clearance (TC) effect was categorised as class I, class II and class III according to the VP rate. The patency rates were < 50% for class I, 0%–90% for class II and > 90% for class III.
Evaluation of treatment effectiveness: Evaluation of treatment (ET) effectiveness was based on clinical healing, swelling of the affected limb and disappearance of pain and other symptoms. Obvious improvement of symptoms was considered significantly effective if symptoms improved and significantly ineffective if there was no improvement or even aggravation of symptoms.
Security assessment: Complications including minor bleeding, extremely severe bleeding, PE and renal damage were observed. Minor bleeding involved hematoma, subcutaneous petechiae or oozing of blood at the puncture site. Severe bleeding involved gastrointestinal haemorrhage, retroperitoneal hematoma or intracranial haemorrhage.
Tolerance: Patients self-rated their tolerance of the two treatments on a scale of 1–12, with 1–4 assigned for experiencing fair or mild discomfort; 5–8 for experiencing moderate but tolerable discomfort and 9–12 for experiencing intolerable discomfort, not allowing the patient to continue treatment. In the tolerance test, there were three grades based on the evaluation scores: 1–4 were graded as A, 5–8 were graded as B and 9–12 were graded as C.
Six-minute walk test: Patients were asked to walk back and forth while wearing comfortable clothes and shoes on a flat surface of a 50-m long linear label in the departmental corridor, and their exercise tolerance was assessed by calculating the distance they walked. In general, the greater the walking distance, the better the exercise tolerance.
Other evaluation indicators: Urokinase dosage, thrombolysis duration and hospitalisation duration during treatment.
For statistical analysis, SPSS ver.22.0 was employed, and the measurement data were represented as. A paired-sample t-test was performed to compare the test indexes in the PMT group before and after surgery, and an independent-sample t-test or Mann–Whitney rank-sum test was used to compare the measurement data between the two groups. The χ2 test was performed for comparing count data.
Table 1 presents a comparison of the general data and clinical characteristics of the two patient groups at the time of admission.
| Item | PMT group (n = 24) | CDT group (n = 32) | P value |
| Sex | |||
| Male | 8 (33.33) | 13 (40.63) | 0.616 |
| Female | 16 (66.67) | 19 (59.37) | |
| Age (years) | 63.19 ± 14.04 | 62.80 ± 12.98 | 0.931 |
| Time of onset (days) | 7.15 ± 3.28 | 7.11 ± 3.39 | 0.961 |
| Lower extremity DVT type | |||
| Central type | 6 (25.00) | 8 (25.00) | 0.936 |
| Mixed type | 18 (75.00) | 24 (75.00) | |
| Distribution of affected limb | |||
| Left lower extremity | 19 (79.17) | 28 (87.50) | 0.604 |
| Right lower extremity | 5 (20.83) | 4 (12.50) | |
| Risk factors | |||
| No obvious cause | 8 (33.33) | 10 (31.25) | 0.857 |
| History of surgical operations within 3 months | 8 (33.33) | 12 (37.50) | 0.623 |
| Stay in bed or sit for a long time | 5 (20.83) | 5 (15.63) | 0.779 |
| Malignant tumor | 2 (8.33) | 3 (9.37) | 0.851 |
| Hyperhomocysteinemia | 2 (8.33) | 1 (3.12) | 0.524 |
| Deep vein valve dysfunction | 0 (0) | 2 (6.25) | 0.101 |
| After delivery | 2 (8.33) | 0 (0) | 0.057 |
| Stent implantation | 12 (50.00) | 16 (50.00) | 0.949 |
| Iliac vein stenosis | 15 (62.50) | 22 (68.75) | 0.686 |
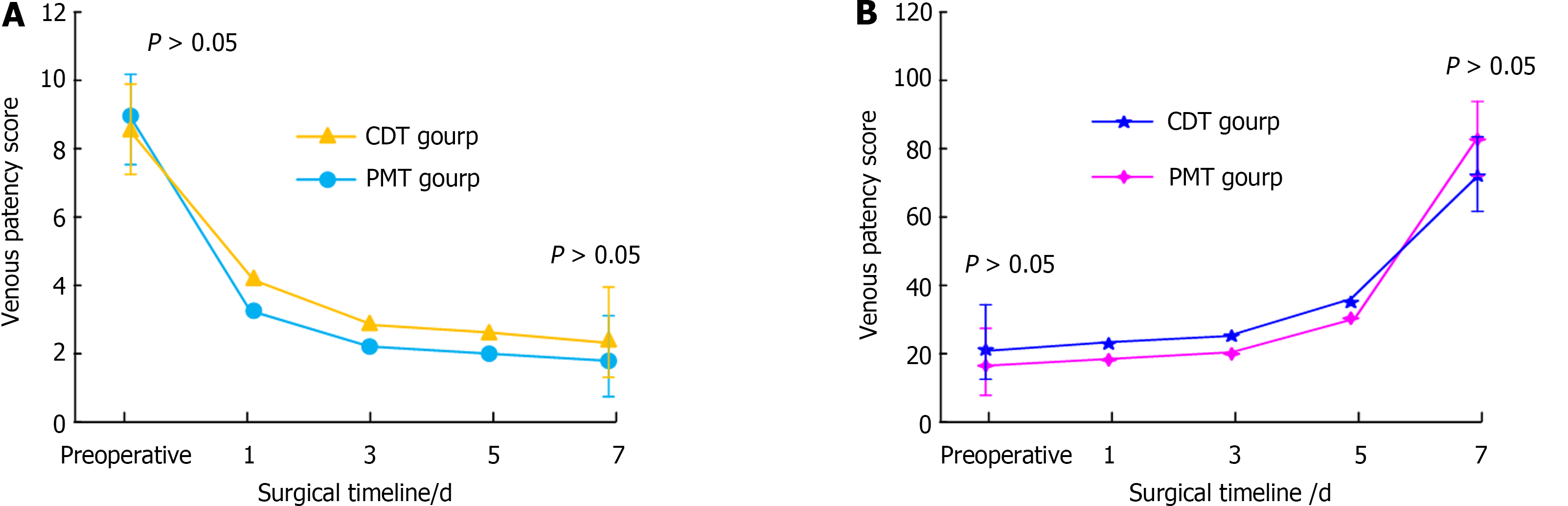
Figure 2 shows the comparison of VP scores and VP rates between the two groups. There was no statistically significant difference in the VP scores between the PMT and CDT groups prior to treatment (t = 0.560, P = 0.578).
Table 2 presents the comparison of TC effects between the two groups. Compared with the PMT group, the CDT group had a decreased rate of grade III TC.
| Argument | PMT group (n = 24) | CDT group (n = 32) | P value |
| Grade I thrombus clearance | 1 (4.16) | 2 (6.25) | 0.732 |
| Grade II thrombus clearance | 13 (54.17) | 22 (68.75) | 0.265 |
| Level III thrombus clearance | 10 (41.67) | 8 (25.00) | 0.186 |
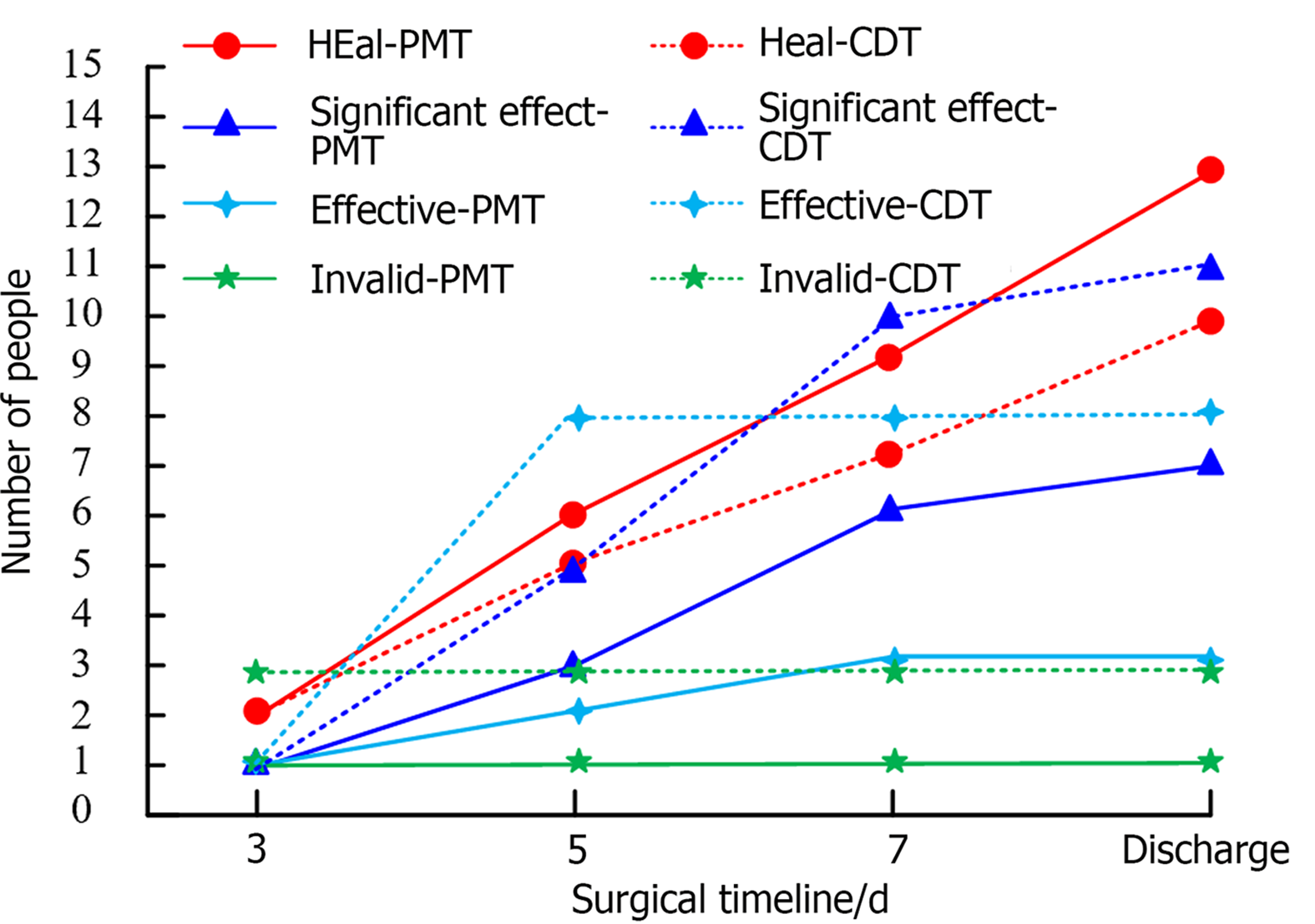
Figure 3 shows the clinical ET outcomes of the two patient groups. The patients underwent ET evaluations at days 3, 5 and 7 after therapy and at discharge.
The safety of patients in both groups was evaluated by the number of patients with complications and postoperative -related test indexes, including the occurrence of complications (Table 3). In the PMT group, there was no statistically significant difference in the safety of patients between postoperative days 1 and 3 (P = 0.551).
| Argument | PMT group (n = 24) | CDT group (n = 32) | P value | ||
| 1 day after surgery | 3 days after surgery | 1 day after surgery | 1 day after surgery | ||
| Minor bleeding | 1 (4.17) | 2 (8.33) | 4 (12.50) | 10 (31.25) | 0.011 |
| Severe bleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Infect | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | |
| Severe pulmonary embolism | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Complication rate | 1 (4.17) | 2 (8.33) | 4 (12.50) | 11 (34.38) | |
| P value | 0.551 | 0.039 | |||
Table 4 displays the findings of the comparison between the patients' preoperative and postoperative test indices in the PMT group. No complications such as renal failure and severe bleeding occurred during the treatment of the PMT group, and the difference between the preoperative.
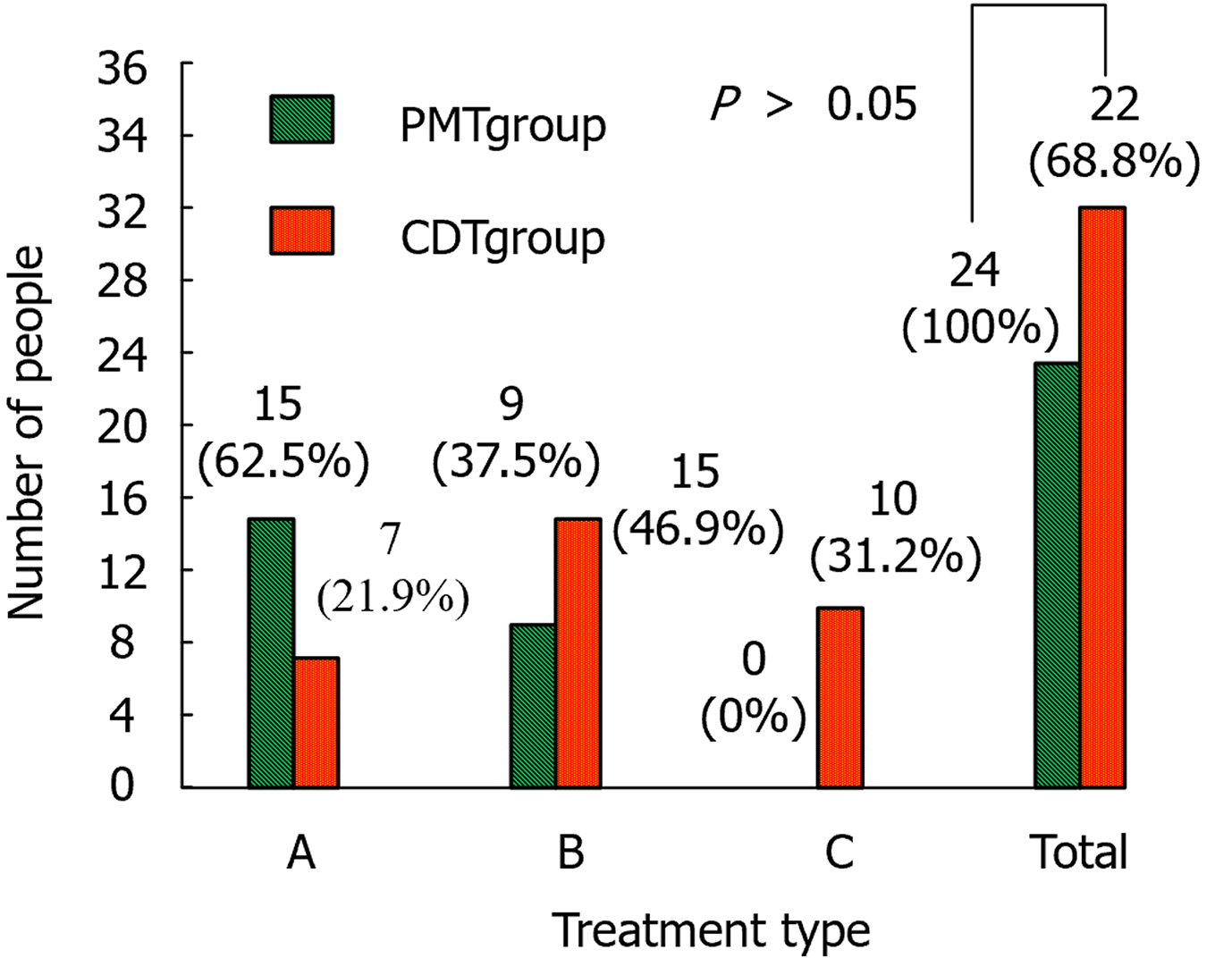
Figure 4 compares the tolerability of the patients in the two groups. In the CDT group, there were ten patients with grade C and zero in the PMT group.
Table 5 shows the comparison of the urokinase dosage, thrombolysis duration and hospitalisation duration between the two groups.
| Argument | PMT group (n = 24) | CDT group (n = 32) | P value | ||
| mean | SD | mean | SD | ||
| Urokinase dosage/10000 U | 18.24 | 7.33 | 345.15 | 45.54 | 0.000 |
| Thrombolytic time/hour | 0.34 | 0.17 | 154.48 | 62.37 | 0.000 |
| Hospitalization time/day | 6.54 | 2.48 | 8.14 | 3.56 | 0.000 |
Table 6 presents the comparison outcomes of the six-minute walk test (6MWT) between the two patient groups.
| Group | 6MWT before treatment/m | 6MWT after treatment/m | t | P value |
| CDT group (n = 32) | 331.24 ± 20.45 | 514.80 ± 24.77 | 32.367 | 0.000 |
| PMT group (n = 24) | 321.56 ± 18.23 | 553.12 ± 18.43 | 43.761 | 0.000 |
| t | 1.866 | 6.638 | - | |
| P value | 0.068 | 0.000 | ||
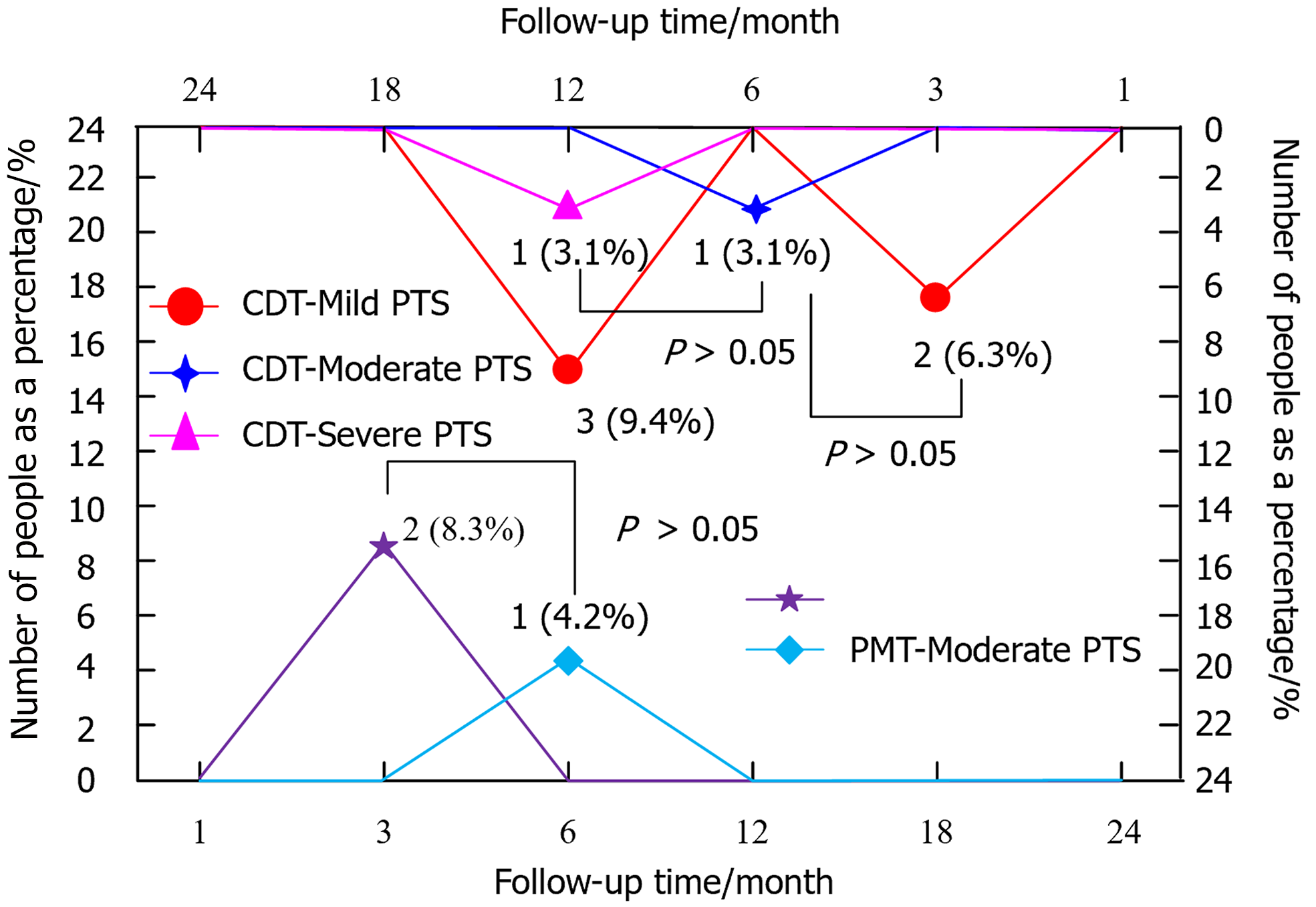
Figure 5 displays the follow-up outcomes of patients in PMT and CDT groups. There was no statistically significant difference in the incidence of PTS between PMT and CDT groups (χ2 = 0.318, P = 0.591).
Deep vein blood clots are a common symptom of DVT, a disease that mostly affects lower extremity deep veins, particularly those in the thighs and calves. If left untreated, DVT affects normal daily life of patients and leads to serious complications[18-20], such as varicose veins, walking difficulties and even PE and PTS. Patients should receive treatment immediately after DVT diagnosis[21,22]. Currently, the common treatment is CDT, which can be precisely performed in the affected deep vein when treating DVT, and the thrombolytic drugs can be delivered directly to the thrombus site, thus ensuring that the local effect is more direct and the effect of thrombus dissolution is more significant. CDT treatment can also control the effect and safety of treatment by adjusting the drug dose to avoid the adverse effects of drugs on the whole body[23,24]. However, there are other problems in the treatment process, such as longer bed rest time, poorer patient tolerance, use of the urokinase dose Deng and contraindications to thrombolysis in some patients[25,26]. With the goal of overcoming the limitations of CDT treatment, we assessed the ET effectiveness of PMT in this study.
Our retrospective analysis compared the data related to patients who received PMT and CDT therapies. The PMT group comprised 24 patients with a mean age of 63 (63.19 ± 14.04) years, with 7 males and 17 females. The mean duration between the development of symptoms and their presentation was 7 (7.15 ± 3.28) days, with a range of 1 to 14 days. Among the types of DVT, 5 cases were of the central type and 19 were of the mixed type. Seven patients underwent surgery within the past 3 months, four were advised post-injury bed rest, two had concomitant malignancy, two had hyperhomocysteinemia, two had symptoms after natural childbirth and seven had unknown onset of symptoms. There were 16 cases of iliac vein stenosis in the PMT group, 3 of them underwent balloon dilatation treatment and 13 received balloon dilatation plus SI. The mean age of the 32 individuals in the CDT group (12 males and 20 females) was 63 (62.80 ± 12.98) years. The mean duration between illness and consultation was 7 (7.11 ± 3.39) days, with a range of 1 to 14 days. In this group, 27 patients suffered from left -leg lesions and 5 suffered from right -leg lesions. Regarding the type of DVT, 7 cases were of the central type and 25 were of the mixed type. Eleven patients in this group underwent surgery in the past 3 months, five were bedridden after injury, three had malignant tumours, one suffered from hyperhomocysteinemia, one developed symptoms after a long-distance bus trip and two had venous valve dysfunction in the lower extremities. The cause of disease remained unknown in nine patients. There were 23 cases of iliac vein stenosis in the CDT group; 5 of them received balloon dilatation and 17 received treatment involving both balloon dilatation and stenting. In the comparison of VP scores and VP rates, the pre-treatment VP scores in PMT and CDT groups were 9.04 ± 1.40 and 8.81 ± 1.60, post-treatment VP scores were 1.75 ± 1.33 and 2.03 ± 1.33 and VP rates were 80.32% ± 15.67% and 76.70% ± 15.90%, respectively. Among the TC outcomes, we reviewed 1 case of TC grade I, 13 cases of TC grade II and 10 cases of TC grade III in the PMT group for imaging before discharge. In the contrast review after CDT completion, we assessed 8 cases of TC grade III, 22 cases of TC grade II and 2 cases of TC grade I. There was only 1 case of TC grade III in the CDT group. Thirteen patients in the PMT group were clinically cured, 7 showed considerable efficacy, 3 showed some efficacy and 1 showed no efficacy in the assessment of clinical results between the two groups. There were 8 cases of effective treatment, 3 cases of ineffective treatment, 10 cases of clinical healing and 11 cases of apparent effect in the CDT group. The findings revealed that, both before and after therapy, the VP scores and VP rates were significantly improved in both groups. Therefore, PMT and CDT therapies had similar ET effectiveness in DVT. Fuller et al[27] demonstrated the effectiveness of CDT therapy via endovascular treatment in 22 patients, reporting a 100% success rate and > 70% thrombus regression rate. A retrospective examination of 160 patients by Yıldız et al[28] revealed that PMT therapy yielded better outcomes in terms of the Villalta scoring and EuroQol-5D-3L score. Both of these results were in agreement with the findings of the current study.
In the safety assessment, there were two cases of haemorrhagic complications in the PMT group, including one case of hematoma at the Fem-V puncture point and one case of subcutaneous ecchymosis, both of which were not treated specifically. Thirteen cases developed carnal haemoglobinuria after surgery, and the urine colour returned to normal within 24 h of treatment with adequate rehydration and hydration. There were 11 cases of bleeding complications in the CDT group, including 5 cases of blood leakage from the puncture point of Pop-V, 1 case of subcutaneous petechiae, 3 cases of haematuria and 1 case of upper gastrointestinal bleeding. In addition, the abovementioned patients were treated for their symptoms by reducing the urokinase dose, suspending thrombolysis, applying compression to the bleeding site to stop bleeding and suppressing acid production to protect the stomach lining. Their prognoses were favourable, but one patient experienced fever and chills associated with a catheter-related infection. The thrombolytic catheter was withdrawn, and the patient fully recovered before being discharged from the hospital following intensive treatment with sensitive antibiotics. Considering that PMT is a minimally invasive procedure, there is less damage to the surrounding tissues during operation. Additionally, the amount of drugs used during PMT was lower, which reduces the occurrence of drug-related complications. In the tolerability assessment, the PMT group could tolerate the treatment. In the CDT group, 10 patients were intolerant and 3 requested early termination of the treatment. The patients who underwent PMT showed higher tolerance and almost all patients could tolerate PMT treatment. In contrast, in the CDT group, some patients requested early termination of treatment due to discomfort. This discomfort may be related to the fact that PMT treatment is shorter and less invasive, making patients feel more comfortable.
Regarding urokinase dosage, the highest dosage was 250000 U in the PMT group and 3.9 million U in the CDT group. The longest thrombolysis time was 0.5 h in the PMT group and 217 h in the CDT group. The longest hospitalisation was 9 h in the PMT group and 12 h in the CDT group. A major advantage of PMT treatment is that its treatment time and hospitalisation duration are significantly shorter than those of CDT treatment. This advantage reduces the financial and psychological burden on patients and decreases the chance of developing decubitus ulcers and pneumonia, which are two conditions related to extended bed rest. In the follow-up results, 21 patients had no PTS, 2 had mild PTS, 1 had moderate PTS and none of the patients had severe PTS in the PMT group. In contrast, 25 patients had no occurrence of PTS, 5 had mild PTS, 1 had moderate PTS and 1 had severe PTS in the CDT group. Compared with the CDT group, patients in the PMT group had a decreased incidence of PTS. The incidence of PTS was marginally reduced in the PMT-treated group even though there was no discernible difference in PTS prevention function between the two treatments. This finding may be due to the ability of PMT to restore vascular patency more rapidly, thereby reducing the risk of PTS caused by long-term hemodynamic changes. These outcomes demonstrated that the ET effectiveness was higher in the PMT group than in the CDT group, as indicated by the safety, tolerability, medication dosage, treatment duration, hospitalisation duration and recovery during the course of DVT treatment. Li et al[29] conducted a retrospective analysis of 82 patients with LEDVT and found that PMT was superior to CDT in terms of poor circumferential diameter of the lower extremity. By comparing the two groups, they could determine that PMT was superior in terms of hospitalisation duration, swelling reduction rate in the affected limb, TC rate and long-term revascularisation[29]. Li et al[30] individually compared PMT and CDT therapies and found that PMT had better ET effectiveness in patients suffering from severe acute hypoplastic iliofemoral DVT.
We found that PMT was comparable to CDT in terms of treating acute LEDVT, as demonstrated by the TC rate, improvement of clinical symptoms and PTS prevention function. However, PMT significantly reduced the urokinase dosage, thrombolysis time and hospitalisation duration and lowered the incidence of minor bleeding events and infections, demonstrating notable efficacy in recent follow-ups. PMT offers considerable advantages in reducing treatment duration and minimising complications related to prolonged bed rest. Future studies should use longer follow-up periods to further validate these findings.
| 1. | Harbsmeier AN, Altintas I, Iversen K, Andersen O, Nehlin JO. Biomarkers and the post-thrombotic syndrome: A systematic review of biomarkers associated with the occurrence of the post-thrombotic syndrome after lower extremity deep venous thrombosis. Phlebology. 2023;38:577-598. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Daniels P, Vlazny D, Meverden R, Bartlett M, Hesley G, Lekah A, Macedo T, Wysokinski WE, Houghton DE. Popliteal cysts are not a risk factor for lower extremity deep vein thrombosis. J Thromb Thrombolysis. 2022;54:492-499. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Hou J, Wang W, Cai H, Chen J, Chen B, Shen Z, Tang Y, Li J, Liu S, Mei Y, Wang J, Lu S. Patients With Right Lower Extremity Deep Vein Thrombosis Have a Higher Risk of Symptomatic Pulmonary Embolism: A Retrospective Study of 1585 Patients. Ann Vasc Surg. 2022;81:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Yuriditsky E, Narula N, Jacobowitz GR, Moreira AL, Maldonado TS, Horowitz JM, Sadek M, Barfield ME, Rockman CB, Garg K. Histologic assessment of lower extremity deep vein thrombus from patients undergoing percutaneous mechanical thrombectomy. J Vasc Surg Venous Lymphat Disord. 2022;10:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Zhao WG, Zhang WL, Zhang YZ. Characteristics of Deep Venous Thrombosis in Isolated Lower Extremity Fractures and Unsolved Problems in Guidelines: A Review of Recent Literature. Orthop Surg. 2022;14:1558-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Rudnin S, Kaminsky J, Ghosh R, Webb R, Fu W, Tama M, Hayim M, Hahn B, Greenstein J. Distribution of Lower Extremity Deep Vein Thrombosis and Implications for Limited Compression Ultrasound Examinations. J Emerg Med. 2022;63:348-354. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Schellong S, Ageno W, Casella IB, Chee KH, Schulman S, Singer DE, Desch M, Tang W, Voccia I, Zint K, Goldhaber SZ. Profile of Patients with Isolated Distal Deep Vein Thrombosis versus Proximal Deep Vein Thrombosis or Pulmonary Embolism: RE-COVERY DVT/PE Study. Semin Thromb Hemost. 2022;48:446-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Lou Z, Ma H, Li X, Zhang F, Du K, Wang B. Hsa_circ_0001020 accelerates the lower extremity deep vein thrombosis via sponging miR-29c-3p to promote MDM2 expression. Thromb Res. 2022;211:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Hekman KE, Chao CL, Morgan CE, Helenowski IB, Eskandari MK. Direct oral anticoagulants decrease treatment failure for acute lower extremity deep venous thrombosis. Vascular. 2022;30:1199-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Valeriani E, Di Nisio M, Porceddu E, Agostini F, Pola R, Spoto S, Donadini MP, Ageno W, Porfidia A. Anticoagulant treatment for upper extremity deep vein thrombosis: A systematic review and meta-analysis. J Thromb Haemost. 2022;20:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Yang B, Zhang Z. Suppression of long intergenic non-protein coding RNA 1123 constrains lower extremity deep vein thrombosis via microRNA-125a-3p to target interleukin 1 receptor type 1. Bioengineered. 2022;13:13452-13461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Li Y, Shan J. Study on the correlation between high density lipoprotein and lower extremities deep venous thrombosis in patients undergoing hip arthroplasty. Phlebology. 2022;37:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Schafer K, Goldschmidt E, Oostra D, Kaminski B, Mattin M, Lurie F. Defining the role of risk stratification and duplex ultrasound in the diagnosis of acute lower extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | İnce S, Özgokçe M, Özkaçmaz S, Dündar İ, Türko E, Ayyıldız VA, Göya C. Comparison of Medical Treatment Efficiency With Shear Wave Elastography Values of Thrombus in Patients With Lower Extremity Deep Vein Thrombosis. Ultrasound Q. 2023;39:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Baytaroglu C, Sevgili E. Learning curve for percutaneous thrombectomy in treatment of acute lower extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10:602-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | de Kleijn RJCMF, Schropp L, van Hattum ES, Ünlu Ç, Middeldorp S, Nijkeuter M, Westerink J, Petri BJ, de Borst GJ. Post-thrombotic syndrome after upper extremity deep vein thrombosis: An international Delphi consensus study. J Thromb Haemost. 2022;20:1880-1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Chen J, Yang Y, Wang S, Zhang K. Crocin improves lower extremity deep venous thrombosis by regulating the PIM1/FOXO3a axis. Cell Mol Biol (Noisy-le-grand). 2023;69:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Tian Y, Shi CH, Lu WL, Zhang BX, Zhou C, Huang YL, Hao JS, Chen Q. Risk factors and outcomes regarding the acute kidney injury after AngioJet thrombectomy for acute lower-extremity deep vein thrombosis. Asian J Surg. 2023;46:3505-3511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Dey R, Vinod KV, Adole PS. Body Iron Store and its Association with Risk of First Episode of Spontaneous Lower Extremity Deep Vein Thrombosis/ Pulmonary Embolism: A Case-Control Study. Indian J Hematol Blood Transfus. 2023;39:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 20. | Cires-Drouet RS, Durham F, Sharma J, Cheeka P, Strumpf Z, Cranston E, Xu C, Mayorga-Carlin M, Sorkin JD, Lal BK. Prevalence and clinical outcomes of hospitalized patients with upper extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Chen F, Huang JG, Liu X, Zhou W. Left iliac vein involvement is a protective factor against symptomatic pulmonary embolism in lower left extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10:1272-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Correia R, Gião N, Bento R, Garcia R, Camacho N, Ferreira ME. Cystic Adventitial Disease of the Popliteal Vein, a Rare Cause of Lower Limb Deep Vein Thrombosis. EJVES Vasc Forum. 2022;54:75-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Musil D, Kováčik F. Delay between clinical presentation and treatment of deep venous thrombosis in the lower limbs and regression of thrombosis. Phlebology. 2022;37:120-124. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Huang J, Kong J, Zhang X, Liu C, Zhao Z, Liu L, Xiao L, Han X. Risk factors for inferior vena cava filter thrombosis in traumatic fracture patients with deep venous thrombosis of lower extremity: A single-center experience. Vascular. 2024;32:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Feng SY, Li Y. Incidence, timing, location, risk factors, and nomogram of lower extremity deep venous thrombosis after acute carbon monoxide poisoning. Ir J Med Sci. 2023;192:417-422. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Teichman AL, Walls D, Choron RL, Butts CA, Krumrei N, Amro C, Swaminathan S, Arcomano N, Parekh A, Romeo P. The Utility of Lower Extremity Screening Duplex for the Detection of Deep Vein Thrombosis in Trauma. J Surg Res. 2022;269:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Fuller T, Neville E, Shapiro J, Muck AE, Broering M, Kulwicki A, Kuhn B, Recht M, Muck P. Comparison of aspiration thrombectomy to other endovascular therapies for proximal upper extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Yıldız Z, Kayğın MA, Özkara T, Limandal HK, Diler MS, Çüçen Dayı HI, Ergün S, Dağ Ö. Effects of Deep Venous Thrombosis Treatments on Early and Long-term Quality of Life: Medical Therapy vs. Systemic Thrombolysis vs. Pharmacomechanical Thrombolysis. Vasc Endovascular Surg. 2024;58:5-12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Li JW, Xue J, Guo F, Han L, Ban RB, Wu XL. [Clinical Comparison of the Efficacy of Systemic Thrombolysis,Catheter-Directed Thrombolysis,and AngioJet Percutaneous Mechanical Thrombectomy in Acute Lower Extremity Deep Venous Thrombosis]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2023;45:410-415. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Li W, Zaid Al-Kaylani A, Zeebregts CJ, El Moumni M, de Vries JPM, van der Doef HPJ, Bokkers RPH. Effectiveness and safety of catheter-directed thrombolysis in conjunction with percutaneous mechanical thrombectomy for acute iliofemoral deep vein thrombosis: A meta-analysis. J Vasc Surg Venous Lymphat Disord. 2023;11:843-853.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |