Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4574
Revised: May 18, 2024
Accepted: June 13, 2024
Published online: July 26, 2024
Processing time: 82 Days and 1.5 Hours
Gastric ulcers (GUs) have a high risk of clinical morbidity and recurrence, and further exploration is needed for the prevention, diagnosis, and treatment of the disease.
To investigated the effects of a diet plan on pepsinogen (PG) I, PG II, gastrin-17 (G-17) levels and nutritional status in patients with GUs.
A total of 100 patients with GUs treated between May 2022 and May 2023 were enrolled, with 47 patients in the control group receiving routine nursing and 53 patients in the experimental group receiving dietary nursing intervention based on a diet plan. The study compared the two groups in terms of nursing efficacy, adverse events (vomiting, acid reflux, and celialgia), time to symptom impro
The experimental group showed a markedly higher total effective rate of nursing, a significantly lower incidence of adverse events, and a shorter time to symptom improvement than the control group. Additionally, the experimental group’s post-intervention PG I, PG II, and G-17 levels were significantly lower than pre-intervention or control group levels, whereas PA and ALB levels were signifi
The diet plan significantly reduced PG I, PG II, and G-17 levels in patients with GUs and significantly improved their nutritional status.
Core Tip: Gastric ulcers (GUs) have high clinical morbidity and recurrence, posing varying degrees of adverse effects on patients’ gastric function, physical and mental health, and quality of life. Such patients have also been a key focus of nursing interventions. This study included 100 patients with GUs and compared the clinical effects of routine nursing vs dietary nursing intervention based on a diet plan. We found that the dietary nursing intervention significantly improved nursing efficacy in patients with GUs, preventing vomiting, acid reflux, and celialgia; promoting symptom improvement; and helping restore gastric function and nutritional status, thus providing an effective new option for the clinical nursing of patients with GUs.
- Citation: Zhang WW, Wang XF, Yu HY, Wang LF. Influence of a diet meal plan on pepsinogen I and II, gastrin-17, and nutritional status in gastric ulcer patients. World J Clin Cases 2024; 12(21): 4574-4581
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4574.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4574
Gastric ulcers (GUs) are a chronic digestive condition with a high risk of morbidity and recurrence. Its clinical symptoms principally include abdominal distention, belching, acid reflux, and epigastric pain[1,2]. Patients with severe disease may exhibit symptoms such as hematemesis and melena, occasionally leading to severe complications such as gastric perforation, gastric bleeding, and even cancer. These complications can negatively affect patients’ gastric function, physical and mental health, and overall quality of life[3,4]. Helicobacter pylori infection, smoking, and certain unhealthy lifestyle and dietary habits can worsen or create more GUs[5-7]. In 2022, the International Epidemiological Association reported an increase in the global incidence of GUs, accompanied by a decline in age-standardized prevalence and mortality. However, the prevention, diagnosis, and treatment of GUs still require further exploration[8].
Although GUs are not considered a critical disease, they have always been the focus of nursing interventions because patients with GUs frequently demonstrate impaired gastric function and abnormal nutritional status[9,10]. This study focused on providing effective intervention for patients with GUs from a nursing perspective. Moreover, current standard nursing practices must be revised to cater to the diverse nursing requirements of patients with GUs[11].
This study proposes a diet plan-based intervention that educates patients on healthy diet and lifestyle principles while offering them scientific and effective dietary guidance at different stages. Additionally, this novel intervention features dynamic tracking and follow-up of the patients’ diet through telephone, QQ, WeChat, and other Internet-based communication methods[12,13]. This care model differs from standard nursing practice as it provides dietary education and tracks and guides the patient on dietary status, plan, and mood and other relevant variables relevant to GU patient care. The intervention focuses on repairing gastric function and improving nutritional status to offer the patient comprehensive recovery[14-16].
At present, there is limited research on how diet plans affect gastric function and nutritional status in patients with GUs. Therefore, the present study aimed to fill this research gap and make a modest contribution to the prevention, diagnosis, and treatment of patients with GUs.
This was a retrospective study. We retrospectively analyzed 100 patients with GUs treated at Lujiang County Hospital of Traditional Chinese Medicine from May 2022 to May 2023. The patients were categorized into the control group (n = 47), which received routine nursing, and the experimental group (n = 53), which received diet plan-based dietary care.
All eligible patients met the diagnostic criteria for GUs and could understand and cooperate with the nursing staff throughout their care. These patients had stable conditions, complete medical records, and no history of ulcer perforation or cancer. Their immediate family members cared for them both during and after hospitalization.
We excluded patients with upper gastrointestinal bleeding, pylorochesis, duodenal ulcer, or other digestive system diseases; serious cardio-cerebrovascular diseases; complete loss of self-care ability; coagulopathy; liver and renal insufficiency; hematological system diseases; history of gastric surgery; and mental disorders or mental illness as well as pregnant or lactating women.
The control group received routine nursing care, including health education, admission guidance, condition monitoring, medication guidance, rehabilitation confidence enhancement, vital sign monitoring, and regular telephone follow-up after discharge.
The experimental group received diet plan-based dietary intervention through the following nursing methods: (1) First, the patients were provided dietary education to let them realize the importance of correct eating habits for physical rehabilitation; each patient was guided and encouraged to correct their maladaptive eating habits. Additionally, the medical staff patiently and carefully introduced a modified diet plan tailored to patients with chronic GUs. This plan emphasized eating fewer calorie-dense foods, avoiding fats and proteins, paying particular attention to nutrition at breakfast, prioritizing intake of easy-to-digest foods at lunch, and swapping greasy and spicy foods for a liquid meal at dinner while avoiding the consumption of excess calories; (2) The patients were encouraged to establish better dietary habits through open discussion of dietary and lifestyle principles. Furthermore, patients were recommended to establish normal daily routines and eat regularly, including (when appropriate) eating small meals, eating more slowly, paying attention to nutrition labels, and limiting intake of fats. Furthermore, patients were advised to exercise moderately, with a recommended duration of 30 min daily; (3) Each patient received a tailored diet plan featuring simple and easy-to-understand diet and lifestyle recommendations, including food “groups”, dietary patterns, and weekly diet plans and guidance (three meals a day). The diet plan was pushed weekly or daily through a WeChat mini-program. Patients’ moods and feelings about the intervention were regularly monitored such that negative impressions could be promptly addressed; (4) The diet plan-based intervention was further customized to patients with different GU severities. Minor diet adjustments were made for patients with mild GUs to ensure a healthy and reasonable diet. Patients with moderate GUs were advised to eat specific foods according to their severity of acid reflux, vomiting, and celialgia. Those with sudden onset and serious symptoms were urged to eat easily digestible and light foods, avoid stimulating foods, and limit the intake of milk and strong tea; (5) Convalescing patients were advised to eat more alkaline foods and increase their intake of zinc and foods that can remove free radicals, such as soy products, fresh fruits, and sweet potatoes, and eat less or no sweets; and (6) All patients were followed-up by nurses via telephone, QQ, WeChat, and other means. Patients who maintained maladaptive diets or failed to present for follow-up appointments were discontinued from the study.
Nursing efficacy: The gradual resolution of clinical symptoms such as acid reflux, abdominal pain, and vomiting, along with the return to a normal diet, was considered marked effectiveness. Significant improvement in clinical symptoms and alleviation of vomiting, acid reflux, and abdominal pain after the dietary intervention were regarded as effectiveness. No change in clinical symptoms or even aggravation was regarded as ineffectiveness.
Occurrence of adverse events: The number of adverse events such as vomiting, acid reflux, and celialgia was observed and recorded and the total incidence rate was calculated.
Time to symptom improvement: The time to improvement of clinical symptoms (burning sensation, acid reflux, and celialgia) was observed and recorded.
Stomach function: Before and after the dietary intervention, 5 mL of venous blood was drawn to determine pepsinogen (PG) I and PG II levels via chemiluminescence immunoassay and gastrin-17 (G-17) level via enzyme-linked immuno
Nutritional status: The levels of prealbumin (PA) and albumin (ALB) were measured via transmission turbidimetry before and after the dietary intervention.
We used SPSS 21.0 software to process data with a statistical significance level of P < 0.05. Quantitative data are presented as mean ± SD. Independent sample t-tests were used for between-group comparisons, whereas paired t-tests were used for comparisons between two time points. Nursing efficacy, adverse event (such as vomiting, acid reflux, and celialgia) incidence, and other count data are expressed as n (%), with between-group comparisons made using the χ2 test.
No significant between-group differences were observed in sex, age, ulcer area, hypertension, diabetes, and dyslipidemia (P > 0.05, Table 1).
| Indicators | Control group (n = 47) | Experimental group (n = 53) | χ2/t | P value |
| Sex (male/female) | 24/23 | 29/24 | 0.133 | 0.715 |
| Age (years) | 40.96 ± 5.43 | 42.45 ± 8.49 | 1.030 | 0.305 |
| Ulcer area (cm2) | 8.02 ± 1.66 | 7.77 ± 2.32 | 0.613 | 0.542 |
| Hypertension (+/-) | 8/39 | 10/43 | 0.058 | 0.810 |
| Diabetes mellitus (+/-) | 6/41 | 12/41 | 1.646 | 0.200 |
| Dyslipidemia (+/-) | 5/42 | 7/46 | 0.156 | 0.693 |
The total effective rate of nursing was significantly higher in the experimental group (96.23%) than in the control group (80.85%) (P < 0.05, Table 2).
| Indicators | Control group (n = 47) | Experimental group (n = 53) | χ2 | P value |
| Marked effectiveness | 16 (34.04) | 27 (50.94) | ||
| Effectiveness | 22 (46.81) | 24 (45.28) | ||
| Ineffectiveness | 9 (19.15) | 2 (3.77) | ||
| Total | 38 (80.85) | 51 (96.23) | 6.015 | 0.014 |
The control and experimental groups had 10 cases and 2 cases, respectively, of vomiting, acid reflux, and celialgia, indicating a significant difference (P < 0.05, Table 3).
| Indicators | Control group (n = 47) | Experimental group (n = 53) | χ2 | P value |
| Vomiting | 4 (8.51) | 0 (0.00) | ||
| Acid reflux | 3 (6.38) | 2 (3.77) | ||
| Celialgia | 3 (6.38) | 0 (0.00) | ||
| Total | 10 (21.28) | 2 (3.77) | 7.227 | 0.007 |
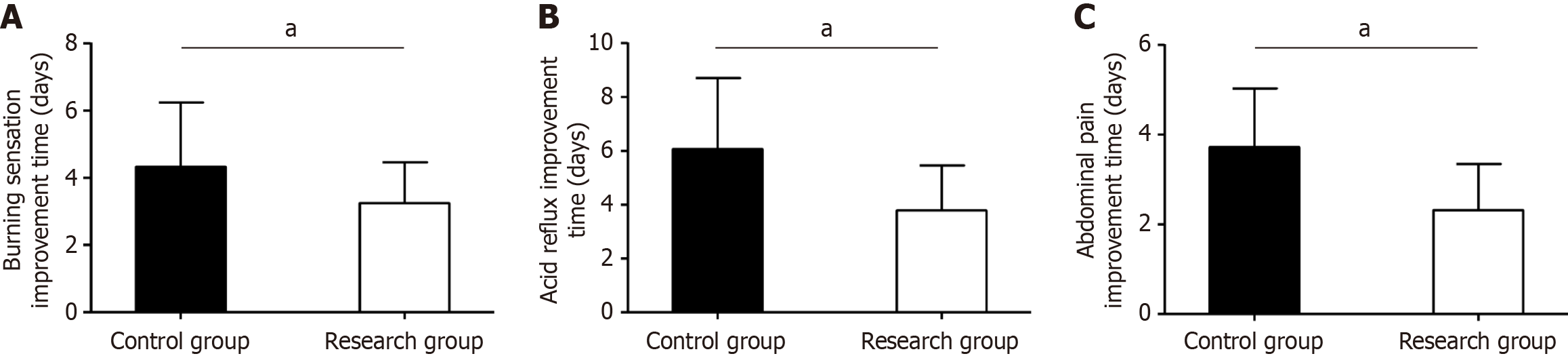
We observed a significant decrease in time to symptom improvement in the experimental group compared with the control group for burning sensation, acid reflux, and celialgia (P < 0.05, Figure 1).
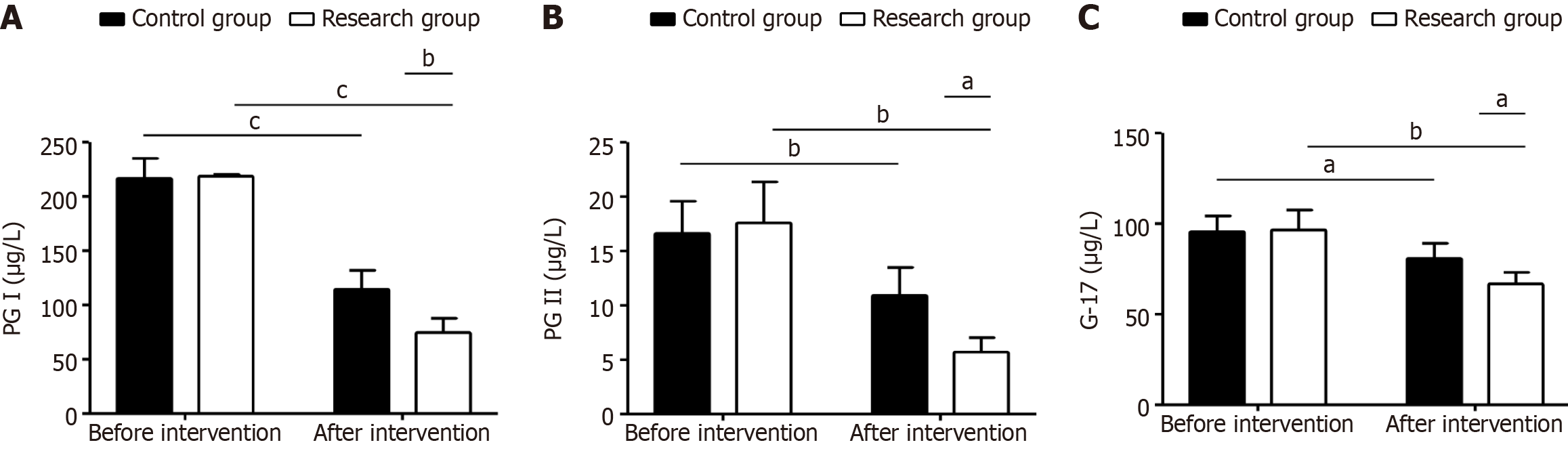
Gastric function indices (PG I, PG II, and G-17) showed no significant between-group differences pre-intervention (P > 0.05); however, post-intervention, there were significant differences in the reductions in PG I, PG II, and G-17 levels (P < 0.05); this change was particularly significant in the experimental group (P < 0.05, Figure 2).
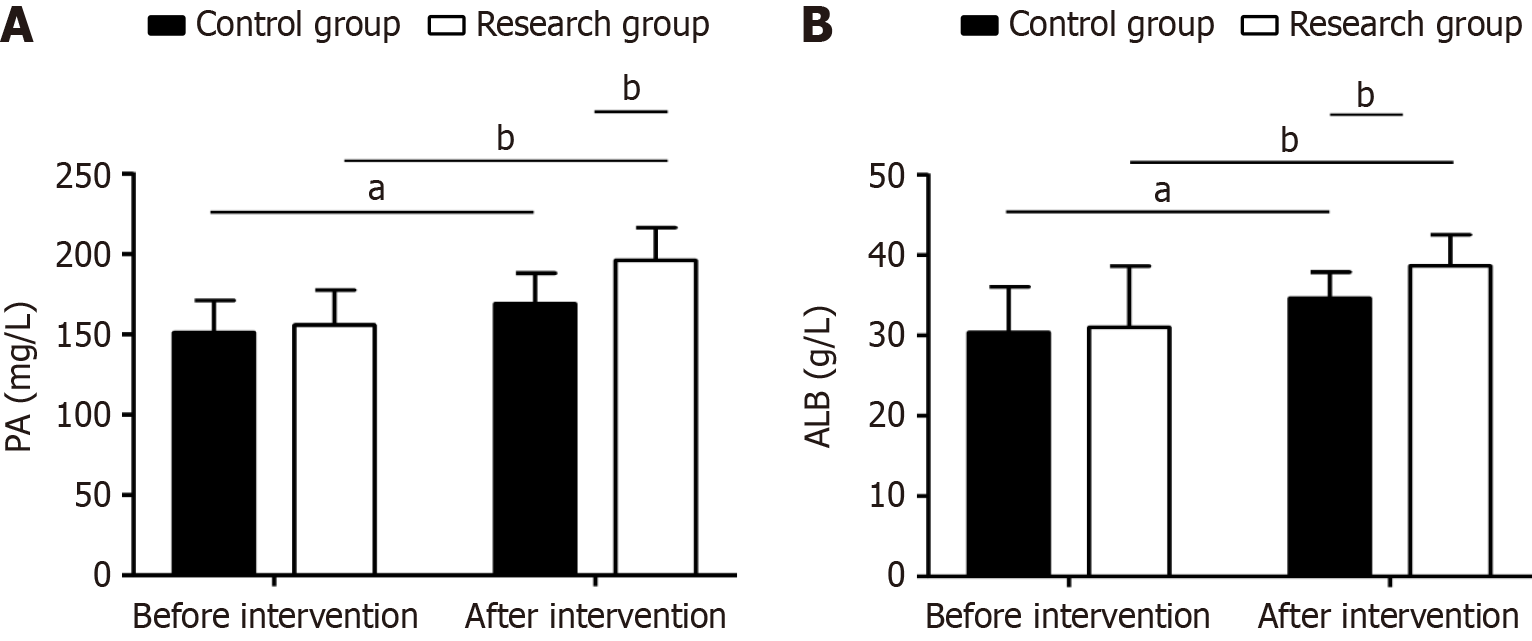
The study assessed each patient’s nutritional status using PA and ALB indices. There were no significant between-group differences at baseline (P > 0.05). However, post-intervention, both groups showed increased PA and ALB (P < 0.05) levels, with significantly higher levels of both in the experimental group than in the control group (P < 0.05, Figure 3).
GUs are caused by damage to the gastric mucosa, frequently causing increased gastric acid and pepsin levels[17]. GUs are frequently accompanied by gastritis, potentially increasing the risk of developing gastric cancer[18]. According to statistics, up to 6.07% of people in China may be at risk of developing GUs. Furthermore, the probability of GUs occurr
Many researchers have analyzed the clinical effects, impacts, and underlying mechanisms of dietary interventions in GUs and related gastric diseases. For instance, Jeong et al[21] demonstrated that dietary interventions with Artemisia argyi and green tea extracts significantly alleviate pathological changes in mice with Helicobacter pylori-associated chronic atrophic gastritis, thereby aiding in the prevention of tumorigenesis. Similarly, Ohara et al[22] proposed the use of a plant-derived compound, citral, in an obesity-related GU mouse model, which not only significantly inhibited scar tissue progression but also actively regulated abnormal indicators such as matrix metalloproteinases. In the present study, the total effective rate of nursing was notably higher in the experimental group than in the control group, suggesting that diet plan-based dietary care interventions employed in the experimental group greatly contribute to improving nursing efficacy in patients with GUs. This improvement may be attributed to the customized diet plan provided to patients, which, built upon routine management, enhanced their understanding of dietary and lifestyle principles, dietary patterns, recommended foods, etc. Additionally, paying close attention to patients’ mood during meals enabled timely soothing[23,24]. Furthermore, the diet of patients with GUs could be dynamically tracked, facilitating timely adjustments by medical staff and providing patients with timely and appropriate feedback to maximize nursing effectiveness[25]. Regarding clinical manifestations such as vomiting, acid reflux, and celialgia, the total incidence of adverse events in the experimental group was 17.51%, lower than that in the control group, suggesting that the diet plan used in the experimental group could help reduce the risk of adverse events in patients with GUs. Regarding the time to symptom improvement, the experimental group showed a significantly shorter improvement time for symptoms such as burning sensation, acid reflux, and celialgia compared with the control group, demonstrating that diet plan-based interventions can effectively promote symptom improvement in patients with GUs. Similar to our findings, Peng et al[26] reported that diet plan-based interventions are effective in alleviating functional gastrointestinal symptoms in patients with inflammatory bowel disease.
PG I, PG II, and G-17 are three important indices that reflect the gastric function of patients with GUs. PGI and PGII are significantly associated with the rate of gastric acid secretion by gastric mucosa and the risk of peptic ulcers, whereas G-17 is closely associated with peptic ulcer bleeding caused by excessive gastric acid secretion[27-29]. As serological indicators, they are often used to screen for precancerous diseases or early-stage gastric cancer, providing valuable screening value for conditions such as chronic nonatrophic gastritis, GUs, and gastric cancer[30]. In the present study, analysis of gastric function indices revealed that PG I, PG II, and G-17 levels in the experimental group were significantly inhibited after intervention, showing significant reductions compared with the control group. This indicates that diet plan-based interventions can help improve gastric function in patients with GUs. Furthermore, nutritional status evaluation revealed that the experimental group demonstrated better nutritional status after intervention, evidenced by more significant increases in PA and ALB levels compared with the control group. This suggests that the nutritional status of patients with GUs can be significantly improved through diet plan-based interventions. In a study by Tang and Fu[31], diet plan-based interventions applied to hemodialysis patients also significantly improved PA and ALB levels and enhanced their nutritional status, consistent with our study results.
The study has several limitations. First, as a small sample analysis, only 100 patients with GUs were included in this study, potentially impacting the generalizability of clinical results. Second, because this study was conducted at a single center, it could inevitably introduce information collection bias, which could affect the accuracy of our results. Finally, we did not analyze risk factors affecting care outcomes or adverse events in patients with GUs. Future efforts will primarily focus on expanding the sample size, diversifying sample sources, and analyzing risk factors affecting nursing effects or adverse events in patients with GUs to gradually enhance the comprehensiveness of this study.
Diet plan-based interventions for patients with GUs have many benefits. They can improve nursing efficacy and help prevent or improve symptoms such as vomiting, acid reflux, and celialgia. Additionally, these plans may correct abnormally high levels of PG I, PG II, and G-17 and deficient levels of PA and ALB. These changes can help restore patients’ gastric function and nutritional status. Diet plan-based interventions provide effective new treatment options for patients with GUs. Our results contribute to existing clinical evidence of the feasibility of these diet plans and serve as a clinical reference for the nutritional care of patients with GUs.
| 1. | Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 398] [Article Influence: 199.0] [Reference Citation Analysis (1)] |
| 2. | Kan J, Cheng J, Xu L, Hood M, Zhong D, Cheng M, Liu Y, Chen L, Du J. The combination of wheat peptides and fucoidan protects against chronic superficial gastritis and alters gut microbiota: A double-blinded, placebo-controlled study. Eur J Nutr. 2020;59:1655-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Sykes BW, Sykes KM, Hallowell GD. A comparison of two doses of omeprazole in the treatment of equine gastric ulcer syndrome: A blinded, randomised, clinical trial. Equine Vet J. 2014;46:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 315] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | Rueda-Robles A, Rubio-Tomás T, Plaza-Diaz J, Álvarez-Mercado AI. Impact of Dietary Patterns on H. pylori Infection and the Modulation of Microbiota to Counteract Its Effect. A Narrative Review. Pathogens. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Mueller L, Ciervo CA. Smoking in women. J Osteopath Med. 1998;98:s7-s10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Søgaard KK, Farkas DK, Pedersen L, Lund JL, Thomsen RW, Sørensen HT. Long-term risk of gastrointestinal cancers in persons with gastric or duodenal ulcers. Cancer Med. 2016;5:1341-1351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Ren J, Jin X, Li J, Li R, Gao Y, Zhang J, Wang X, Wang G. The global burden of peptic ulcer disease in 204 countries and territories from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Int J Epidemiol. 2022;51:1666-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Xue Z, Shi G, Fang Y, Liu X, Zhou X, Feng S, Zhao L. Protective effect of polysaccharides from Radix Hedysari on gastric ulcers induced by acetic acid in rats. Food Funct. 2019;10:3965-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Vomero ND, Colpo E. Nutritional care in peptic ulcer. Arq Bras Cir Dig. 2014;27:298-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Møller MH, Larsson HJ, Rosenstock S, Jørgensen H, Johnsen SP, Madsen AH, Adamsen S, Jensen AG, Zimmermann-Nielsen E, Thomsen RW; Danish Clinical Register of Emergency Surgery. Quality-of-care initiative in patients treated surgically for perforated peptic ulcer. Br J Surg. 2013;100:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Sin D, Harasemiw O, Curtis S, Iman Y, Buenafe J, DaCosta J, Mollard RC, Tangri N, Protudjer JLP, Mackay D. Dietary Patterns and Perceptions in Older Adults With Chronic Kidney Disease in the Canadian Frailty Observation and Interventions Trial (CanFIT): A Mixed-Methods Study. Can J Kidney Health Dis. 2022;9:20543581221140633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Liu D, Shi G, Yin C, Liu Z, Yang A. Effect of Psychological Intervention Combined with Dietary Guidance on Quality of Life and Long-Term Efficacy of Bushen Quyu Decoction in Treatment of Patients with Advanced Ovarian Cancer. Evid Based Complement Alternat Med. 2021;2021:1075513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Kamada T, Satoh K, Itoh T, Ito M, Iwamoto J, Okimoto T, Kanno T, Sugimoto M, Chiba T, Nomura S, Mieda M, Hiraishi H, Yoshino J, Takagi A, Watanabe S, Koike K. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J Gastroenterol. 2021;56:303-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 15. | Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2022;71:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 194] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 16. | Castro-Espin C, Agudo A. The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 17. | Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191-5204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (3)] |
| 18. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2439] [Article Influence: 487.8] [Reference Citation Analysis (3)] |
| 19. | Bi WP, Man HB, Man MQ. Efficacy and safety of herbal medicines in treating gastric ulcer: a review. World J Gastroenterol. 2014;20:17020-17028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 20. | Li Z, Zou D, Ma X, Chen J, Shi X, Gong Y, Man X, Gao L, Zhao Y, Wang R, Yan X, Dent J, Sung JJ, Wernersson B, Johansson S, Liu W, He J. Epidemiology of peptic ulcer disease: endoscopic results of the systematic investigation of gastrointestinal disease in China. Am J Gastroenterol. 2010;105:2570-2577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Jeong M, Park JM, Han YM, Kangwan N, Kwon SO, Kim BN, Kim WH, Hahm KB. Dietary Intervention of Artemisia and Green Tea Extracts to Rejuvenate Helicobacter pylori-Associated Chronic Atrophic Gastritis and to Prevent Tumorigenesis. Helicobacter. 2016;21:40-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Ohara R, Dario FL, Emílio-Silva MT, Assunção R, Rodrigues VP, Bueno G, Raimundo PR, da Rocha LRM, Hiruma-Lima CA. Citral Modulates MMP-2 and MMP-9 Activities on Healing of Gastric Ulcers Associated with High-Fat Diet-Induced Obesity. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Ding W, Jian M, Xu RJ. The impact of standardized perioperative care management on improving outcomes in patients with peptic ulcer disease. Medicine (Baltimore). 2023;102:e33769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Nestel PJ, Mori TA. Dietary patterns, dietary nutrients and cardiovascular disease. Rev Cardiovasc Med. 2022;23:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Pitta MR, Campos FM, Monteiro AG, Cunha AGF, Porto JD, Gomes RR. Tutorial on Diarrhea and Enteral Nutrition: A Comprehensive Step-By-Step Approach. JPEN J Parenter Enteral Nutr. 2019;43:1008-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Peng Z, Yi J, Liu X. A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Zhou B, Chen X, Huang B, Hu Y, Tang G, Zhang J, Lin Q. Changes in serum pepsinogen levels and their value as a predictor of treatment outcomes in children with peptic ulcer. J Paediatr Child Health. 2019;55:1103-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Schubert ML. Physiologic, pathophysiologic, and pharmacologic regulation of gastric acid secretion. Curr Opin Gastroenterol. 2017;33:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Schubert ML, Rehfeld JF. Gastric Peptides-Gastrin and Somatostatin. Compr Physiol. 2019;10:197-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Dong Z, Zhang X, Chen X, Zhang J. Significance of Serological Gastric Biopsy in Different Gastric Mucosal Lesions: an Observational Study. Clin Lab. 2019;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Tang L, Fu Z. The effects of the transtheoretical model combined with nutritional intervention in hemodialysis patients. Am J Transl Res. 2021;13:7898-7905. [PubMed] |