Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4372
Revised: May 13, 2024
Accepted: May 21, 2024
Published online: July 16, 2024
Processing time: 102 Days and 13 Hours
The role of diverse antibodies in mediating peripheral nerve injury in Guillain-Barré syndrome (GBS) is becoming clearer, but positivity for multiple antibodies in one case is uncommon. To our knowledge, this is the first case involving GBS with positive anti-sulfatide, anti-GT1a, and anti-GT1b antibodies.
A 20-year-old female patient was admitted to the hospital due to weakness of limbs for 5 d, and deterioration of the weakness and muscle aches for 1 d. The patient's limbs were weak, but the tendon reflexes in the part of the limbs were normal. There was no comorbid peripheral nociception or deep sensory dys
In this article, the clinical manifestations, neurophysiological examination, and auxiliary examination findings of a GBS patient positive for multiple antibodies were analyzed to improve the identification of the disease by clinical physicians at an early stage.
Core Tip: Guillain-Barré syndrome (GBS) is an acquired acute autoimmune polyneuropathy. Different positive antibodies have different clinical characteristics, and multiple positive antibodies in a case is very rare. This paper reports the clinical characteristics of a GBS case positive for multiple antibodies, to guide clinicians in early identification and diagnosis of such rare cases.
- Citation: Tan FF, Liu HX, Huang XY, Yu CY, Yang XY. Atypical Guillain-Barré syndrome with positive anti-sulfatide, anti-GT1b, and anti-GT1a antibodies: A case report. World J Clin Cases 2024; 12(20): 4372-4376
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4372.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4372
Guillain-Barré syndrome (GBS) is an acquired acute autoimmune polyradiculoneuropathy, in which complement-mediated multiple antibodies attack nerves may play an important role. It is usually caused by an immune response following an infection with Campylobacter jejuni, cytomegalovirus, Mycoplasma pneumoniae, or influenza A virus. At present, cases simultaneously positive for anti-sulfatide, anti-GT1b, and anti-GT1a antibodies in serum and cerebrospinal fluid have not been reported.
A 20-year-old female patient was admitted to the hospital with 5 d of weakness in the limbs, and worsening of the weakness and muscle soreness for 1 d.
Five days before admission, the patient developed fatigue of the extremities, difficulty in walking, and inability to grasp objects. She had no symptoms such as numbness, headache, speech disorders, coughing when drinking water, difficulty in swallowing, fever, or dysfunction of urination and defecation. The weakness of the limbs gradually worsened until she was unable to walk, and the symptoms did not improve after potassium supplementation. Muscle soreness in both lower limbs developed 1 d ago. The patient did not have symptoms such as difficulty breathing or tachypnea. One week before the onset of the illness, she had experienced an acute upper respiratory tract infection.
The patient denied any past medical history.
The patient denied any family history of genetic diseases or tumors.
At the time of admission, consciousness, language, memory, and cranial nerve examinations were normal. The proximal and distal limb strength of the patient was grade 4 and grade 2, respectively. Muscle tone and tendon reflex were normal. She had no sensitive lesions and pathologic signs were negative.
The tests of anti-cyclic citrullinated peptide antibody, immunoglobulin, rheumatoid factor, anti-nuclear antibody spectrum, anti-neutrophil cytoplasmic antibody, creatine kinase (CK), CK isoenzyme (CK-MB), lactic dehydrogenase, α-hydroxybutyrate dehydrogenase, potassium, sodium, and chlorine were normal. The outcome of arterial blood gas analysis was normal. The electrocardiogram showed sinus bradycardia, with a heart rate of 51 beats per minute. Lumbar puncture was performed 4 d after admission (9 d after onset). The intracranial pressure was 120 mmH2O. The number of cells and protein in cerebrospinal fluid were 5 × 106/L and 567 mg/L, respectively. The patient was anti-sulfatide antibody IgG+ and anti-GT1b antibody IgG+ in both cerebrospinal fluid and serum, but she was anti-GT1a antibody IgM- in cerebrospinal fluid but + in serum. Neuro-electrophysiological examination results (3 d after admission) were as follows: The initiation latency of compound muscle action potential (CMAP) following stimulation of the bilateral ulnar nerve at the wrist were prolonged, and CMAP amplitude and waveform were significantly decreased in the elbow and upper arm segment. Motor conduction velocity (MCV) in the elbow slowed down, and MCV in the upper arm slowed down slightly. The waveform of CMAP of the right ulnar nerve in the whole arm were discrete, and the initial latency of CMAP following stimulation of the left median nerve at the wrist was prolonged. The frequency of F-wave of the left ulnar nerve decreased, and the incubation period was prolonged. Electrophysiological examination showed demyeli
Magnetic resonance imaging examination of the head was normal.
The patient was diagnosed as having GBS.
Six days after admission, the patient was given intravenous immunoglobulin 0.4 mg/kg for 5 consecutive days, together with intramuscular vitamin B1 and vitamin B12.
From the second day of immunoglobulin injection, the patient's symptoms gradually improve.
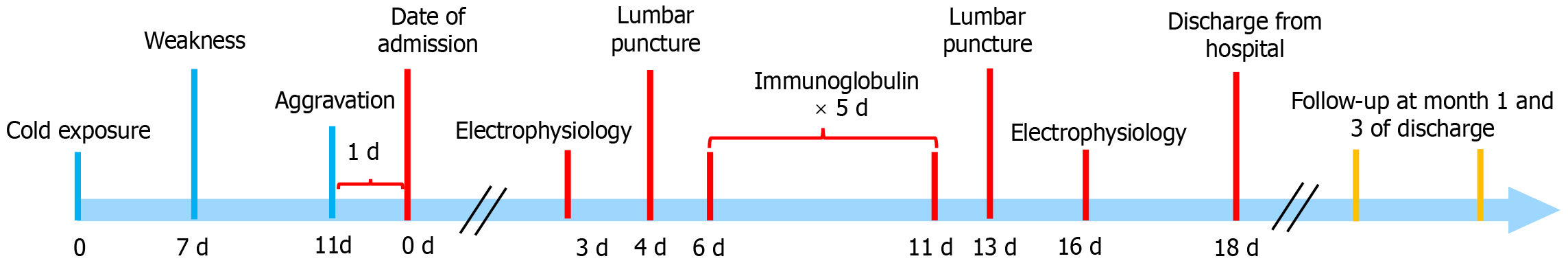
The neuro-electrophysiological examination on day 16 showed improvement in demyelination changes after 5 d of intravenous immunoglobulin. The electrocardiogram was normal, with a heart rate of 68 beats per minute. She was discharged on August 8, 2023. Physical examination showed that limb muscle strength was grade 5, and muscle tone and tendon reflex were normal yet. The patient was followed by telephone for 3 mo (Figure 1), and there was no weakness.
GBS is an acute immune-mediated disease of the peripheral nervous system. It is reported that the pathogenesis of GBS is related to many antibodies, such as ganglioside antibodies (GM, GD, GQ, and GT) containing sialic acid residues[1] and sulfatide antibodies containing sulfuric acid residues[2]. These antibodies are closely related to the clinical manifestations and subtypes of GBS and are also important biomarkers for its diagnosis[3]. In this case, the clinical manifestations, the number of cells and protein in cerebrospinal fluid, and demyelinating changes in neurophysiological examination, were consistent with typical GBS. However, it is different from typical GBS. First, the tendon reflexes were normal; second, the patient had obvious muscle pain, which is easily misdiagnosed as polymyositis; third, ECG examination showed sinus bradycardia. The heart rate returned to normal after treatment; lastly, the intravenous immunoglobulin treatment was satisfactory and resulted in a fine prognosis. In the classification and diagnostic criteria of 2014, the reduction and/or absence of tendon reflexes is no longer regarded as core clinical features, but a clinical feature supporting diagnosis[4]. About 10% of patients with GBS have normal reflex or hyperreflexia, which is consistent with our report. The pathogenesis of GBS involves diverse antibodies. Serum antibodies against gangliosides and other antigens have been found in GBS, particularly in motor GBS[5]. Anti-sulfatide antibody is a kind of acid glycolipid which widely exists in brain, spinal cord[6], and peripheral nerve tissues, and plays an important role in maintaining the structure and physiological function of nerve sheaths[7]. Thiamine-selectin interactions are thought to play a key role in inflammatory pain. This is because elevated thiamine activates selectins in the spinal cord, leading to abnormal pain[6]. This is consistent with myalgia in the case. GT1a and GT1b are subtypes of gangliosides. GT1a is highly expressed in the human glossopharyngeal and vagus nerve[8]. In this case, anti-GT1a antibody is positive in serum and cerebrospinal fluid. The electrocardiogram showed that the heart rate changed, and it returned to normal after treatment. We speculate that GBS patients may experience damage to the sympathetic and parasympathetic nerves, with more significant sympathetic nerve loss than parasympathetic nerve damage, resulting in a slower heart rate. GT1b and GD1a on the axon bind to myelin-associated glycoprotein on myelin, which help to ensure the long-term stability of axon-myelin, protect axons from toxic damage, and regulate axon regeneration[9]. This may be one of the reasons for the favorable prognosis of this patient. Multiple antibody-positive GBS has been reported in the literature, but cases positive for anti-sulfatide, anti-GT1a, and anti-GT1b antibodies have not been reported. In this case, in addition to the characteristics of GBS, the patient also exhibited myalgia with anti-sulfatide antibody positivity, autonomic nerve dysfunction, and sensitivity to immunoglobulin therapy, demonstrating a combination of three antibody-positive symptoms. Due to the rarity of multi-antibody-positive cases, it is necessary to analyze and summarize the clinical characteristics and prognosis of different antibody-positive cases in GBS, so as to improve the ability of clinicians in diagnosis and treatment of this kind of disease.
Due to the rarity of multi-antibody-positive cases, it is necessary to analyze and summarize the clinical characteristics and prognosis of different antibody-positive cases in GBS, to improve the diagnosis and treatment ability of clinical physicians for this type of disease.
We would like to express our sincere gratitude to the patient for her support.
| 1. | Wahatule R, Dutta D, Debnath M, Nagappa M, Mahadevan A, Sinha S, Sundaravadivel P, Rao U, Periyavan S, Binu VS, Rao S, Taly AB. Ganglioside complex antibodies in an Indian cohort of Guillain-Barré syndrome. Muscle Nerve. 2020;62:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Lee KP, Abdul Halim S, Sapiai NA. A Severe Pharyngeal-Sensory-Ataxic Variant of Guillain-Barré Syndrome With Transient Cardiac Dysfunction and a Positive Anti-sulfatide IgM. Cureus. 2022;14:e29261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Lleixà C, Martín-Aguilar L, Pascual-Goñi E, Franco T, Caballero M, de Luna N, Gallardo E, Suárez-Calvet X, Martínez-Martínez L, Diaz-Manera J, Rojas-García R, Cortés-Vicente E, Turón J, Casasnovas C, Homedes C, Gutiérrez-Gutiérrez G, Jimeno-Montero MC, Berciano J, Sedano-Tous MJ, García-Sobrino T, Pardo-Fernández J, Márquez-Infante C, Rojas-Marcos I, Jericó-Pascual I, Martínez-Hernández E, Morís de la Tassa G, Domínguez-González C, Juárez C, Illa I, Querol L. Autoantibody screening in Guillain-Barré syndrome. J Neuroinflammation. 2021;18:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Wakerley BR, Uncini A, Yuki N; GBS Classification Group; GBS Classification Group. Guillain-Barré and Miller Fisher syndromes--new diagnostic classification. Nat Rev Neurol. 2014;10:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 390] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 5. | van Doorn PA, Van den Bergh PYK, Hadden RDM, Avau B, Vankrunkelsven P, Attarian S, Blomkwist-Markens PH, Cornblath DR, Goedee HS, Harbo T, Jacobs BC, Kusunoki S, Lehmann HC, Lewis RA, Lunn MP, Nobile-Orazio E, Querol L, Rajabally YA, Umapathi T, Topaloglu HA, Willison HJ. European Academy of Neurology/Peripheral Nerve Society Guideline on diagnosis and treatment of Guillain-Barré syndrome. Eur J Neurol. 2023;30:3646-3674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 86] [Reference Citation Analysis (0)] |
| 6. | Morita M, Watanabe S, Nomura N, Takano-Matsuzaki K, Oyama M, Iwai T, Tanabe M. Sulfatide-selectin signaling in the spinal cord induces mechanical allodynia. J Neurochem. 2023;164:658-670. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Palavicini JP, Wang C, Chen L, Ahmar S, Higuera JD, Dupree JL, Han X. Novel molecular insights into the critical role of sulfatide in myelin maintenance/function. J Neurochem. 2016;139:40-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Kaida K, Kusunoki S. Antibodies to gangliosides and ganglioside complexes in Guillain-Barré syndrome and Fisher syndrome: mini-review. J Neuroimmunol. 2010;223:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Sturgill ER, Aoki K, Lopez PH, Colacurcio D, Vajn K, Lorenzini I, Majić S, Yang WH, Heffer M, Tiemeyer M, Marth JD, Schnaar RL. Biosynthesis of the major brain gangliosides GD1a and GT1b. Glycobiology. 2012;22:1289-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |