Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4365
Revised: May 7, 2024
Accepted: May 20, 2024
Published online: July 16, 2024
Processing time: 98 Days and 19.9 Hours
Anti-contactin-associated protein-like 2 (CASPR2) antibody encephalitis is an autoimmune disorder characterized by the presence of antibodies against the voltage-gated potassium channel. This leads to neurological symptoms, such as seizures, cognitive decline, and neuropathic pain, primarily affecting the limbic system. The prognosis of this disorder varies among individuals.
The patient, a girl aged nine years and nine months, underwent treatment for 14 to 21 d. The main clinical manifestations were vomiting and unclear conscious
Anti-CASPR2 antibody autoimmune encephalitis in children is rare, mainly manifested as convulsions, mental abnormalities, cognitive impairment, and neuropathic pain, among others. Timely evaluation for autoimmune encephalitis antibodies is crucial, especially in cases of recurrent central nervous system involvement in children.
Core Tip: Anti-contactin-associated protein-like 2 (CASPR2) antibody encephalitis is a subtype of autoimmune encephalitis targeting the CASPR2 protein in the voltage-gated potassium channels complex, mainly affecting the limbic system and related neuronal structures. While it is sensitive to immunotherapy, it carries a risk of relapse and potentially poor prognosis in patients with underlying tumors. Herein, we focus on a patient presenting with vomiting, unconsciousness, recurrent seizures, cognitive decline, and limb pain, underlining the diagnostic importance of these symptoms. This investigation accentuates that early detection and management are crucial in achieving favorable outcomes in anti-CASPR2 antibody encephalitis, requiring clinician awareness and a high index of suspicion.
- Citation: Chen HY, Wang J, Song DY, Wang B, Xu ZY, Wu Q, Wang ZL. Anti-contact protein-associated protein 2 antibody encephalitis in children: A case report. World J Clin Cases 2024; 12(20): 4365-4371
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4365.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4365
Anti-contactin-associated protein-like 2 (CASPR2) antibody encephalitis, i.e. anti-CASPR2 antibody encephalitis, is an autoimmune encephalitis (AE) with an antibody against the voltage-gated potassium channels (VGKC) protein, which is expressed in the axons, cytosol and dendritic spines of neurons, mainly in the limbic system, hippocampus, endocasts and dendritic spines[1,2]. CASPR2 is expressed in neuronal axons, cytosol and dendritic spines, mainly in the limbic system, hippocampus, internal capsule, cerebellum, spinal ganglia, and the cortex[3,4]. Anti-CSPR2 antibody encephalitis is most common in limbic encephalitis and Morvan’s syndrome, which are more common in males than in females. It is sensitive to immunotherapy, but has the potential for relapse and has a poor outcome and prognosis in a small number of patients with combined tumora[5,6]. Common clinical manifestations include limbic system symptoms, memory loss, cerebellar symptoms, seizures, cognitive impairment, peripheral neuroleptic stimulation, autonomic dysfunction, insomnia, neuropathic pain, and weight loss[7]. In this case, recurrent epileptic seizures, cognitive decline, and unre
The patient, female, 9 years old, was admitted to our hospital on November 6, 2021 for “vomiting with unconsciousness for 2 h”.
The patient was a fourth-grade student, who suddenly developed nausea and vomiting 2 h before admission, about 6 times during the lunch break at school, non-projectile, with gastric contents at the beginning of the vomit, each time ranging from about 30-100 mL, and then yellow bile-like mucus, each time in small quantities, accompanied by paroxysms of dizziness, and then appeared to be accompanied by the call of no response, unconsciousness, paroxysms of crying, restlessness, no fever and convulsions, no bruising, no cough and wheezing, no abdominal pain and diarrhea, and was not treated locally. No fever and convulsions, no cyanosis, no coughing and wheezing, no abdominal pain and diarrhea, no local treatment, urgently came to our hospital, 10% chloral hydrate 10 mL enema was given as an emergency to sedate the patient and then admitted to our department. She was incontinent once after the enema. After admission, the child still had frequent nausea and vomiting with yellow mucus, and had fever twice during the night on the day of hospitalization, with a temperature as high as 38.9 ℃, without convulsions.
The child was physically fit, and on October 31, he had received the first injection of the new coronary vaccine.
Personal history: full-term pregnancy, second child, second birth, vaginal delivery, new method of delivery, postnatal denial of history of ventricular respiration, breastfeeding, complementary feeding at 6 months, weaning at 1 year and 6 months. Physical and mental development was normal for her age group, and she was up to date with her vaccination schedule, having received the first dose of the new coronary vaccine on 31 October. Family history: healthy parents, no consanguineous marriages, no history of sexually transmitted diseases. Family history denies hereditary or infectious paralysis.
Body temperature of 36.8 ℃, restlessness, paroxysmal crying, not call, the whole body skin and mucous membranes did not see a rash on the hemorrhagic spots, pupils about 2 mm in diameter, equal round, light reflex exists, pharynx is not congested, the neck is not resisted, the lungs respiratory sounds rough, did not hear dry and wet rhonchi, heart sounds strong, abdominal soft, no pressure pain, bowel sounds are normal, bilateral Babinski’s sign negative, bilateral Kerning’s sign negative, Brilliant’s sign is negative, the heart is strong. Kerning’s sign was negative, Brudzinski’s sign was negative. Capillary refill time was < 2 s. The child had a modified Glasgow Coma Score of 7 (best verbal response 1, eyes open 2, best motor response 4). Neck resistance was present on the night of admission.
Blood routine: absolute neutrophil value was 11.97 × 109/L; C-reactive protein was 2.1 mg/L; antinuclear antibody was negative. Ultrasound of the thyroid gland and lymph nodes in the neck: the size and shape of the thyroid gland were normal, the gland was moderately echogenic, and there was no obvious cystic or space-occupying nodule, and CDFI showed no abnormality in the distribution of blood flow in the gland. CDFI: blood flow distribution of the gland was not abnormal. There were no obvious enlarged lymph nodes in the neck bilaterally. Suggestion: no obvious abnormality of the thyroid gland (TI-RADS class I).
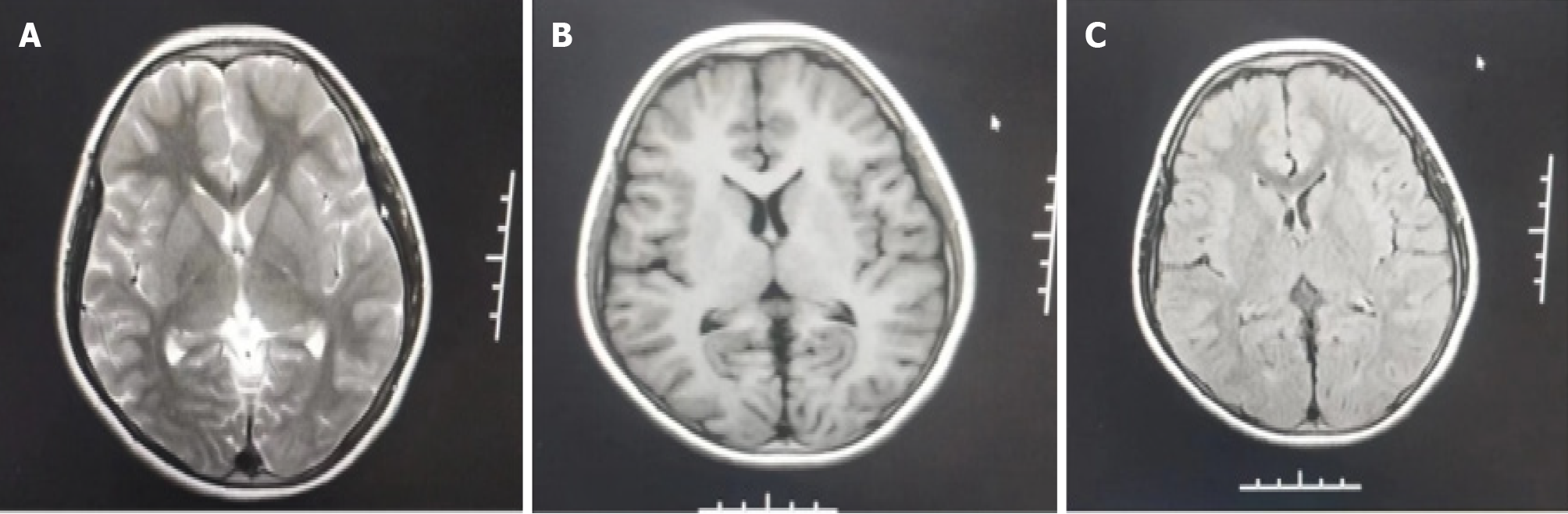
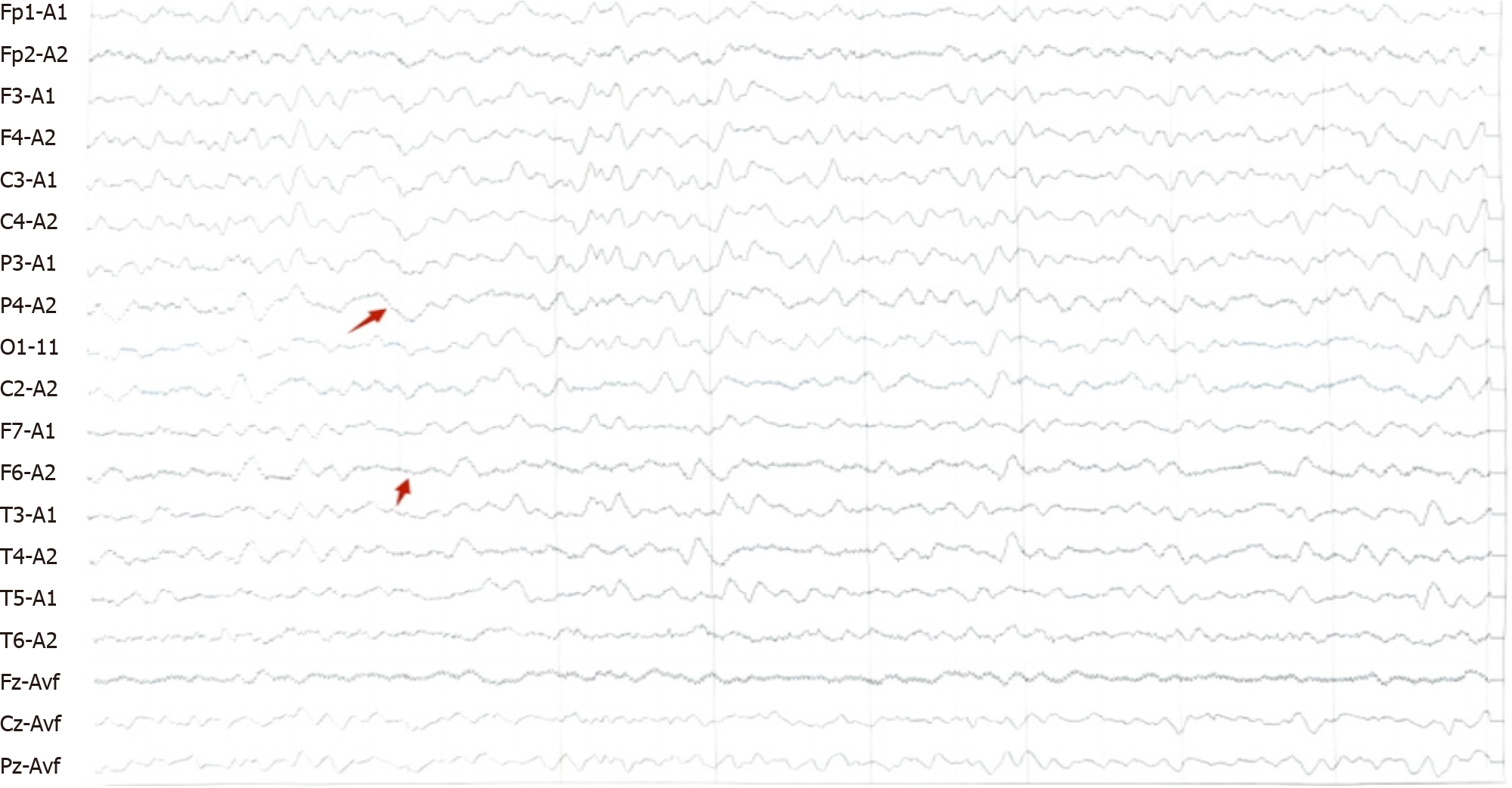
On November 8, 2021, cranial NMR + cervical spine NMR + MRA (Figure 1): symmetry of intracerebral structures, clear gray-white matter structures, no abnormal signals (Figure 1B). There was no enlargement of the ventricle brain pool, no widening of the sulcus, and no displacement of the midline structures. The bilateral auditory nerves were well displayed, with no occupancy; no abnormal signals were seen in the bilateral mastoid airspaces (Figure 1C). T2WI high signal was seen in the left maxillary sinus (Figure 1A). Bilateral internal carotid arteries, middle cerebral arteries, and anterior cerebral arteries were well displayed, with no stenosis or limited dilatation. Bilateral posterior communicating arteries were open, basilar arteries and bilateral posterior cerebral arteries were well displayed without stenosis or limited dilatation. There was no compression and displacement of the intracranial vessels seen. The sequence of cervical vertebrae was neat, with no hyperplasia at the edge of the vertebral body, no compression and flattening of the vertebral body, and no abnormal signal in the bone. The intervertebral discs were well displayed, with no herniation and no compression of the dural sac. No abnormal signal was seen in the cervical cord. Hints: no abnormalities were seen in the cranial magnetic resonance imaging (MRI) scan; no abnormalities were seen in the cranial MRA; no abnormalities were seen in the cervical MR scan. Abnormal electroencephalogram (EEG): background rhythm slightly generalized; There were more sharp waves and sharp slow waves in the left frontotemporal region during sleep (Figure 2).
Autoimmune encephalitis (CASPR2 encephalitis).
Administered static 20% mannitol 5 mL/kg q6h to reduce cerebral edema, acyclovir (10 mg/kg/times q12h) antiviral, temporary static paramivir antiviral, static meropenem (1.0 g q8h, together with 34 mg/kg/times q8h) anti-infective, given a total of human immunoglobulin 2 g/kg divided into 5 d, static methylprednisolone 20 mg/kg. The total amount of human immunoglobulin was 2 g/kg for 5 d, and methylprednisolone 20 mg/kg/d was administered as a shock treatment for 2 times × 3 d, and then the amount was reduced to 10 mg/kg/d once a day × 3 d, and to 2.5 mg/kg/d once a day × 3 d. Intravenous polyenphosphatidylcholine injection to protect hepatocytes.
After the first A-strength shock treatment (28 h after admission, coma for about 30 h) combined with gammaglobulin treatment, the child gradually turned from coma to consciousness (with cognitive impairment in the early stage after waking up), and the cognitive impairment gradually disappeared as the treatment progressed. At present, the child is conscious, with no cognitive impairment, no fever or vomiting, no headache or dizziness, no convulsions, no motor impairment, no amnesia, and normal speech and communication, and the neurological examination shows no abnor
Occasional hair sinking, dizziness, wanting to vomit (similar to the initial symptoms of the first onset), can be relieved on its own by resting for half a day or 1 d, frequency: about 1 time in 2-3 months, no triggers. 2022-7-6 cranial magnetic resonance plain scanning: localized mucosal thickening of the left sieve sinus, the rest of the abnormalities were not seen. 2023-2-15 cranial magnetic resonance plain scanning + MRA + MRV: a few foci of subfrontal cortical degeneration on the bilateral frontal lobes; Inflammation of the left sieve sinus and maxillary sinus; adenoid hypertrophy; multiple moderate stenosis of the A3 segment of the right anterior cerebral artery; no significant abnormality on cranial MRV.
Anti-CASPR2 encephalitis is a subtype of AE in which the target antigen is located on the cell surface and the antibody causes humoral immunity resulting in relatively reversible neurological deficits. VGKC is a membrane protein complex of which CASPR2 is only one component, and which also includes contactin-2 and leucine-rich glioma inactivating protein 1. The main subtype of anti-CASPR2 immunoglobulin is IgG4, and patients with anti-CASPR2 encephalitis are predominantly male, and the specific mechanism of this gender bias is not yet fully understood[8,9].
Autoimmune encephalitis with anti-CSPR2 antibodies often has an acute, subacute, or chronic onset, with a course of days to years, and can be distinguished from antibody-associated encephalitis, which has a subacute onset. The clinical picture of the disease is diverse, with fewer cases reported in children, and is characterized by a combination of seven core symptoms, including: limbic system symptoms, cerebellar ataxia, increased peripheral nerve excitability[10] (muscle twitching, spasticity, fasciculations), autonomic dysfunction[11], insomnia, neuropathic pain, and weight loss[12]. van Sonderen et al[13] reported that the most common symptom in the course of CASPR2 antibody-associated encephalitis was neuropathic pain, and the mechanism of the pain in both lower limbs of the child in the present study may be that the CASPR2 antibody inhibits the function of potassium channels and acts on sensory neurons, causing peripheral nerve hypersensitivity. In addition, the acute onset of the disease in this case, with vomiting and symptoms of antireflux infection, was in line with previous reports[14,15] that anti-CASPR2 antibody-associated encephalitis could be secondary to infection; the first symptom in this case was limbic symptoms, and the vomiting was accompanied by cognitive disorders, and poor mental health and increased sleep in two cases, which were accompanied by chills, easy to startle, headache, dull eyes, paroxysmal orbital tilting, and other symptoms. In addition to symptoms such as chills, headache, headache, dull eyes, and paroxysmal crooked mouth, there were also clinical symptoms such as pain in both lower limbs, weakness of the lower limbs, unsteady walking, optic neuritis, diarrhea, and abdominal distension, which were consistent with the clinical symptoms reported above. Therefore, the possibility of this disease should be considered when children present with first symptoms such as convulsions and cognitive deficits, accompanied by sleep abnor
Most of the laboratory tests about the disease such as routine blood, biochemistry, and pathogenetics were normal, and only 1/4 antibody-positive patients could have cerebrospinal fluid abnormalities, but the antibodies in the blood were more sensitive, probably related to the fact that the antibodies were mostly produced in the periphery . The cerebrospinal fluid of this child was normal in terms of routine cell count and average chlorinated water. Anti-CASPR2 antibodies were positive in both cerebrospinal fluid and serum[16], consistent with the report of Boyko et al[17]. The EEG may not be abnormal, and epileptic waves may be observed in some cases[18,19]. The EEG abnormality of this child mainly mani
For the treatment of the disease[21], mainly divided into: (1) First-line treatment for hormone therapy, gammaglobulin therapy, second-line treatment for cyclophosphamide and other drugs, but the specific diagnosis and treatment, should also be adjusted according to the patient’s clinical performance and the effect of drugs; and (2) symptomatic supportive treatment. Some patients with severe clinical manifestations, in addition to the treatment of the cause, should also be based on the clinical manifestations of the child, timely and early symptomatic treatment, such as accompanied by epileptic seizures, the patient should be timely application of antiepileptic drugs; mental disorders of patients with serious, timely application of psychotropic drugs, accompanied by cardiac arrhythmia patients, usually serious, should be closely monitored for changes in the condition of the disease, timely application of relevant drug therapy, if the discovery of tumors, can be symptomatic treatment according to the type of tumor, and the treatment can be adjusted according to the type of tumor. If tumor is found, symptomatic treatment can be carried out according to the tumor type, and if surgical treatment is possible, it should be carried out as early as possible , and if the tumor is not combined with the tumor, it can be cured by symptomatic treatment . In clinical work, for patients with severe conditions, close supervision and monitoring of vital signs should be carried out to prevent secondary damage to other organs. In this case, the children received immunomodulatory therapy, mainly intravenous gammaglobulin and glucocorticoid shock therapy. Follow-up was 15 months and there was no recurrence.
In summary, a subset of patients with anti-CSPR2 antibody encephalitis present with singular clinical symptoms, no significant abnormalities in laboratory tests, and no typical findings on imaging studies. If conventional treatments are ineffective and relapses occur, the possibility of this disease should be considered. It is crucial to promptly improve the assessment for antibodies associated with autoimmune encephalitis to facilitate early diagnosis and treatment. Furthermore, given the prolonged disease course in diagnosed children, long-term follow-up is necessary. If there are changes in the condition or relapses, timely treatment is essential to improve prognosis and reduce the incidence of sequelae such as epilepsy, cognitive impairments, and psychiatric and behavioral abnormalities.
| 1. | Joubert B, Gobert F, Thomas L, Saint-Martin M, Desestret V, Convers P, Rogemond V, Picard G, Ducray F, Psimaras D, Antoine JC, Delattre JY, Honnorat J. Autoimmune episodic ataxia in patients with anti-CASPR2 antibody-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Bien CG, Mirzadjanova Z, Baumgartner C, Onugoren MD, Grunwald T, Holtkamp M, Isenmann S, Kermer P, Melzer N, Naumann M, Riepe M, Schäbitz WR, von Oertzen TJ, von Podewils F, Rauschka H, May TW. Anti-contactin-associated protein-2 encephalitis: relevance of antibody titres, presentation and outcome. Eur J Neurol. 2017;24:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Patterson KR, Dalmau J, Lancaster E. Mechanisms of Caspr2 antibodies in autoimmune encephalitis and neuromyotonia. Ann Neurol. 2018;83:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Giannoccaro MP, Crisp SJ, Vincent A. Antibody-mediated central nervous system diseases. Brain Neurosci Adv. 2018;2:2398212818817497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Lang B, Makuch M, Moloney T, Dettmann I, Mindorf S, Probst C, Stoecker W, Buckley C, Newton CR, Leite MI, Maddison P, Komorowski L, Adcock J, Vincent A, Waters P, Irani SR. Intracellular and non-neuronal targets of voltage-gated potassium channel complex antibodies. J Neurol Neurosurg Psychiatry. 2017;88:353-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol. 2014;9:S143-S147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Qin X, Yang H, Zhu F, Wang Q, Shan W. Clinical Character of CASPR2 Autoimmune Encephalitis: A Multiple Center Retrospective Study. Front Immunol. 2021;12:652864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Lancaster E, Huijbers MG, Bar V, Boronat A, Wong A, Martinez-Hernandez E, Wilson C, Jacobs D, Lai M, Walker RW, Graus F, Bataller L, Illa I, Markx S, Strauss KA, Peles E, Scherer SS, Dalmau J. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol. 2011;69:303-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 9. | Saint-Martin M, Pieters A, Déchelotte B, Malleval C, Pinatel D, Pascual O, Karagogeos D, Honnorat J, Pellier-Monnin V, Noraz N. Impact of anti-CASPR2 autoantibodies from patients with autoimmune encephalitis on CASPR2/TAG-1 interaction and Kv1 expression. J Autoimmun. 2019;103:102284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Teixeira NB, Picolo G, Giardini AC, Boumezbeur F, Pottier G, Kuhnast B, Servent D, Benoit E. Alterations of peripheral nerve excitability in an experimental autoimmune encephalomyelitis mouse model for multiple sclerosis. J Neuroinflammation. 2020;17:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Palma JA, Kaufmann H. Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov Disord. 2018;33:372-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 12. | van Sonderen A, Schreurs MW, Wirtz PW, Sillevis Smitt PA, Titulaer MJ. From VGKC to LGI1 and Caspr2 encephalitis: The evolution of a disease entity over time. Autoimmun Rev. 2016;15:970-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | van Sonderen A, Ariño H, Petit-Pedrol M, Leypoldt F, Körtvélyessy P, Wandinger KP, Lancaster E, Wirtz PW, Schreurs MW, Sillevis Smitt PA, Graus F, Dalmau J, Titulaer MJ. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 2016;87:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 14. | Yadlapati R, Hungness ES, Pandolfino JE. Complications of Antireflux Surgery. Am J Gastroenterol. 2018;113:1137-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Surana S, Kumar R, Pitt M, Hafner P, Mclellan A, Davidson J, Prabakhar P, Vincent A, Hacohen Y, Wright S. Acquired neuromyotonia in children with CASPR2 and LGI1 antibodies. Dev Med Child Neurol. 2019;61:1344-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Seery N, Butzkueven H, O'Brien TJ, Monif M. Contemporary advances in antibody-mediated encephalitis: anti-LGI1 and anti-Caspr2 antibody (Ab)-mediated encephalitides. Autoimmun Rev. 2022;21:103074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 17. | Boyko M, Au KLK, Casault C, de Robles P, Pfeffer G. Systematic review of the clinical spectrum of CASPR2 antibody syndrome. J Neurol. 2020;267:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Braczkowski M, Soszyński D, Sierakowska A, Braczkowski R, Kufel K, Łabuz-Roszak B. Autoimmune Encephalitis with Antibodies: Anti-NMDAR, Anti-AMPAR, Anti-GQ1b, Anti-DPPX, Anti-CASPR2, Anti-LGI1, Anti-RI, Anti-Yo, Anti-Hu, Anti-CV2 and Anti-GABAAR, in the Course of Psychoses, Neoplastic Diseases, and Paraneoplastic Syndromes. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Freund B, Ritzl EK. A review of EEG in anti-NMDA receptor encephalitis. J Neuroimmunol. 2019;332:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Kim H, Lim YM, Lee EJ, Kim HW, Ahn HS, Kim KK. Anti-CASPR2-Antibody-Positive Isaacs' Syndrome Presenting with Myokymia, Neuropathic Pain, and Hyperhidrosis. J Clin Neurol. 2020;16:699-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Wang DZ, Li BH, Deng BL, Guo FQ, Hu SS, Yu NW, Liu J. Anti-CASPR2 encephalitis in a liver posttransplant patient receiving immune-suppression and lenvatinib: a case report and literature review. Neurol Sci. 2023;44:1069-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |