Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4348
Revised: May 18, 2024
Accepted: June 4, 2024
Published online: July 16, 2024
Processing time: 115 Days and 21.3 Hours
Pituitary stalk interruption syndrome (PSIS) is a rare anatomical defect of the pituitary gland falling under the spectrum of holoprosencephaly phenotypes. It is characterized by a deficiency in anterior pituitary hormones, such as growth hormone, gonadotropins, and thyroid hormones. Due to the syndrome's rarity and nonspecific manifestations, there is a lack of standardized treatment strate
A 30-year-old man presented with absent secondary sexual characteristics and azoospermia. Laboratory evaluation revealed a deficiency in gonadotropins, while thyroid function was mostly within normal ranges. Magnetic resonance imaging of the pituitary gland showed pituitary stalk agenesis, hypoplasia of the anterior pituitary, and ectopic posterior pituitary, leading to the diagnosis of PSIS. Initially, the patient underwent 6 mo of gonadotropin therapy without significant changes in hormone levels and secondary sexual characteristics. Pulsatile gonadotropin-releasing hormone therapy was then administered, resulting in the detection of sperm in the semen analysis within 3 mo. After 6 mo, routine semen tests showed normal semen quality. The couple faced challenges in conceiving due to abstinence and underwent three cycles of artificial insemination, which was unsuccessful. They also attempted in vitro fertilization, but unfortunately, the woman experienced a miscarriage 10 wk after the embryo transfer.
Early detection, accurate diagnosis, and timely treatment are crucial in improving the quality of life and fertility of PSIS patients.
Core Tip: Pituitary stalk interruption syndrome (PSIS) is a rare anatomical defect affecting the pituitary gland, falling within the spectrum of holoprosencephaly phenotypes. In cases where fertility is a concern, treatment options typically involve pulsatile gonadotropin-releasing hormone (GnRH) therapy and gonadotropin therapy. This case study highlights a patient with PSIS who initially did not respond to gonadotropin therapy but showed successful spermatogenesis upon switching to pulsatile GnRH therapy. Timely identification, accurate diagnosis, and tailored treatment play a vital role in enhancing the quality of life and fertility outcomes for individuals with PSIS.
- Citation: Xie JL, Zhu HY, Dong Y, Sun PP, Qi DD, Luan SX, Zhang Y, Ma HG. Pulsatile gonadotropin-releasing hormone therapy induces spermatogenesis in pituitary stalk interruption syndrome: A case report and review of the literature. World J Clin Cases 2024; 12(20): 4348-4356
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4348.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4348
Pituitary stalk interruption syndrome (PSIS) is a congenital anatomical defect of the pituitary, clinically characterized by various symptoms related to pituitary hormone deficiency. Radiological findings from pituitary magnetic resonance imaging (MRI) often show a thin or absent pituitary stalk, aplasia, or hypoplasia of the anterior pituitary gland, or an ectopic posterior lobe, forming a typical triad. Through a literature review, we explore the etiology, genetic characteristics, clinical manifestations, diagnosis, and management of PSIS based on a single patient case. Our goal is to raise awareness of PSIS within the medical community, particularly among endocrinologists, pediatricians, and reproductive physicians. By doing so, we aim to reduce misdiagnosis, promote early detection, provide appropriate treatments like hormone replacement therapy, minimize adverse effects, improve patient prognosis, enhance quality of life, and advance reproductive health outcomes for individuals affected by PSIS.
The present case involves a 30-year-old male patient who presented with absent secondary sexual characteristics and azoospermia. He visited our hospital at the age of 30 and complained of azoospermia and infertility.
This patient presented with absent secondary sexual characteristics and azoospermia. Up to June 16, 2022, the patient was 168 cm tall and weighed 70 kg, characterized by a broad face, short neck, low hairline, sparse hair, and slightly high-pitched voice. There were no reported olfactory disorders, and the patient denied any history of cleft lip and palate surgery, cryptorchidism surgery, or heart disease.
The patient had a history of delayed growth and shorter stature compared to his peers since childhood. At the age of 17, the patient sought medical care due to his height measuring 150 cm. The results of the pituitary hormone tests indicated decreased levels of thyroid hormone, growth hormone, and adrenal cortex function. The bone age assessment revealed a 2-year delay, with a predicted height of 178 cm. Subsequently, the patient underwent growth hormone, thyroid hormone, prednisone, and testosterone replacement therapy, which resulted in a significant increase in height. Within 2 years, the patient's height reached 165 cm. However, the patient did not consistently adhere to the prescribed medication thereafter. At the age of 27, the patient was diagnosed with PSIS at a local hospital. He underwent gonadotropin therapy (HCG 2000U-4000U twice a week, HMG 75U twice a week) for nearly 3 years. However, hormonal tests during this period revealed an inadequate hormonal response.
The patient's parents are non-consanguineous, and the patient was born with a height of 50 cm and weight of 3650 g. He was delivered vaginally in a breech position with no history of postpartum asphyxia or rescue. The patient experienced delayed growth and development and was notably shorter than his peers. His intellectual development was within normal range, achieving a specialized college education.
Upon physical examination, inadequate secondary sexual characteristics were observed, with Tanner staging at stage 2, P2G2. At this point, the patient had a stretched penile length of 6 cm and increased pubic hair. Testicular volume was measured at 5 mL using the Prader orchidometer.
To evaluate the function of the pituitary gonadal axis and pituitary thyroid axis, the thyroid-stimulating hormone (TSH), T3, T4, free T4 (FT4), free T3, follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T), prolactin (PRL), estradiol, and growth hormone were measured.
The level of FT4 was slightly below the normal range, and the TSH level was within the normal range, and other parameters were normal. There is no requirement for additional supplementation of thyroid hormones. The specific results are shown in Table 1.
| Parameter | Result | Range |
| FT3 | 4.45 | 3.5-6.5 pmol/L |
| FT4 | 8.07 | 11.5-22.7 pmol/L |
| TSH | 3.02 | 0.55-4.78 uIU/mL |
| A-TG | 26.6 | 0-60 IU/mL |
| A-TPO | 40.6 | 0-60 IU/mL |
The levels of FSH, LH, and T were significantly lower than the normal range, while the estradiol and PRL were within the normal range (Table 2). Following a prolonged gonadotropin-releasing hormone (GnRH) stimulation test, hormone levels were assessed one week post pulsatile GnRH treatment. These measurements were taken in the morning at around 7:30 AM on an empty stomach upon waking. Results showed that the FSH level was 2.3 IU/L and LH level was 1.2IU/L.
| Parameter | Result | Range |
| FSH | 0.31 | 1.4-18.1 IU/L |
| LH | 0.15 | 1.5-9.3 IU/L |
| T | 0.24 | 4.27-28.24 nmol/L |
| E2 | 18.35 | 57-232 pmol/L |
| PRL | 231.13 | 45-375 uIU/mL |
The chromosomal karyotype revealed a standard 46, XY pattern. The investigation into Y chromosome microdeletion showed no deletions within the AZF regions. Utilizing the IDT xGen Exome Research Panel V1.0, we specifically targeted known pathogenic genes linked to PSIS and HH in this patient. However, no pathogenic/likely pathogenic/clinically significant variants were identified that could fully or partially explain the patient's clinical phenotype.
Negative outcomes could be attributed to constraints inherent within genetic testing modalities, the evolving landscape of genetic disorder exploration, or the intricate nature of the disease in question. Within this framework, genetic determinants may not serve as the principal etiological agents, given the absence of identified gene mutations associated with PSIS (Table 3).
| Gene | Chromosomal position (GRCh37/hg19) | Nucleotide change | Amino acid change | Transcript | Exon/intron | gnomAD-EAS gene frequency | Disease and inheritance | ACMG variation rating |
| NA | NA | NA | NA | NA | NA | NA | NA | NA |
Other examinations such as liver and kidney function and electrolytes did not show any significant abnormalities.
Pituitary MRI was used to assess pituitary and hypothalamic function and to rule out any other brain lesions. PSIS is characterized by a triad of thin, interrupted, or absent pituitary stalk, hypoplastic or aplastic anterior pituitary, and an absent or ectopic posterior pituitary observed on pituitary MRI. A computed tomography scan of the adrenal glands showed no abnormalities.
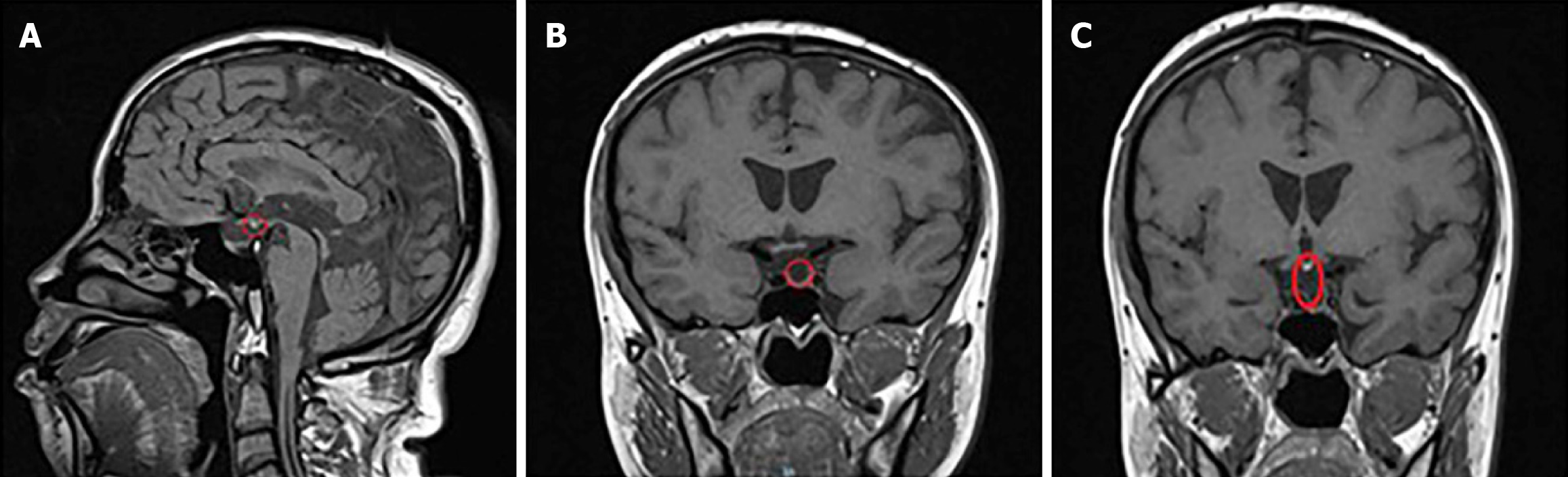
The sagittal and coronal T1-weighted images (spin-echo sequence, 2 mm thickness) revealed the absence of hyperintensity in the pituitary saddle region. The posterior pituitary lobe was ectopic, situated in the infundibular recess of the third ventricle, appearing hyperintense (highlighted by the red circle in Figure 1A). The pituitary structure appeared flattened, with the anterior lobe being indistinct and showing minimal flocculent signal (normal value 8 mm) (highlighted by the red circle in Figure 1B). The pituitary stalk was interrupted and not visible (highlighted by the red circle in Figure 1C). The coronal T2-weighted images (fast spin-echo sequence, 2 mm thickness) demonstrated normal brain gyri morphology and a structurally intact, normal cavernous sinus area. These MRI findings of an ectopic posterior pituitary lobe, underdeveloped anterior lobe, and an interrupted, non-visible pituitary stalk are consistent with the characteristic triad of findings associated with PSIS.
The results of the ultrasound of the kidneys, urinary tract, and bladder indicated no abnormalities within the genitou
Semen volume, sperm concentration, progressive motility, and total sperm number were assessed according to World Health Organization 5th guidelines, with the results presented in Table 4.
| Time | Semen parameters | |||
| Semen volume (mL) | Sperm concentration (106 per mL) | Progressive motility (PR, %) | Total sperm number (106 per ejacculate) | |
| After 3 months of pulsatile GnRH therapy | 1.5 | 10.17 | 50.35 | 15.26 |
| After 6 months of pulsatile GnRH therapy | 2 | 51.66 | 47.56 | 103.32 |
| After 12 months of pulsatile GnRH therapy | 1.3 | 12.22 | 61.35 | 15.89 |
| After 18 months of pulsatile GnRH therapy | 1.5 | 54.02 | 50.44 | 81.03 |
| After 24 months of pulsatile GnRH therapy | 1.6 | 59.3 | 25.9 | 94.88 |
Primary male infertility: Non-obstructive azoospermia; hypogonadotropic hypogonadism; pituitary stalk interruption syndrome.
The patient's treatment journey has been arduous, but significant progress has been made due to the dedicated efforts of the clinical team. Initially, dual gonadotropin stimulation therapy (HCG 4000 U twice a week, HMG 150 U twice a week) for 6 mo did not result in improvements, prompting a transition to pulsatile GnRH therapy. Pulsatile gonadorelin (Fengyuan Pharmaceutical Co.) was administered at a dose of 10-15 μg per 90 min using a portable infusion pump (Weichuang Medical Science Co.).
Notably, within 3 mo of starting this therapy, sperm was detected in the patient's semen samples. Sperm concentration and motility were assessed following the 5th edition of the World Health Organization guidelines, showing normal semen quality at 6 and 12 mo. Despite achieving normal semen quality, the couple faced challenges conceiving due to personal reasons leading to abstinence from sexual intercourse. To address this, three cycles of artificial insemination treatment was administered without resulting in pregnancy. A thorough physical evaluation revealed a body mass index of 29.14 kg/m2 and an anti-Mullerian hormone concentration of 5.69 ng/mL in the female partner. Subsequently, the couple decided to undergo in vitro fertilization assistance. Ovarian stimulation was carried out using the luteal phase short-acting GnRH agonist long protocol, resulting in the retrieval of 16 oocytes, 13 of which reached the mature metaphase II stage. This process led to the development of two cleavage-stage embryos and six blastocysts. Conception was achieved after the transfer of a blastocyst, but unfortunately, the female partner experienced a miscarriage at the tenth week of gestation. Currently, the couple is undergoing treatments involving frozen-thawed embryo transplantation. It is crucial for them to remain resilient and hopeful during these challenges. The clinical team is likely to continue exploring effective treatment options to assist the couple in achieving their goal of parenthood.
Post-marketing database analyses by Deal et al[1] and Maghnie et al[2], involving over 8000 and 13000 patients, respectively, found a PSIS incidence of 4%-8% in these populations. PSIS cases are mostly sporadic and occasionally familial. PSIS was first described in 1987, characterized by an ectopic posterior lobe and interrupted pituitary stalk[3]. The condition has an incidence rate of approximately 0.5/100000[4], with males showing higher susceptibility than females[5,6]. Studies have shown a male to female ratio of 2.5:1 and even 8.5:1 in some cases among patients with PSIS[6,7]. The gender difference in PSIS incidence may be influenced by geographic factors or gender-specific disease determinants.
The etiology of PSIS may be attributed to perinatal abnormalities, such as breech delivery and postnatal asphyxia. A prospective cross-sectional study indicated a high prevalence of adverse perinatal events in children with PSIS, including breech birth, neonatal distress, cryptorchidism, midline defects, and nystagmus[7]. While initial research focused on perinatal factors, recent studies have highlighted the significance of genetic factors in the development of pituitary abnormalities[8]. Early gene mutations within the hypothalamic-pituitary development pathway, involving genes like Wnt, Notch, and Sonic hedgehog, are now considered crucial[9,10]. Various inheritance patterns, including autosomal dominant or recessive, X-linked, and potentially digenic[11] or polygenic[12], have been proposed for these genes.
Genes related to signal pathway abnormalities include mutations in genes involved in anterior pituitary development, such as PIT1, PROP1[13], LHX3/LHX4[14], PROKR2[11], OTX2, TGIF, HESX1[15,16], ROBO1[17], and GPR161[18]. CORREA-SILVA et al[19] assessed copy number variation (CNV) in 21 non-syndromic PSIS patients using array-based comparative genomic hybridization technology. They identified 35 distinct rare CNVs in 18 patients, with chromosomes X, 18, 20, and 1 being most commonly affected. This suggests that CNVs may be a potential mechanism for genetic anomalies in PSIS individuals. Additionally, Fang et al[20] conducted whole-exome sequencing on 59 sporadic PSIS patients and found heterozygous mutations in PTCH1 and PTCH2, inhibitors of Hedgehog signaling, in 22% of the cohort. PSIS is a polygenic condition with significant phenotypic variability due to gene-environment interactions[21]. Despite this, genetic factors have only been identified in 5% of PSIS cases[22]. Overall, current evidence points to genetic origins involving mutations in early developmental genes with complex inheritance patterns and environmental influences, placing PSIS at the crossroads between Mendelian and multifactorial conditions[21].
Newborns with PSIS commonly exhibit symptoms like respiratory distress, jaundice, hypoglycemia[23], cholestasis[24], micropenis, and cryptorchidism[7]. Studies have reported stunted growth in a significant proportion of PSIS patients, with the median age of diagnosis typically around four years[25]. Multiple pituitary hormone deficiencies are also frequently observed in these patients[26]. Specific research on Chinese PSIS patients highlighted a high male proportion, a history of breech delivery, and multiple pituitary hormone deficiencies in the majority of cases[27]. Additional clinical features may include overweight and obesity[28]. Variations in clinical presentation, including differences in sex distribution and age at diagnosis, have been noted among various ethnic groups. Patients with PSIS display a wide range of physical characteristics. Some may experience seizures, intellectual disability, micropenis, or cryptorchidism. These manifestations should not be viewed as a separate clinical entity, but rather as part of the range of physical traits seen in established genetic syndromes[22].
PSIS is a congenital anatomical defect characterized by a typical triad: A thin or interrupted pituitary stalk, absence or ectopic posterior lobe, and hypoplasia or regenerative disturbances of the anterior lobe. MRI typically shows absence of the pituitary stalk, anterior pituitary hypoplasia (98.3%), and ectopic posterior pituitary (91.2%). Neurohypophyseal ectopy is often found in the infundibular remnant (60.4%)[29]. Enhanced MRI diagnostics using gadopentetate dimeglumine (Gd-DTPA) have proven to be more sensitive in identifying the vascular components of the pituitary stalk, offering new insights into the partial preservation of hypothalamo-hypophyseal portal circulation[30]. Guo et al[31] conducted a study comparing the metabolomics and lipidomics of seminal plasma in 21 PSIS patients and 23 healthy controls, identifying altered metabolites such as triacylglycerols, phosphatidylethanolamines, sphingomyelins, ceramides, and phosphatidylcholines in PSIS patients. Through bioinformatics assessments and receiver operating characteristic curve modeling, they identified pregnenolone and L-saccharopine as potential biomarkers for PSIS, emphasizing the need for further validation in future research. Clinical manifestations in our patient include poor development of secondary sexual characteristics, particularly sparse facial and pubic hair, a small penis, and small testes. Hormonal assays revealed low levels of follicle-stimulating hormone, luteinizing hormone, and testosterone. MRI findings align with the classical PSIS triad, showing an ectopic posterior pituitary, hypoplastic anterior pituitary, and an absent pituitary stalk.
Previous studies inferred that pulsatile GnRH therapy proved more effective for patients with hypogonadotropic hypogonadism[32], with mixed results in PSIS cases. Recent scholarly contributions suggest that post-pulsatile GnRH therapy in PSIS patients led to normalized serum LH and FSH levels, indicating the persisting functionality of anterior pituitary gonadotrophs. Pulsatile GnRH therapy motifs can potentially 'awaken' these gonadotrophs and retrieve hypothalamus-pituitary-gonad axis function[33,34]. A retrospective study involving patients with PSIS suggested that pulsatile GnRH therapy was superior to gonadotropin therapy in inducing spermatogenesis[35].
Promising prognostic indicators include a responsive increase in hormonal levels and a gradual increase in testicular volume during therapy. A study by a Chinese scholar found that a serum LH level of ≥ 2 IU/L within a month of starting pulsatile GnRH therapy is a valuable predictive marker for stimulating spermatogenesis[36]. Additionally, baseline testicular volume [hazard ratio (HR): 1.13, 95% confidence interval (CI): 1.01-1.27] and LH peak (HR: 1.11, 95%CI: 1.0-1.23) were identified as early predictors of spermatogenesis in PSIS patients undergoing pulsatile GnRH therapy[34]. A retrospective study established an LH level of 1 IU/L as the cutoff value after 1 mo of pulsatile GnRH therapy, associated with a favorable clinical outcome[35]. This indicates a positive prognosis for the patient, with improvements in sexual function noted, such as increased frequency of morning erections and enhanced libido. After 6 mo of therapy, all parameters in the patient's semen analysis normalized, and no adverse effects like skin allergies or acne were observed during treatment. Andrologists are advised to consider recommending fertility preservation in human sperm banks once sperm are detected. Given the significant health implications of multi-pituitary hormonal deficiencies, it is crucial to ensure long-term follow-ups and regular monitoring of hormonal levels for these patients[37].
PSIS is a rare congenital abnormality of the pituitary gland leading to anterior pituitary deficiency. This condition is characterized by both clinical and genetic heterogeneity. PSIS is an uncommon disorder presenting with complex clinical features and variability. It is considered a prenatal developmental defect related to holoprosencephaly phenotypes and can be identified through MRI. The exact causes of PSIS are currently unknown, but they may be linked to perinatal adverse events and genetic mutations resulting in hereditary abnormalities. The disease onset can vary, making it challenging to detect abnormalities in childhood. After puberty, individuals with PSIS may experience delayed growth, insufficient development of secondary sexual characteristics, osteoporosis, and significant impacts on physical and mental health, as well as reproductive issues like fertility in adulthood. Diagnosis of PSIS is primarily based on clinical symptoms, deficiency of multiple pituitary hormones, and specific MRI findings. Therefore, timely identification of causes and targeted interventions are essential to promote normal height development, fertility, and overall well-being. Long-term monitoring of hormone levels is crucial, and future research should prioritize investigating the etiology of PSIS, identifying reliable biological markers for diagnosis, and developing more effective treatment strategies.
We thank the patient and his family for their participation in this study. We thank Professor Zhe Zhang from the Reproductive Medicine Center of Peking University Third Hospital for his guidance and assistance with this article.
| 1. | Deal C, Hasselmann C, Pfäffle RW, Zimmermann AG, Quigley CA, Child CJ, Shavrikova EP, Cutler GB Jr, Blum WF. Associations between pituitary imaging abnormalities and clinical and biochemical phenotypes in children with congenital growth hormone deficiency: data from an international observational study. Horm Res Paediatr. 2013;79:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Maghnie M, Lindberg A, Koltowska-Häggström M, Ranke MB. Magnetic resonance imaging of CNS in 15,043 children with GH deficiency in KIGS (Pfizer International Growth Database). Eur J Endocrinol. 2013;168:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Fujisawa I, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, Minami S, Sagoh T, Hiraoka T, Momoi T. Transection of the pituitary stalk: development of an ectopic posterior lobe assessed with MR imaging. Radiology. 1987;165:487-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 153] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | El Chehadeh-Djebbar S, Callier P, Masurel-Paulet A, Bensignor C, Méjean N, Payet M, Ragon C, Durand C, Marle N, Mosca-Boidron AL, Huet F, Mugneret F, Faivre L, Thauvin-Robinet C. 17q21.31 microdeletion in a patient with pituitary stalk interruption syndrome. Eur J Med Genet. 2011;54:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Guo QH, Wang CZ, Wu ZQ, Qin Y, Han BY, Wang AP, Wang BA, Dou JT, Wu XS, Mu YM. Multi-genic pattern found in rare type of hypopituitarism: a whole-exome sequencing study of Han Chinese with pituitary stalk interruption syndrome. J Cell Mol Med. 2017;21:3626-3632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Han BY, Zhang Q, Li LL, Guo QH, Wang CZ, Cang L, Jin N, Chen F, Zhao L, Cui J, Gu XL, Ma FL, Zhang SC, Mu YM, Dou JT. Clinical Features of Pituitary Stalk Interruption Syndrome in 114 Cases. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Sridhar S, Raja BR, Priyanka R, Natarajan S, Soundararajan S, Natarajan V. Clinico-radiological correlation of pituitary stalk interruption syndrome in children with growth hormone deficiency. Pituitary. 2023;26:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Simon D, Hadjiathanasiou C, Garel C, Czernichow P, Léger J. Phenotypic variability in children with growth hormone deficiency associated with posterior pituitary ectopia. Clin Endocrinol (Oxf). 2006;64:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Alatzoglou KS, Gregory LC, Dattani MT. Development of the Pituitary Gland. Compr Physiol. 2020;10:389-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Castinetti F, Reynaud R, Saveanu A, Jullien N, Quentien MH, Rochette C, Barlier A, Enjalbert A, Brue T. MECHANISMS IN ENDOCRINOLOGY: An update in the genetic aetiologies of combined pituitary hormone deficiency. Eur J Endocrinol. 2016;174:R239-R247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | McCormack SE, Li D, Kim YJ, Lee JY, Kim SH, Rapaport R, Levine MA. Digenic Inheritance of PROKR2 and WDR11 Mutations in Pituitary Stalk Interruption Syndrome. J Clin Endocrinol Metab. 2017;102:2501-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Zwaveling-Soonawala N, Alders M, Jongejan A, Kovacic L, Duijkers FA, Maas SM, Fliers E, van Trotsenburg ASP, Hennekam RC. Clues for Polygenic Inheritance of Pituitary Stalk Interruption Syndrome From Exome Sequencing in 20 Patients. J Clin Endocrinol Metab. 2018;103:415-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Cheung LYM, Camper SA. PROP1-Dependent Retinoic Acid Signaling Regulates Developmental Pituitary Morphogenesis and Hormone Expression. Endocrinology. 2020;161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Gregory LC, Cionna C, Cerbone M, Dattani MT. Identification of genetic variants and phenotypic characterization of a large cohort of patients with congenital hypopituitarism and related disorders. Genet Med. 2023;25:100881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Budny B, Karmelita-Katulska K, Stajgis M, Żemojtel T, Ruchała M, Ziemnicka K. Copy Number Variants Contributing to Combined Pituitary Hormone Deficiency. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Wang CZ, Guo LL, Han BY, Su X, Guo QH, Mu YM. Pituitary Stalk Interruption Syndrome: From Clinical Findings to Pathogenesis. J Neuroendocrinol. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Liu Z, Chen X. A Novel Missense Mutation in Human Receptor Roundabout-1 (ROBO1) Gene Associated with Pituitary Stalk Interruption Syndrome. J Clin Res Pediatr Endocrinol. 2020;12:212-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Karaca E, Buyukkaya R, Pehlivan D, Charng WL, Yaykasli KO, Bayram Y, Gambin T, Withers M, Atik MM, Arslanoglu I, Bolu S, Erdin S, Buyukkaya A, Yaykasli E, Jhangiani SN, Muzny DM, Gibbs RA, Lupski JR. Whole-exome sequencing identifies homozygous GPR161 mutation in a family with pituitary stalk interruption syndrome. J Clin Endocrinol Metab. 2015;100:E140-E147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Correa-Silva SR, Kunii I, Mitne-Neto M, Moreira CM, Dias-da-Silva MR, Abucham J. Copy number variation in pituitary stalk interruption syndrome: A large case series of sporadic non-syndromic patients and literature review. J Neuroendocrinol. 2023;35:e13221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Fang X, Zhang Y, Cai J, Lu T, Hu J, Yuan F, Chen P. Identification of novel candidate pathogenic genes in pituitary stalk interruption syndrome by whole-exome sequencing. J Cell Mol Med. 2020;24:11703-11717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Vergier J, Castinetti F, Saveanu A, Girard N, Brue T, Reynaud R. DIAGNOSIS OF ENDOCRINE DISEASE: Pituitary stalk interruption syndrome: etiology and clinical manifestations. Eur J Endocrinol. 2019;181:R199-R209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Brauner R, Bignon-Topalovic J, Bashamboo A, McElreavey K. Pituitary stalk interruption syndrome is characterized by genetic heterogeneity. PLoS One. 2020;15:e0242358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Wang Q, Meng X, Sun Y, Liu F, Xu C, Qiao Y, Yang J, Li G, Wang Y. Hypoglycemia and jaundice in newborns with pituitary stalk interruption syndrome. Medicine (Baltimore). 2021;100:e25843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Mauvais FX, Gonzales E, Davit-Spraul A, Jacquemin E, Brauner R. Cholestasis Reveals Severe Cortisol Deficiency in Neonatal Pituitary Stalk Interruption Syndrome. PLoS One. 2016;11:e0147750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Bar C, Zadro C, Diene G, Oliver I, Pienkowski C, Jouret B, Cartault A, Ajaltouni Z, Salles JP, Sevely A, Tauber M, Edouard T. Pituitary Stalk Interruption Syndrome from Infancy to Adulthood: Clinical, Hormonal, and Radiological Assessment According to the Initial Presentation. PLoS One. 2015;10:e0142354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Diwaker C, Thadani P, Memon SS, Sarathi V, Lila AR, Arya S, Krishnappa B, Karlekar M, Patil VA, Shah N, Bandgar T. Pituitary stalk interruption syndrome: phenotype, predictors, and pathophysiology of perinatal events. Pituitary. 2022;25:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Guo Q, Yang Y, Mu Y, Lu J, Pan C, Dou J, Lv Z, Ba J, Wang B, Zou X, Yang L, Ouyang J, Yang G, Wang X, Du J, Gu W, Jin N, Chen K, Zang L, Erickson BJ. Pituitary stalk interruption syndrome in Chinese people: clinical characteristic analysis of 55 cases. PLoS One. 2013;8:e53579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Nannette G, Bar C, Diene G, Pienkowski C, Oliver-Petit I, Jouret B, Cartault A, Porquet-Bordes V, Salles JP, Grunenwald S, Edouard T, Molinas C, Tauber M. Obesity, Overweight, and Pituitary Stalk Interruption Syndrome in Children and Young Adults. J Clin Endocrinol Metab. 2023;108:323-330. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Wang Q, Hu Y, Li G, Sun X. Pituitary stalk interruption syndrome in 59 children: the value of MRI in assessment of pituitary functions. Eur J Pediatr. 2014;173:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Maghnie M, Genovese E, Villa A, Spagnolo L, Campan R, Severi F. Dynamic MRI in the congenital agenesis of the neural pituitary stalk syndrome: the role of the vascular pituitary stalk in predicting residual anterior pituitary function. Clin Endocrinol (Oxf). 1996;45:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Guo Y, Li X, Wang X, Li H, Luo G, Si Y, Wu X, Li Y. Seminal plasma metabolomics and lipidomics profiling to identify signatures of pituitary stalk interruption syndrome. Orphanet J Rare Dis. 2022;17:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Hao M, Mao JF, Guan QB, Tian L, Han H, Lei HE, Zheng DM, Tian ZH, Nie M, Wang X, Yu BQ, Gao YJ, Wu XY. Efficacy and safety of pulsatile gonadotropin-releasing hormone therapy in patients with congenital hypogonadotropic hypogonadism: a multicentre clinical study. Ann Transl Med. 2021;9:962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Gu Y, Zhang S, Li X, Dou J, Lyu Z, Ba J, Guo Q, Zang L, Chen K, Du J, Pei Y, Mu Y, Gu W. The efficacy of pulsatile gonadotropin-releasing hormone therapy in male patients with hypogonadism caused by hypopituitarism. Ann Palliat Med. 2021;10:4642-4651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Huang Q, Mao J, Wang X, Yu B, Ma W, Ji W, Zhu Y, Zhang R, Sun B, Zhang J, Nie M, Wu X. Efficacy of Pulsatile Gonadotropin-Releasing Hormone Therapy in Male Patients: Comparison between Pituitary Stalk Interruption Syndrome and Congenital Hypogonadotropic Hypogonadism. Endocr Pract. 2022;28:521-527. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Zhang J, Zhu Y, Zhang R, Liu H, Sun B, Zhang W, Wang X, Nie M, Mao J, Wu X. Pulsatile Gonadotropin-Releasing Hormone Therapy Is Associated With Better Spermatogenic Outcomes than Gonadotropin Therapy in Patients With Pituitary Stalk Interruption Syndrome. Endocr Pract. 2024;30:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Mao JF, Wang X, Zheng JJ, Liu ZX, Xu HL, Huang BK, Nie M, Wu XY. Predictive factors for pituitary response to pulsatile GnRH therapy in patients with congenital hypogonadotropic hypogonadism. Asian J Androl. 2018;20:319-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Winkler I, Steichen E, Kapelari K, Wöckinger P, Neubauer V, Kiechl-Kohlendorfer U, Griesmaier E. Pituitary Stalk Interruption Syndrome - Clinical Presentation and Management of a Potentially Life-threatening Disease in Newborns. J Clin Res Pediatr Endocrinol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |