Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4272
Revised: May 10, 2024
Accepted: June 3, 2024
Published online: July 16, 2024
Processing time: 171 Days and 17.8 Hours
Education, cognition, and intelligence are associated with cholelithiasis occur
To explore the causal associations between education, cognition, and intelligence and cholelithiasis, and the cardiometabolic risk factors that mediate the associations.
Applying genome-wide association study summary statistics of primarily European individuals, we utilized two-sample multivariable Mendelian rando
Inverse variance weighted Mendelian randomization results from the FinnGen consortium showed that genetically higher education, cognition, or intelligence were not independently associated with cholelithiasis and cholecystitis; when adjusted for cholelithiasis, higher education still presented an inverse effect on cholecystitis [odds ratio: 0.292 (95%CI: 0.171-0.501)], which could not be induced by cognition or intelligence. Five out of 21 cardiometabolic risk factors were perceived as mediators of the association between education and cholelithiasis, including body mass index (20.84%), body fat percentage (40.3%), waist circumference (44.4%), waist-to-hip ratio (32.9%), and time spent watching television (41.6%), while time spent watching television was also a mediator from cognition (20.4%) and intelligence to cholelithiasis (28.4%). All results were robust to sensitivity analyses.
Education, cognition, and intelligence all play crucial roles in the development of cholelithiasis, and several cardiometabolic mediators have been identified for prevention of cholelithiasis due to defects in each exposure.
Core Tip: In this study, we investigated the independent causal effects of education, cognition, and intelligence on cholelithiasis and cholecystitis. Subsequently, we estimated the independent association between each exposure and cholecystitis, after adjustment for cholelithiasis. Finally, the ultimate aims were to screen out the mediator(s) and clarify the mediating effects of several correlated risk factors in the pathogenesis of cholelithiasis to instrument clinical practice.
- Citation: Li CL, Liu YK, Lan YY, Wang ZS. Association of education with cholelithiasis and mediating effects of cardiometabolic factors: A Mendelian randomization study. World J Clin Cases 2024; 12(20): 4272-4288
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4272.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4272
Cholelithiasis (gallstone disease) harasses approximately 10%-20% of the global population and places burdens on their health and socioeconomic status[1,2]. Cholecystitis, cholangitis, and pancreatitis, as the most ubiquitous complications derived from cholelithiasis, markedly influence patients’ quality of life and physical condition extensively and severely[3,4]. Concurrently, laparoscopic cholecystectomy is the standard treatment for gallstone-related diseases that comply with guideline standards[5-7]. With the increasing occurrence of cholelithiasis and its broad complications, identifying their potential risk factors is of great significance. For instance, emerging epidemiological evidence shows that several cardiometabolic risk factors—metabolic traits (adiposity[1,2,8-10], glucose-related traits[11,12], and lipid levels[13,14]), dietary behaviors[15-17], physical activity[18-20], and socioeconomic factors[21] might be correlated with the develop
Educational attainment has been demonstrated to be adversely related to gallbladder diseases, notably cholelithiasis, in numerous observational and Mendelian randomization (MR) studies[1,22-24]. In addition, recent studies have demon
MR is a well-acknowledged method to infer causal relationships, by perceiving genetic variants as proxies for exposure(s), parallel to performing a classical randomized control trial, and simultaneously avoiding several confounding biases and reverse causality that occur in observational studies[28]. Multivariable Mendelian randomization (MVMR) is an extensive approach to estimating the independent effects of several relevant or interrelated exposures on one outcome by combining genetic variants of each exposure simultaneously[29]. Subsequently, a two-step MVMR study (mediation MR) is performed to determine whether one putative mediator has a significant mediating effect in the pathway from a certain exposure to an outcome, markedly eliminating confounding bias and measurement error existing between exposure, mediator, and outcome[30].
In this study, we investigated the independent causal effects of education, cognition, and intelligence on cholelithiasis and cholecystitis, using the two-sample MR and MVMR methods. Subsequently, we estimated the independent asso
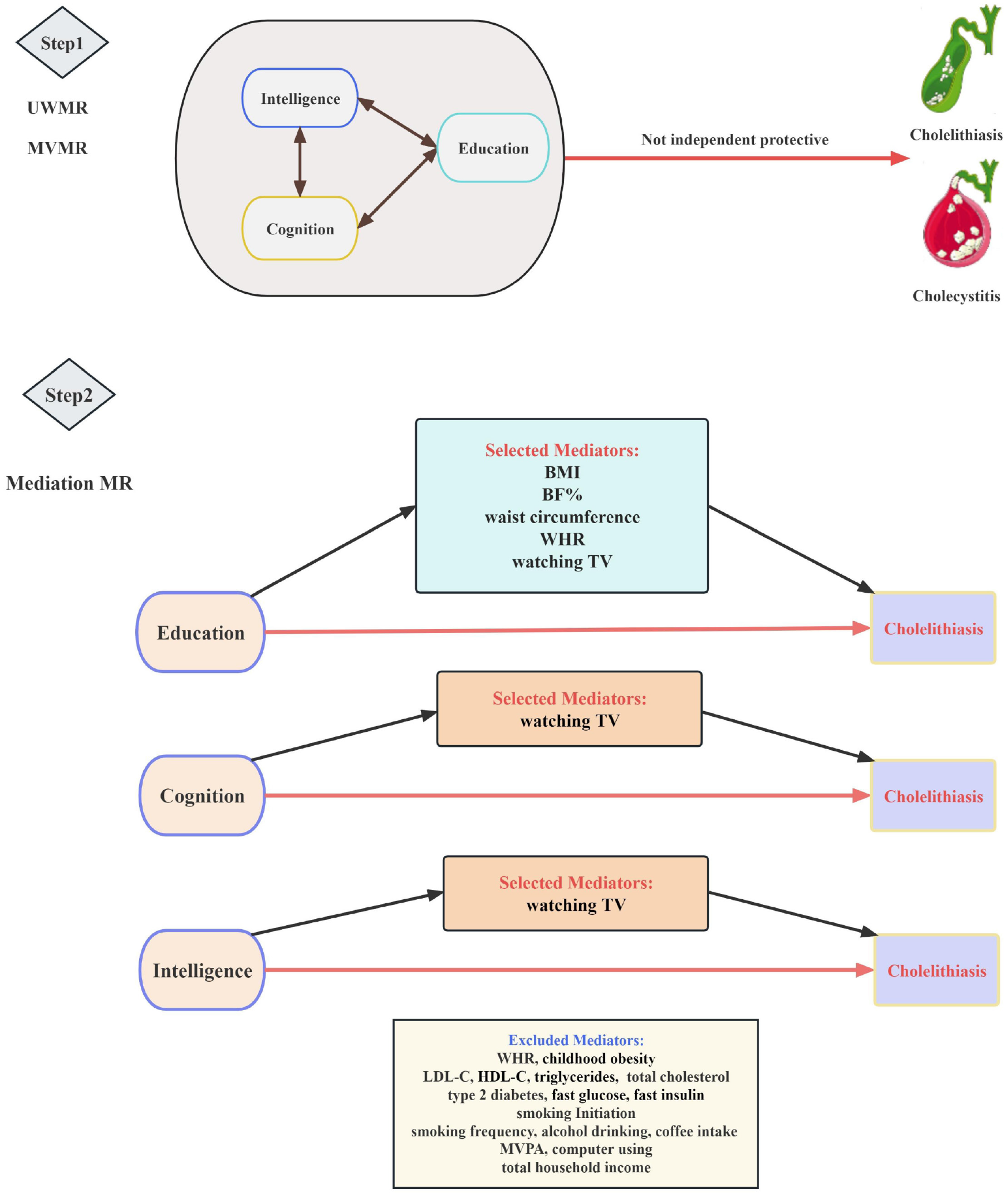
This study is mainly composed of two-stage analyses (Figure 1). First, we estimated the causal relationship of education, cognition, and intelligence with cholelithiasis and cholecystitis utilizing UVMR and MVMR, by selecting single nucleo
In this MR analysis, data of exposures, mediators, and outcomes were all extracted from summary-level data from genome-wide association studies (GWASs) performed primarily in individuals of European ancestry (Table 1).
| Phenotype | Unit | No. of participants | Ancestry | Consortium/cohort | Ref. | GWAS resources |
| Education | SD (4.2 years) | 1131881 | European | SSGAC | Lee et al[32], 2018 | |
| Cognition | SD (0.99 point) | 257841 | European | COGENT | Lee et al[32], 2018 | |
| Intelligence | SD | 269867 | European | Meta | Savage et al[25], 2018 | |
| Cholelithiasis | Event | 330903 | European | FinnGen | Not available | https://r9.finngen.fi/ |
| Cholecystitis | Event | 330903 | European | FinnGen | Not available | https://r9.finngen.fi/ |
| BMI | SD (4.7 kg/m2) | 681275 | European | GIANT | Yengo et al[34], 2018 | |
| BF% | SD (6.6%) | 65831 | European | Meta | Lu et al[35], 2016 | |
| WHR | SD (0.09) | 212244 | European | GIANT | Shungin et al[36], 2015 | |
| Waist circumference | SD (12.5 cm) | 231353 | European | GIANT | Shungin et al[36], 2015 | |
| Watching TV | SD (1.5 h) | 408815 | European | United Kingdom Biobank | Van de Vegte et al[44], 2020 | |
| Childhood obesity | Event | 13848 | European | EGG | Bradfield et al[37], 2012 | |
| Type 2 diabetes | Event | 70127 | European | Meta | Sílvia Bonàs-Guarch et al[38], 2018 | |
| Fasting insulin | SD (0.79 pmol/L) | 108557 | European | MAGIC | Scott et al[39], 2012 | |
| Fasting glucose | SD (0.73 mmol/L) | 133010 | European | MAGIC | Scott et al[39], 2012 | |
| LDL-C | SD (38.7 mg/dL) | 173082 | Mixed1 | GLGC | Willer et al[40], 2013 | |
| HDL-C | SD (15.5 mg/dL) | 187167 | Mixed1 | GLGC | Willer et al[40], 2013 | |
| Triglycerides | SD (90.7 mg/dL) | 177861 | Mixed1 | GLGC | Willer et al[40], 2013 | |
| Total cholesterol | SD (41.8 mg/dL) | 187365 | Mixed1 | GLGC | Willer et al[40], 2013 | |
| Smoking initiation | Event | 607291 | European | GSCAN | Liu et al[41], 2019 | |
| Smoking heaviness | SD (8 cigarettes) | 337334 | European | GSCAN | Liu et al[41], 2019 | |
| Alcohol drinking | SD (9 drinks/wk) | 335394 | European | GSCAN | Liu et al[41], 2019 | |
| Coffee intake | SD (1% change) | 375833 | European | United Kingdom Biobank | Zhong et al[42], 2019 | |
| Tea intake | SD (2.85 cups/d) | 447485 | European | United Kingdom Biobank | Zhong et al[42], 2019 | UKB-b-6066 |
| MVPA | SD (2084 MET-min/wk) | 377234 | European | United Kingdom Biobank | Klimentidis et al[43], 2018 | |
| Computer using | SD (1.2 h) | 408815 | European | United Kingdom Biobank | Van de Vegte et al[44], 2020 | |
| Total house income | SD | 397751 | European | United Kingdom Biobank | Hemani et al[45], 2018 |
Exposures: Genetic instruments for education were selected from the Social Science Genetic Association Consortium[32]. GWAS of education attainment (years of schooling) enrolled 1131881 individuals of European ancestry with summary data, and after excluding participants from 23 and Me, 766345 of these participants were made available for while data can only be exhibited for up to 10000 SNPs[32]. Genetic instruments for cognition (cognitive performance) were derived from a meta-analysis in 257841 individuals from the Cognitive Genomics Consortium and United Kingdom Biobank, by calculating broadband index (g) or verbal-numerical reasoning scores with less statistically significant values of heterogeneity in meta-analytic tests[32,33]. Genetic instruments for intelligence were extracted from a GWAS meta-analysis of 269867 European individuals via neurocognitive tests (primarily gauging fluid domains of cognitive functioning) to assess intelligence, without evidence of heterogeneity between cohorts in the genetic associations[25].
After conducting linkage disequilibrium analyses using related criteria (r2 < 0.001; distance threshold: 10000 kb), 393/1271, 132/225, and 165/242 independent genome-wide significant (P < 5 × 10-8) SNPs were singled out as the primary genetic instruments for education, cognition, and intelligence, respectively.
Mediators: Based on observational studies, we selected 21 candidate mediators of cholelithiasis-related risk factors (Table 1), which may exist in the pathway from education, cognition, or intelligence to cholelithiasis. All of the genetic instruments were availably derived from the GWAS database, including adiposity characteristics (body mass index (BMI)[34], body fat percentage (BF%)[35], waist circumference[36], waist-to-hip ratio (WHR)[36], and childhood obesity[37]), glucose-related traits (type 2 diabetes[38], fasting glucose[39], and fast insulin[39]), serum lipid traits (low-density lipoprotein cholesterol (LDL-C)[40], high-density lipoprotein cholesterol (HDL-C)[40], triglycerides[40], and total cholesterol (TC)[40]), dietary behaviors (smoking initiation[41], smoking frequency[41], alcohol drinking[41], coffee intake[42], and tea intake [from IEU OpenGWAS project (mrcieu.ac.uk); GWAS ID: ukb-b-6066]), physical activity (moderate to vigorous physical activity (MVPA)[43], watching TV, and computer use[44]), and socioeconomic factor (total household income[45]).
Subsequently, we screened out mediators of the independent association of education, cognition, and intelligence with cholelithiasis, according to the following rigorous criteria: (1) A causal association exists between education, cognition, or intelligence and the mediator, and the effect of education, cognition, or intelligence on the mediator should be unidirectional, to avoid possible bidirectionality influencing the mediation; (2) a causal relationship consistently exists between the mediator and cholelithiasis with or without adjustment for education, cognition, or intelligence; and (3) based on common scientific evidence, the association between education, cognition, or intelligence and the mediator and the association between the mediator and cholelithiasis should be in inverse directions. Ultimately, five, one, and one risk factor met all criteria and were included in the Mediation analyses to evaluate their mediating effects on the independent causal associations of education, cognition, and intelligence with cholelithiasis, respectively. During MVMR analyses, we selected genetic instruments by combining SNPs, which were of genome-wide significance in either the GWAS of education or the GWAS of mediator after clumping, based on linkage disequilibrium threshold r2 < 0.001 and window > 10000 kb.
Outcomes: Summary-level genetic data for cholelithiasis (gallstone disease, defined by the International Classification of Diseases 10th Revision code K80) and cholecystitis (defined by the International Classification of Diseases 10th Revision code K81) was obtained from the FinnGen consortium (https://r9.finngen.fi/). The FinnGen study included 37041 European individuals with cholelithiasis and 4299 individuals with cholecystitis among a total of 330903 participants.
All the above GWAS data have obtained ethical approval from the related institutional review boards, stringent quality control, and participant informed consent. All participants have given consent to each GWAS. In addition, the database used in our study is publicly available in Table 1 (Consortium/Cohort column).
UVMR and MVMR analyses: We conducted a two-sample UVMR to determine the total effect of education, cognition, or intelligence on cholelithiasis and cholecystitis. We performed MVMR to: (1) Estimate the independent direct effects of education, cognition, and intelligence on cholelithiasis and cholecystitis by mutually adjusting to determine which exposure exhibited a causal association with cholelithiasis and cholecystitis, independent of the other two exposures; and (2) determine whether education, cognition, or intelligence had a causal effect on cholecystitis after adjusted for cholelithiasis. More importantly, all MR analyses should fulfill three critical assumptions as follows: (1) SNPs must be closely associated with the exposure in the UVMR procedure and should be closely associated with at least one of the several exposures in the MVMR procedure; (2) SNPs should not be relevant to confounders of the associations between instruments of each exposure and cholelithiasis and cholecystitis; and (3) the effects of genetic variants on cholelithiasis or cholecystitis must pass through each exposure[46]. In addition, we searched proxy SNPs in high linkage disequilibrium
Mediation MR analyses: When carefully filtering the mediator(s), we utilized GWAS data from the FinnGen study as the primary source for cholelithiasis, which had no or few overlapping samples with the mediator GWASs. Given that cholecystitis is mostly derived from cholelithiasis, we abnegated cholecystitis in the mediation MR analysis to reduce the confounding bias caused by cholelithiasis. A two-step MR approach was performed to determine whether a putative intermediate risk factor plays a mediating role between education, cognition, or intelligence and cholelithiasis[47]. The first step was conducted to calculate the causal effects of genetically determined education, cognition, and intelligence on the mediator (β1) using UVMR. Then, the second step was to calculate the causal effect of the mediator on cholelithiasis by applying GWASs from the FinnGen Study with adjustment for education, cognition, or intelligence (β2) using MVMR. Furthermore, the proportion of the total effect of education, cognition, or intelligence on cholelithiasis that was mediated by each mediator was calculated. Then, the indirect effect was estimated by multiplying the estimates from the two-step results (β1 × β2) by the total effect. The Delta method was applied to obtain SEs using effect estimates acquired from two-sample MR analyses[48].
MR sensitivity analyses: To guarantee the strength of the IVW results, we also performed MR Egger, Weighted Median, and MR pleiotropy residual sum and outlier methods in UVMR analyses, and applied the MVMR Egger method in MVMR analyses. The MR Egger method has been widely applied to estimate whether selected genetic variants obtained directional pleiotropic effects on the outcome, differing on average from zero and providing a similar inference of the causal effect, under the InSIDE (Instrument Strength Independent of Direct Effect) hypothesis[49]. The Weighted Median method is utilized to provide consistent estimates under the assumption that more than 50% of the content leading to the analysis comes from solid instrumental variables[50]. In addition, the MR pleiotropy residual sum and outlier method can detect whether outlying SNPs exist, which can potentially induce horizontal pleiotropy, and estimate whether the exclusion of outlying SNPs can influence the causal effects, under the assumption that the candidate instruments in the largest group of with similar estimates compose a robust and valid group of instrumental variables[51,52].
The intercepting estimate of MR Egger was applied to test for pleiotropy, indicating potential infiltrations of the IV assumptions during the two-sample MR process. In addition, we also calculated the Q′ heterogeneity statistic to estimate the heterogeneity between instruments. Conditional F-statistics were used to test instrumental validity, with an F < 10 indicating for low instrument validity.
Collectively, we considered IVW results as causal inferences only when they exhibited the same direction and statistical significance when conducting at least one sensitivity analysis, with no evidence of pleiotropy (P > 0.05). All effect sizes are presented as odds ratios (ORs), beta coefficients (βs), or proportions, with corresponding 95% confidence intervals (CIs). All MR analyses were performed using R packages “TwoSampleMR,” “MRPRESSO,” “MendelianRandomization,” “MVMR,” and “easyMR” in R software (version 4.2.3; the R Foundation for Statistical Computing, Vienna, Austria).
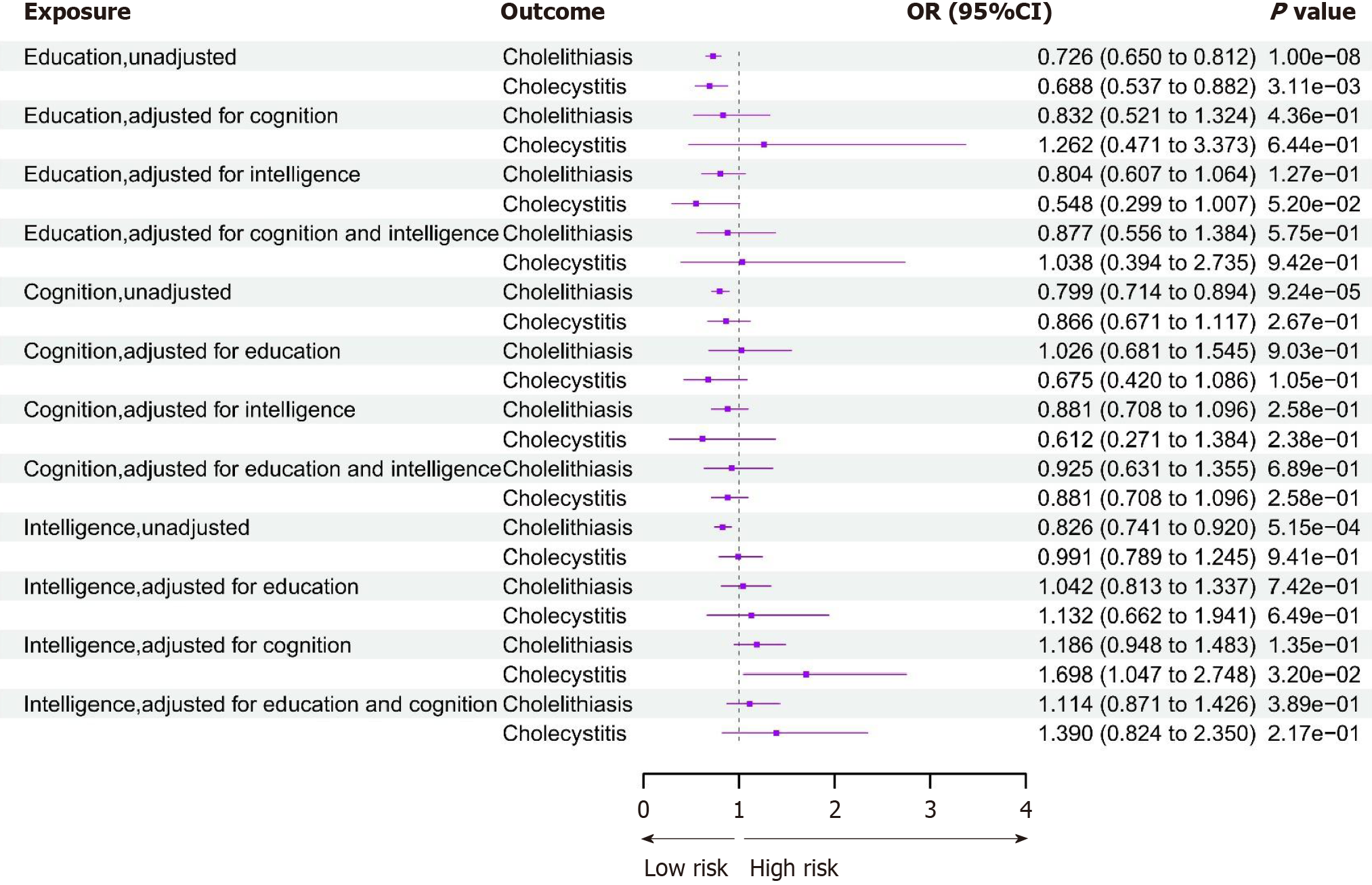
First, we conducted UVMR analysis to estimate the total effect of the three exposures—education, cognition, or intelligence on cholelithiasis and its secondary complication—cholecystitis. We applied the IVW method as the main criterion, as presented in Figure 2. Each 1-SD (4.2 years) increase in years of schooling [OR: 0.73 (95%CI: 0.65-0.81)], better cognitive performance [OR: 0.80 (95%CI: 0.71-0.89)], and higher intelligence [OR: 0.83 (95%CI: 0.74-0.92)] were all associated with a lower risk of cholelithiasis. Similarly, it was genetically predicted that each 1-SD increase for years of schooling [OR: 0.69 (95%CI: 0.54-0.88)] was also negatively associated with cholecystitis. However, neither better cognitive performance nor higher intelligence was associated with cholecystitis, distinct from cholelithiasis above. Several sensitivity analyses were robust in all MR results. MR Egger and weight median results were both consistent with those of the IVW method (Supplementary Table 1). Genetic instrumental variables of all three exposures displayed certain heterogeneity and no pleiotropy with those of cholelithiasis and cholecystitis (Supplementary Tables 2 and 3).
Subsequently, we performed MVMR to calculate the independent causal relationship after adjusting for other interrelated phenotypes. Nevertheless, no causal association was observed between education and cholelithiasis or cholecystitis after adjusting for cognition, intelligence, or both. Meanwhile, cognitive performance did not exert a genetically causal effect on cholelithiasis or cholecystitis when adjusted for education, intelligence, or both of them. Similarly, intelligence did not show significant causal inference on the two outcomes, except when adjusted for cognition (OR: 1.70 [95%CI: 1.05-2.75]) which exhibited a positive association with cholecystitis, although it presented an insignificant statistical estimate (Supplementary Table 4). In addition, all directions and most of the statistical significance of IVW results resembled MVMR Egger sensitivity analyses, demonstrating a minor risk of bias for less horizontal pleiotropy (Supplementary Table 4).
We conducted the MVMR method to dissect the independent effect between education, cognition, or intelligence and cholecystitis, adjusting for cholelithiasis. To eliminate the overlap between the populations of cholelithiasis and cholecystitis, GWAS from the FinnGen consortium and United Kingdom Biobank cohort were applied, individually. In the MVMR results, each SD (4.2-year) increase in years of schooling [IVW OR: 0.292; (95%CI: 0.171-0.501)] was inversely associated with the occurrence of cholecystitis after adjustment for cholelithiasis (Table 2). However, after adjustment for cholelithiasis, neither cognition [OR: 0.942 (95%CI: 0.600-1.667)] or intelligence [OR: 0.834 (0.506-1.374)] was significantly negatively correlated with cholecystitis. The instrument strength of the SNPs for all variables in the MVMR models was estimated to be sufficient by instrument validity test, with an F-statistic > 10 (Supplementary Tables 5 and 6).
| Exposure | Method | β (95%CI) | OR (95%CI) | P value |
| Education | MR MV_IVW | -0.534 (-0.767 to -0.300) | 0.292 (0.171-0.501) | 1.73E-43 |
| MR MV_Egger | -0.614 (-0.853 to -0.376) | 0.243 (0.140-0.421) | < 0.001 | |
| MR MV_Median | -0.608 (-0.942 to -0.275) | 0.247 (0.114-0.531) | < 0.001 | |
| MR MV_Lasso | -0.513 (-0.738 to -0.288) | 0.307 (0.183-0.515) | < 0.001 | |
| Cognition | MR MV_IVW | -0.026 (-0.222 to 0.222) | 0.942 (0.600-1.667) | 0.839 |
| MR MV_Egger | -0.894 (-1.590 to -0.197) | 0.128 (0.026-1.574) | 0.012 | |
| MR MV_Median | 0.094 (-0.234 to 0.421) | 1.242 (0.583-2.636) | 0.576 | |
| MR MV_Lasso | -0.025 (-0.247 to 0.196) | 0.944 (0.566-1.570) | 0.822 | |
| Intelligence | MR MV_IVW | -0.079 (-0.296 to 0.138) | 0.834 (0.506-1.374) | 0.475 |
| MR MV_Egger | -0.454 (-1.171 to 0.264) | 0.352 (0.067-1.837) | 0.215 | |
| MR MV_Median | -0.105 (-0.414 to 0.203) | 0.785 (0.385-1.596) | 0.504 | |
| MR MV_Lasso | -0.122 (-0.328 to 0.084) | 0.755 (0.470-1.213) | 0.246 |
Among all 21 candidate mediators, we screened five, one, and one mediating risk factor for the independent associations of education, cognition, and intelligence with cholelithiasis in mediation MR analyses, respectively, all of which met the selecting criteria. Then, we conducted UVMR to figure out the effect size between each exposure and mediators (Table 3). In UVMR analyses, each 1-SD longer years of schooling was associated with a lower BMI [IVW β: −0.36; (95%CI: −0.42 to −0.31)], lower BF% [−0.28 SD; (95%CI: −0.32 to −0.24)], lower waist circumference [−0.30 SD; (95%CI: −0.36 to −0.23)], lower WHR [−0.28 SD; (95%CI: −0.34 to −0.23)], and less time spent watching TV [−0.41 SD; (95%CI: −0.44 to −0.39)]. Similarly, better cognitive performance [−0.20 SD; (95%CI: −0.24 to −0.16)] and higher intelligence [−0.21 SD; (95%CI: −0.25 to −0.18)] were both robustly associated with less time spent watching TV. MR Egger, weighed Median, and MR PRESSO results were consistent with those of the IVW method (Table 3). In addition, genetic instrumental variables of education, cognition, and intelligence showed persistent heterogeneity and no pleiotropy with those of mediators (Supplementary Tables 7 and 8).
| Exposure | Mediator | Method | No. of SNPs | β (95%CI) | P value |
| Education | BMI | IVW | 332 | -0.36 (-0.42 to -0.31) | 9.57E-39 |
| MR Egger | 332 | -0.30 (-0.50 to -0.10) | 2.92E-03 | ||
| Weighted Median | 332 | -0.29 ( -0.33 to -0.25) | 1.56E-46 | ||
| MR PRESSO | 651 | -0.35 (-0.37 to -0.33) | 1.44E-60 | ||
| BF% | IVW | 332 | -0.28 (-0.32 to -0.24) | 1.10E-41 | |
| MR Egger | 332 | -0.24 (-0.38 to -0.10) | 1.11E-03 | ||
| Weighted Median | 332 | -0.24 (-0.28 to -0.21) | 7.71E-51 | ||
| MR PRESSO | 541 | -0.29 (-0.30 to -0.28) | 5.98E-66 | ||
| Waist circumference | IVW | 271 | -0.30 (-0.36 to -0.23) | 5.57E-20 | |
| MR Egger | 271 | -0.41 (-0.66 to -0.17) | 1.11E-03 | ||
| Weighted Median | 271 | -0.26 (-0.34 to -0.18) | 1.96E-10 | ||
| MR PRESSO | 91 | -0.27 (-0.30 to -0.24) | 3.59E-22 | ||
| WHR | IVW | 271 | -0.28 (-0.34 to -0.23) | 5.91E-26 | |
| MR Egger | 271 | -0.32 (-0.53 to -0.12) | 1.89E-03 | ||
| Weighted Median | 271 | -0.25 (-0.32 to -0.17) | 2.26E-10 | ||
| MR PRESSO | 21 | -0.28 (-0.31 to -0.25) | 1.31E-25 | ||
| Watching TV | IVW | 332 | -0.41 (-0.44 to -0.39) | 9.10E-226 | |
| MR Egger | 332 | -0.44 (-0.53 to -0.35) | 9.50E-19 | ||
| Weighted Median | 332 | -0.38 (-0.41 to -0.35) | 7.23E-145 | ||
| MR PRESSO | 121 | -0.42 (-0.44 to -0.40) | 2.22E-127 | ||
| Cognition | Watching TV | IVW | 106 | -0.20 (-0.24 to -0.16) | 5.11E-25 |
| MR Egger | 106 | -0.26 (-0.42 to -0.10) | 1.57E-03 | ||
| Weighted Median | 106 | -0.20 (-0.18 to -0.11) | 1.43E-20 | ||
| MR PRESSO | 171 | -0.18 (-0.20 to -0.16) | 1.78E-26 | ||
| Intelligence | Watching TV | IVW | 136 | -0.21(-0.25 to -0.18) | 1.77E-36 |
| MR Egger | 136 | -0.32(-0.47 to -0.16) | 9.16E-05 | ||
| Weighted Median | 136 | -0.19(-0.22 to -0.16) | 3.76E-42 | ||
| MR PRESSO | 211 | -0.20(-0.22 to -0.18) | 4.11E-32 |
We performed the MVMR method to uncover the independent effects of the mediators on cholelithiasis, adjusting for education, cognition, or intelligence. In the MVMR results, each 1-SD unit increase in BMI [IVW OR: 1.60; (95%CI:1.45-1.77)]; BF% [OR: 1.94; (95%CI: 1.69-2.24)]; waist circumference [OR: 1.56; (95%CI: 1.33-1.84)]; WHR [OR: 1.43; (95%CI: 1.14-1.81)], and time spent watching TV [OR: 1.45; (95%CI: 1.06-1.75)] was associated with a high risk of cholelithiasis after adjustment for education (Table 4). In addition, after adjustment for cognition [OR: 1.42; (95%CI: 1.07-1.90)] or intelligence [OR: 1.46; (95%CI: 1.05-2.03)], time spent watching TV was also associated with cholelithiasis. Instrument strength of SNPs for all variables in MVMR models was estimated to be sufficient by instrument validity test, with F-statistic > 10.
| Exposure | Mediator | β (95%CI) | OR (95%CI) | P value |
| Education | BMI | 0.469 (0.369-0.569) | 1.60 (1.45-1.77) | 4.97E-20 |
| BF% | 0.665 (0.525-0.805) | 1.94 (1.69-2.24) | 1.45E-20 | |
| Waist circumference | 0.447 (0.282-0.612) | 1.56 (1.33-1.84) | 1.13E-07 | |
| WHR | 0.361 (0.130-0.592) | 1.43 (1.14-1.81) | 2.19E-03 | |
| Time spent watching TV | 0.372 (0.173-0.571) | 1.45 (1.06-1.75) | 4.29E-02 | |
| Cognition | Time spent watching TV | 0.357 (0.071-0.643) | 1.42 (1.07-1.90) | 1.44E-02 |
| Intelligence | Time spent watching TV | 0.382 (0.056-0.708) | 1.46 (1.05-2.03) | 2.17E-02 |
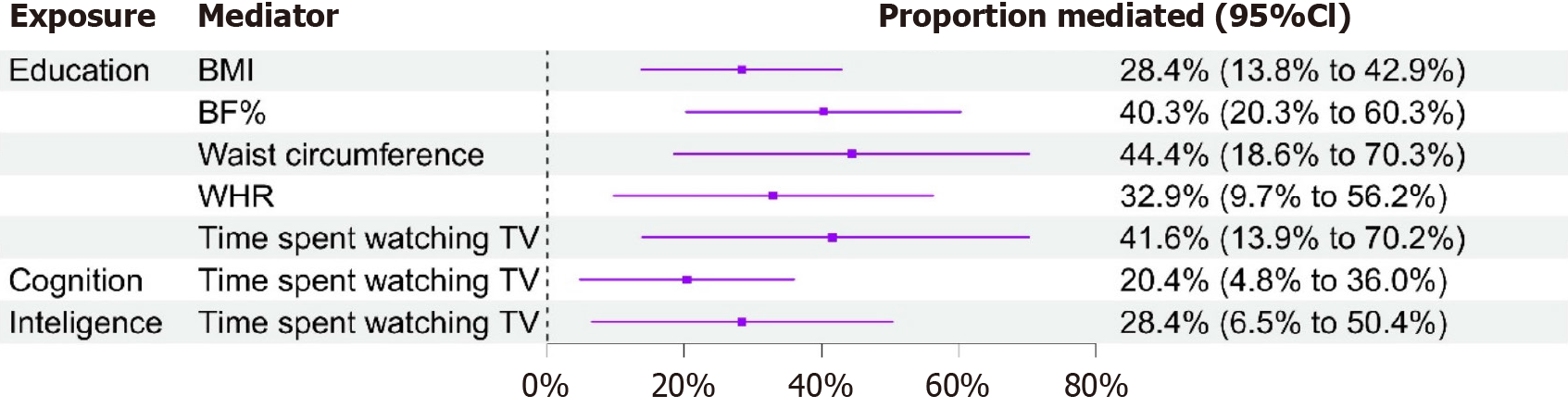
Subsequently, we conducted a two-step MR analysis to estimate the mediating effects induced by the mediators. Five candidate mediators met the criteria and were thus selected as the final mediators in the process from education to cholelithiasis, while only one was selected as the final mediator from cognition or intelligence. The largest causal mediator from education to cholelithiasis was waist circumference [44.4%; (95%CI: 18.6%-70.3%)], followed by time spent watching TV [41.6%; (95%CI: 13.9%-70.2%)], BF% [40.3%; (95%CI: 20.3%-60.3%)], WHR [32.9%; (95%CI: 9.7%-56.2%)], and BMI [28.4%; (95%CI: 13.8%-42.9%)] (Figure 3). Coincidentally, time spent watching TV also mediated the causal role from both cognition to cholelithiasis [20.4%; (95%CI: 4.8%-36.0%)] and intelligence to cholelithiasis [28.4%; (95%CI: 6.5%-50.4%)].
In this MR study, we provided novel genetic evidence for the causal effect of education on cholelithiasis and cholecystitis, with each 4.2-year-increment of schooling attenuating an approximately 27% risk of cholelithiasis and 31% risk of cholecystitis. In addition, we demonstrated causal associations of cognition and intelligence with cholelithiasis and cholecystitis. Each increasing 0.99 point of cognitive performance and each higher SD of intelligence resulted in a decreased risk of cholelithiasis of approximately 20% and 17%, respectively, with neither being significantly less than the educational effect nor showing a causal association with cholecystitis, which was inconsistent with education. Nevertheless, none of the above causal effects persisted when the associations were adjusted for other one or two other exposures. These results show that each of the three exposures—education, cognition, and intelligence—plays a crucial role in the causal process of cholelithiasis and can be influenced by other factors. Thus, we explored the mediating factor from each exposure to cholelithiasis, and ultimately selected five, one, and one out of 21 factors, respectively, in the process from education, cognition, or intelligence to cholelithiasis.
In our MR study, three broadly concerned events—educational attainment, cognitive performance, and intelligence level—were perceived to be the exposures. These exposures are interrelated with others phenotypically and genetically and hard to separate, strongly based on a previous GWAS and an MR study, demonstrating the mutually bidirectional associations among them[25,27]. Emerging evidence from observational studies and MR studies has illustrated that higher educational attainment (more years of schooling) is a protective factor for cholelithiasis[22,24,53,54]. To date, this novel research area, which dissects the association between cognitive performance or intelligence level and cholelithiasis, has not yet been entirely elaborated. For the first time, we used genetic inference to reveal that either better cognitive performance or a higher intelligence level is a protective factor for cholelithiasis. These results are in tune with our general perspectives, for those who have higher cognition and intelligence may be less likely to enjoy high-calorie, high-carbohydrate, and low-fiber diets, or neglect physical activities, all of which prompt gallstone formation[55,56]. As recent epidemiological study showed that gallstone diseases mainly affected adult individuals, notably elderly individuals[1], whose education, cognitive performance, and intelligence level have already been determined. However, earlier educational attainment profoundly affects later life, including cognitive training, knowledge acquisition, and notably health promotion[57]. This study elucidates the importance of improving academic qualifications, cognitive performance, and intelligence level to reduce the risk of gallstone diseases as much as possible. Nonetheless, considering the difficulty and near-impossibility of altering one’s the living environment owing to poverty and other difficult obstacles, a better lifestyle and a healthier diet are strongly recommended for those who cannot obtain prioritized educational sources to prevent the onset of gallstone diseases.
Cholecystitis is the most ubiquitous complication of cholelithiasis and is typically ascribed to calculus in the gallbladder, disrupting patients’ quality of life and threatening their lives[3,58]. Due to the bile duct obstruction caused by gallstones or sludge or lithogenic bile, these individuals are subjected to suffer from cholecystitis-induced right upper quadrant mass, pain, tenderness, and fever[59]. Once diagnosed with acute cholecystitis, within-3-day laparoscopic cholecystectomy (to remove the inflammatory or diseased gallbladder) is the first-line therapy to prevent severe complications[5,6]. Due to the economic burden and postsurgical psychiatric disorders, prevention should receive more attention than the treatment procedure[60,61]. From a clinical perspective, among the general population with gallstone diseases, approximately 80% are asymptomatic[62]. Thus, patients might pay little attention to lifestyle improvement and dieting alteration, despite being diagnosed with cholelithiasis. Suboptimal daily lifestyle habits, especially high-calorie and low-fiber eating habits, predispose individuals to the process from gallstone formation to inflammation in the gallbladder or in the bile duct. Among the three exposures, only education attainment played a causal role in attenuating the prevalence of cholecystitis. Furthermore, after adjustment for cholelithiasis, education still acted as an independent protective factor (Table 2), while neither cognition nor intelligence could do the same. In tune with the general acknowledgment, our results convey that individuals obtaining high educational qualifications may have a lower risk of cholecystitis, due to their predominant awareness, optimal lifestyles, balanced diets, etc. Nonetheless, the Nationwide Inpatient Sample database has indicated that White patients have a significantly lower rate of emergent admission of acute cholecystitis compared with non-White patients, suggesting the disparities in health care[63]. Our results are therefore not suppressed to be suitable for other ethnic populations and non-White community dwellers.
Concurrently, massive cardiometabolic risk factors are considered in many syndromes, including cardiovascular diseases[64,65], hepatobiliary disorders[66,67], nervous system disruption[68,69], and reproductive disturbance[70]. For instance, metabolic traits[1,2,8,9], dietary behaviors[15-17], physical activity[18,19], and socioeconomic factors[21] have attracted ubiquitous attention, playing protective roles or serving as risk factors in the process of gallstones. Previous MR studies and observational research have demonstrated the inverse causal association between educational attainment and gallstone disease[24,54], while whether these putative mediators mediate in this process remains unclear. Cardiome
In this study, three major metabolic stratifications were incorporated into the candidate mediators: Adiposity, glucose-related traits, and serum lipid levels. Adiposity can be displayed in several hierarchies—BMI, BF%, waist circumference, WHR, childhood obesity, etc. The detrimental role of these phenotypes in gallstone disease has been emphasized in previous observational and MR studies[71,72]. Four of these factors—BMI, BF%, waist circumference, and WHR—are perceived as the mediators from education to gallstones, excluding childhood obesity, which might account for the cumulative formation process of lithiasis requiring tens of years. Moreover, after adjustment for education, each mediator remained to be a statistically significant risk factor for gallstones. Among them, waist circumference had the highest mediating proportion (44.4%), followed by BF% (40.9%), WHR (32.9%), and BMI (28.4%) (Table 3), which is equivalent to mediating the corresponding extent of the risk of gallstones ascribed to low educational attainment. Commonly, adiposity traits are easily able to reflect the defects of long-range lifestyles and dieting habits. In addition, obesity is closely associated with high-fat, high-carbohydrate, and low-fibre intakes and less movement. Notably, these obesity-relevant and interrelated indexes carry substantial public health implications, as they are prone to exist as comorbidities and have common biological mechanisms, including genetics, energy metabolism, neuroendocrine regulation, and immunoinflammatory activation[73,74]. Thus, to attenuate the prevalence of gallstones, none of these phenotypes should receive less attention, but instead, they deserve more consideration.
Glucose-related traits have been extensively discussed as risk factors for gallstone disease in previous studies[11,12]. Yuan et al[24] validated the causal association between type 2 diabetes and gallstone disease [OR: 1.13 (95%CI: 1.09-1.17)] through the two-sample MR method, demonstrating its facilitating effectiveness. Considering that the foremost presence of pancreatic impairment in type 2 diabetes is the abnormal serum sugar-relevant level[75], fasting glucose and fasting insulin alike were embodied in our candidate mediators. However, none of the putative mediators were estimated to be definite mediators, which does not indicate a lack of the unimportance or necessity for taking precautions against diabetes-induced factors, which might concurrently promote gallstone shaping.
In general, the major proportion of gallstones in the Western world are cholesterol gallstones, instead of pigment components. Surprisingly, serum lipid levels were excluded as mediators in our study, although hyperlipidemia has been illustrated to be involved in gallstone diseases, based on compelling observational studies[13,14,76,77]. To our know
Physical activity has been demonstrated to play a preventive role in the process of gallstone formation, based on recent observational and MR studies[20,79,80]. Generally, watching TV is an intriguing way to ease anxiety, obtain pleasure, and kill time. Nonetheless, a long-term sedentary lifestyle increases the risk of subhealth problems, notably obesity, nonalcoholic fatty liver diseases (NAFLD), and even cancer[81-84]. This study first illustrated that increasing TV watching time has a causal effect on the formation of gallstones, from a genetic perspective. Importantly, after adjustment for educational attainment, cognitive performance, or intelligence, long-term watching TV still causally increased the risk of gallstone disease. Moreover, it played a mediating role in the process from education to gallstone (mediated proportion: 41.6%), as well as from cognitive performance (20.4%) or intelligence level to the counterpart (28.4%). It has been acknowledged that those who have undergone many years of schooling are more able to perceive the detrimental effects of watching TV, and instead, they prefer other beneficial events. A putative inference of the hazardous pathogenesis can be explained as follows: When absorbed into the colorful scenes in TV series, individuals are more likely to stay motionless for several hours, inhibiting intestinal motility and restraining cholecystokinin-dependent gallbladder emptying[85,86]. Apart from the time spent watching TV, we simultaneously focused on two other candidate mediators: MVPA and computer use. Although neither factor was not perceived to be a mediator, they deserve to be considered for individuals at homes, who should perform certain physical activities frequently and compulsorily avoid long-term concentrated computer use.
In conclusion, this is the first MR study to reveal that the causal effects of education on gallstone disease are independent of cognition and intelligence, although either can play a causal role in gallstone pathogenesis. Second, we are also the first to illustrate that individuals with high-education attainment may be substantially less likely suffer from cholecystitis, after being diagnosed with gallstone disease. Third, we strictly screened out the causal mediator, meeting the set rigorous criteria, in the independent association of education, cognition, or intelligence with gallstones, and afterward estimated the mediated proportion individually.
Nevertheless, this study also has several limitations to be addressed. First, several prevalent and crucial cardiome
Education, cognition, and intelligence all play crucial roles in the development of cholelithiasis, and several cardiometabolic mediators have been identified for prevention of cholelithiasis due to defects in each exposure.
The authors thank all the studies and participants contributing the data used in this work.
| 1. | Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ, Wang DQ. Gallstones. Nat Rev Dis Primers. 2016;2:16024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 516] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65:146-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (2)] |
| 3. | Elwood DR. Cholecystitis. Surg Clin North Am. 2008;88:1241-1252, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract Res Clin Gastroenterol. 2006;20:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 5. | McMahon AJ, Fischbacher CM, Frame SH, MacLeod MC. Impact of laparoscopic cholecystectomy: a population-based study. Lancet. 2000;356:1632-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Chen AY, Daley J, Pappas TN, Henderson WG, Khuri SF. Growing use of laparoscopic cholecystectomy in the national Veterans Affairs Surgical Risk Study: effects on volume, patient selection, and selected outcomes. Ann Surg. 1998;227:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Pucher PH, Brunt LM, Davies N, Linsk A, Munshi A, Rodriguez HA, Fingerhut A, Fanelli RD, Asbun H, Aggarwal R; SAGES Safe Cholecystectomy Task Force. Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc. 2018;32:2175-2183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (1)] |
| 8. | Wirth J, Joshi AD, Song M, Lee DH, Tabung FK, Fung TT, Chan AT, Weikert C, Leitzmann M, Willett WC, Giovannucci E, Wu K. A healthy lifestyle pattern and the risk of symptomatic gallstone disease: results from 2 prospective cohort studies. Am J Clin Nutr. 2020;112:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Aune D, Norat T, Vatten LJ. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol. 2015;30:1009-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Ruhl CE, Everhart JE. Relationship of serum leptin concentration and other measures of adiposity with gallbladder disease. Hepatology. 2001;34:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Aune D, Vatten LJ. Diabetes mellitus and the risk of gallbladder disease: A systematic review and meta-analysis of prospective studies. J Diabetes Complications. 2016;30:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Sasazuki S, Kono S, Todoroki I, Honjo S, Sakurai Y, Wakabayashi K, Nishiwaki M, Hamada H, Nishikawa H, Koga H, Ogawa S, Nakagawa K. Impaired glucose tolerance, diabetes mellitus, and gallstone disease: an extended study of male self-defense officials in Japan. Eur J Epidemiol. 1999;15:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Chang CM, Chiu THT, Chang CC, Lin MN, Lin CL. Plant-Based Diet, Cholesterol, and Risk of Gallstone Disease: A Prospective Study. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Einarsson K, Hellström K, Kallner M. Gallbladder disease in hyperlipoproteinaemia. Lancet. 1975;1:484-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Zhang YP, Li WQ, Sun YL, Zhu RT, Wang WJ. Systematic review with meta-analysis: coffee consumption and the risk of gallstone disease. Aliment Pharmacol Ther. 2015;42:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Cha BH, Jang MJ, Lee SH. Alcohol Consumption Can Reduce the Risk of Gallstone Disease: A Systematic Review with a Dose-Response Meta-Analysis of Case-Control and Cohort Studies. Gut Liver. 2019;13:114-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Aune D, Vatten LJ, Boffetta P. Tobacco smoking and the risk of gallbladder disease. Eur J Epidemiol. 2016;31:643-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Aune D, Leitzmann M, Vatten LJ. Physical Activity and the Risk of Gallbladder Disease: A Systematic Review and Meta-Analysis of Cohort Studies. J Phys Act Health. 2016;13:788-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 1386] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 20. | Qian Q, Jiang H, Cai B, Chen D, Jiang M. Physical activity and risk of gallstone disease: A Mendelian randomization study. Front Genet. 2022;13:943353. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Gonzalez Villalpando C, Rivera Martinez D, Arredondo Perez B, Martinez Diaz S, Gonzalez Villalpando ME, Haffner SM, Stern MP. High prevalence of cholelithiasis in a low income Mexican population: an ultrasonographic survey. Arch Med Res. 1997;28:543-547. [PubMed] |
| 22. | Pak M, Lindseth G. Risk Factors for Cholelithiasis. Gastroenterol Nurs. 2016;39:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Ibrahim M, Sarvepalli S, Morris-Stiff G, Rizk M, Bhatt A, Walsh RM, Hayat U, Garber A, Vargo J, Burke CA. Gallstones: Watch and wait, or intervene? Cleve Clin J Med. 2018;85:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Yuan S, Gill D, Giovannucci EL, Larsson SC. Obesity, Type 2 Diabetes, Lifestyle Factors, and Risk of Gallstone Disease: A Mendelian Randomization Investigation. Clin Gastroenterol Hepatol. 2022;20:e529-e537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (1)] |
| 25. | Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, Nagel M, Awasthi S, Barr PB, Coleman JRI, Grasby KL, Hammerschlag AR, Kaminski JA, Karlsson R, Krapohl E, Lam M, Nygaard M, Reynolds CA, Trampush JW, Young H, Zabaneh D, Hägg S, Hansell NK, Karlsson IK, Linnarsson S, Montgomery GW, Muñoz-Manchado AB, Quinlan EB, Schumann G, Skene NG, Webb BT, White T, Arking DE, Avramopoulos D, Bilder RM, Bitsios P, Burdick KE, Cannon TD, Chiba-Falek O, Christoforou A, Cirulli ET, Congdon E, Corvin A, Davies G, Deary IJ, DeRosse P, Dickinson D, Djurovic S, Donohoe G, Conley ED, Eriksson JG, Espeseth T, Freimer NA, Giakoumaki S, Giegling I, Gill M, Glahn DC, Hariri AR, Hatzimanolis A, Keller MC, Knowles E, Koltai D, Konte B, Lahti J, Le Hellard S, Lencz T, Liewald DC, London E, Lundervold AJ, Malhotra AK, Melle I, Morris D, Need AC, Ollier W, Palotie A, Payton A, Pendleton N, Poldrack RA, Räikkönen K, Reinvang I, Roussos P, Rujescu D, Sabb FW, Scult MA, Smeland OB, Smyrnis N, Starr JM, Steen VM, Stefanis NC, Straub RE, Sundet K, Tiemeier H, Voineskos AN, Weinberger DR, Widen E, Yu J, Abecasis G, Andreassen OA, Breen G, Christiansen L, Debrabant B, Dick DM, Heinz A, Hjerling-Leffler J, Ikram MA, Kendler KS, Martin NG, Medland SE, Pedersen NL, Plomin R, Polderman TJC, Ripke S, van der Sluis S, Sullivan PF, Vrieze SI, Wright MJ, Posthuma D. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 758] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 26. | Mocan N, Altindag DT. Education, cognition, health knowledge, and health behavior. Eur J Health Econ. 2014;15:265-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Ye C, Kong L, Zheng J, Xu M, Xu Y, Li M, Zhao Z, Lu J, Chen Y, Wang W, Ning G, Bi Y, Wang T. Independent Associations of Education, Intelligence, and Cognition With Hypertension and the Mediating Effects of Cardiometabolic Risk Factors: A Mendelian Randomization Study. Hypertension. 2023;80:192-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 28. | Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3525] [Cited by in RCA: 3008] [Article Influence: 176.9] [Reference Citation Analysis (0)] |
| 29. | Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48:713-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 737] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 30. | Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, Taylor AE, Davies NM, Howe LD. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36:465-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 653] [Article Influence: 163.3] [Reference Citation Analysis (0)] |
| 31. | Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, Langenberg C, Golub RM, Loder EW, Gallo V, Tybjaerg-Hansen A, Davey Smith G, Egger M, Richards JB. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326:1614-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 2081] [Article Influence: 520.3] [Reference Citation Analysis (0)] |
| 32. | Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, Nguyen-Viet TA, Bowers P, Sidorenko J, Karlsson Linnér R, Fontana MA, Kundu T, Lee C, Li H, Li R, Royer R, Timshel PN, Walters RK, Willoughby EA, Yengo L; 23andMe Research Team; COGENT (Cognitive Genomics Consortium); Social Science Genetic Association Consortium, Alver M, Bao Y, Clark DW, Day FR, Furlotte NA, Joshi PK, Kemper KE, Kleinman A, Langenberg C, Mägi R, Trampush JW, Verma SS, Wu Y, Lam M, Zhao JH, Zheng Z, Boardman JD, Campbell H, Freese J, Harris KM, Hayward C, Herd P, Kumari M, Lencz T, Luan J, Malhotra AK, Metspalu A, Milani L, Ong KK, Perry JRB, Porteous DJ, Ritchie MD, Smart MC, Smith BH, Tung JY, Wareham NJ, Wilson JF, Beauchamp JP, Conley DC, Esko T, Lehrer SF, Magnusson PKE, Oskarsson S, Pers TH, Robinson MR, Thom K, Watson C, Chabris CF, Meyer MN, Laibson DI, Yang J, Johannesson M, Koellinger PD, Turley P, Visscher PM, Benjamin DJ, Cesarini D. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1491] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 33. | Trampush JW, Yang ML, Yu J, Knowles E, Davies G, Liewald DC, Starr JM, Djurovic S, Melle I, Sundet K, Christoforou A, Reinvang I, DeRosse P, Lundervold AJ, Steen VM, Espeseth T, Räikkönen K, Widen E, Palotie A, Eriksson JG, Giegling I, Konte B, Roussos P, Giakoumaki S, Burdick KE, Payton A, Ollier W, Horan M, Chiba-Falek O, Attix DK, Need AC, Cirulli ET, Voineskos AN, Stefanis NC, Avramopoulos D, Hatzimanolis A, Arking DE, Smyrnis N, Bilder RM, Freimer NA, Cannon TD, London E, Poldrack RA, Sabb FW, Congdon E, Conley ED, Scult MA, Dickinson D, Straub RE, Donohoe G, Morris D, Corvin A, Gill M, Hariri AR, Weinberger DR, Pendleton N, Bitsios P, Rujescu D, Lahti J, Le Hellard S, Keller MC, Andreassen OA, Deary IJ, Glahn DC, Malhotra AK, Lencz T. GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: a report from the COGENT consortium. Mol Psychiatry. 2017;22:336-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 34. | Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, Frayling TM, Hirschhorn J, Yang J, Visscher PM; GIANT Consortium. Meta-analysis of genome-wide association studies for height and body mass index in ~700000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641-3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1484] [Article Influence: 247.3] [Reference Citation Analysis (0)] |
| 35. | Lu Y, Day FR, Gustafsson S, Buchkovich ML, Na J, Bataille V, Cousminer DL, Dastani Z, Drong AW, Esko T, Evans DM, Falchi M, Feitosa MF, Ferreira T, Hedman ÅK, Haring R, Hysi PG, Iles MM, Justice AE, Kanoni S, Lagou V, Li R, Li X, Locke A, Lu C, Mägi R, Perry JR, Pers TH, Qi Q, Sanna M, Schmidt EM, Scott WR, Shungin D, Teumer A, Vinkhuyzen AA, Walker RW, Westra HJ, Zhang M, Zhang W, Zhao JH, Zhu Z, Afzal U, Ahluwalia TS, Bakker SJ, Bellis C, Bonnefond A, Borodulin K, Buchman AS, Cederholm T, Choh AC, Choi HJ, Curran JE, de Groot LC, De Jager PL, Dhonukshe-Rutten RA, Enneman AW, Eury E, Evans DS, Forsen T, Friedrich N, Fumeron F, Garcia ME, Gärtner S, Han BG, Havulinna AS, Hayward C, Hernandez D, Hillege H, Ittermann T, Kent JW, Kolcic I, Laatikainen T, Lahti J, Mateo Leach I, Lee CG, Lee JY, Liu T, Liu Y, Lobbens S, Loh M, Lyytikäinen LP, Medina-Gomez C, Michaëlsson K, Nalls MA, Nielson CM, Oozageer L, Pascoe L, Paternoster L, Polašek O, Ripatti S, Sarzynski MA, Shin CS, Narančić NS, Spira D, Srikanth P, Steinhagen-Thiessen E, Sung YJ, Swart KM, Taittonen L, Tanaka T, Tikkanen E, van der Velde N, van Schoor NM, Verweij N, Wright AF, Yu L, Zmuda JM, Eklund N, Forrester T, Grarup N, Jackson AU, Kristiansson K, Kuulasmaa T, Kuusisto J, Lichtner P, Luan J, Mahajan A, Männistö S, Palmer CD, Ried JS, Scott RA, Stancáková A, Wagner PJ, Demirkan A, Döring A, Gudnason V, Kiel DP, Kühnel B, Mangino M, Mcknight B, Menni C, O'Connell JR, Oostra BA, Shuldiner AR, Song K, Vandenput L, van Duijn CM, Vollenweider P, White CC, Boehnke M, Boettcher Y, Cooper RS, Forouhi NG, Gieger C, Grallert H, Hingorani A, Jørgensen T, Jousilahti P, Kivimaki M, Kumari M, Laakso M, Langenberg C, Linneberg A, Luke A, Mckenzie CA, Palotie A, Pedersen O, Peters A, Strauch K, Tayo BO, Wareham NJ, Bennett DA, Bertram L, Blangero J, Blüher M, Bouchard C, Campbell H, Cho NH, Cummings SR, Czerwinski SA, Demuth I, Eckardt R, Eriksson JG, Ferrucci L, Franco OH, Froguel P, Gansevoort RT, Hansen T, Harris TB, Hastie N, Heliövaara M, Hofman A, Jordan JM, Jula A, Kähönen M, Kajantie E, Knekt PB, Koskinen S, Kovacs P, Lehtimäki T, Lind L, Liu Y, Orwoll ES, Osmond C, Perola M, Pérusse L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Rivadeneira F, Rudan I, Salomaa V, Sørensen TI, Stumvoll M, Tönjes A, Towne B, Tranah GJ, Tremblay A, Uitterlinden AG, van der Harst P, Vartiainen E, Viikari JS, Vitart V, Vohl MC, Völzke H, Walker M, Wallaschofski H, Wild S, Wilson JF, Yengo L, Bishop DT, Borecki IB, Chambers JC, Cupples LA, Dehghan A, Deloukas P, Fatemifar G, Fox C, Furey TS, Franke L, Han J, Hunter DJ, Karjalainen J, Karpe F, Kaplan RC, Kooner JS, McCarthy MI, Murabito JM, Morris AP, Bishop JA, North KE, Ohlsson C, Ong KK, Prokopenko I, Richards JB, Schadt EE, Spector TD, Widén E, Willer CJ, Yang J, Ingelsson E, Mohlke KL, Hirschhorn JN, Pospisilik JA, Zillikens MC, Lindgren C, Kilpeläinen TO, Loos RJ. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016;7:10495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 36. | Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, Strawbridge RJ, Pers TH, Fischer K, Justice AE, Workalemahu T, Wu JMW, Buchkovich ML, Heard-Costa NL, Roman TS, Drong AW, Song C, Gustafsson S, Day FR, Esko T, Fall T, Kutalik Z, Luan J, Randall JC, Scherag A, Vedantam S, Wood AR, Chen J, Fehrmann R, Karjalainen J, Kahali B, Liu CT, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bragg-Gresham JL, Buyske S, Demirkan A, Ehret GB, Feitosa MF, Goel A, Jackson AU, Johnson T, Kleber ME, Kristiansson K, Mangino M, Leach IM, Medina-Gomez C, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Stančáková A, Sung YJ, Tanaka T, Teumer A, Van Vliet-Ostaptchouk JV, Yengo L, Zhang W, Albrecht E, Ärnlöv J, Arscott GM, Bandinelli S, Barrett A, Bellis C, Bennett AJ, Berne C, Blüher M, Böhringer S, Bonnet F, Böttcher Y, Bruinenberg M, Carba DB, Caspersen IH, Clarke R, Daw EW, Deelen J, Deelman E, Delgado G, Doney AS, Eklund N, Erdos MR, Estrada K, Eury E, Friedrich N, Garcia ME, Giedraitis V, Gigante B, Go AS, Golay A, Grallert H, Grammer TB, Gräßler J, Grewal J, Groves CJ, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heikkilä K, Herzig KH, Helmer Q, Hillege HL, Holmen O, Hunt SC, Isaacs A, Ittermann T, James AL, Johansson I, Juliusdottir T, Kalafati IP, Kinnunen L, Koenig W, Kooner IK, Kratzer W, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lobbens S, Lorentzon M, Mach F, Magnusson PK, Mahajan A, McArdle WL, Menni C, Merger S, Mihailov E, Milani L, Mills R, Moayyeri A, Monda KL, Mooijaart SP, Mühleisen TW, Mulas A, Müller G, Müller-Nurasyid M, Nagaraja R, Nalls MA, Narisu N, Glorioso N, Nolte IM, Olden M, Rayner NW, Renstrom F, Ried JS, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Sennblad B, Seufferlein T, Sitlani CM, Smith AV, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tayo BO, Thorand B, Thorleifsson G, Tomaschitz A, Troffa C, van Oort FV, Verweij N, Vonk JM, Waite LL, Wennauer R, Wilsgaard T, Wojczynski MK, Wong A, Zhang Q, Zhao JH, Brennan EP, Choi M, Eriksson P, Folkersen L, Franco-Cereceda A, Gharavi AG, Hedman ÅK, Hivert MF, Huang J, Kanoni S, Karpe F, Keildson S, Kiryluk K, Liang L, Lifton RP, Ma B, McKnight AJ, McPherson R, Metspalu A, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Olsson C, Perry JR, Reinmaa E, Salem RM, Sandholm N, Schadt EE, Scott RA, Stolk L, Vallejo EE, Westra HJ, Zondervan KT; ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGC; ICBP; International Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE Consortium; ReproGen Consortium, Amouyel P, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Blangero J, Brown MJ, Burnier M, Campbell H, Chakravarti A, Chines PS, Claudi-Boehm S, Collins FS, Crawford DC, Danesh J, de Faire U, de Geus EJ, Dörr M, Erbel R, Eriksson JG, Farrall M, Ferrannini E, Ferrières J, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gieger C, Gudnason V, Haiman CA, Harris TB, Hattersley AT, Heliövaara M, Hicks AA, Hingorani AD, Hoffmann W, Hofman A, Homuth G, Humphries SE, Hyppönen E, Illig T, Jarvelin MR, Johansen B, Jousilahti P, Jula AM, Kaprio J, Kee F, Keinanen-Kiukaanniemi SM, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuulasmaa K, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Musk AW, Möhlenkamp S, Morris AD, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Palmer LJ, Penninx BW, Peters A, Pramstaller PP, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schwarz PE, Shuldiner AR, Staessen JA, Steinthorsdottir V, Stolk RP, Strauch K, Tönjes A, Tremblay A, Tremoli E, Vohl MC, Völker U, Vollenweider P, Wilson JF, Witteman JC, Adair LS, Bochud M, Boehm BO, Bornstein SR, Bouchard C, Cauchi S, Caulfield MJ, Chambers JC, Chasman DI, Cooper RS, Dedoussis G, Ferrucci L, Froguel P, Grabe HJ, Hamsten A, Hui J, Hveem K, Jöckel KH, Kivimaki M, Kuh D, Laakso M, Liu Y, März W, Munroe PB, Njølstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sinisalo J, Slagboom PE, Snieder H, Spector TD, Stefansson K, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Veronesi G, Walker M, Wareham NJ, Watkins H, Wichmann HE, Abecasis GR, Assimes TL, Berndt SI, Boehnke M, Borecki IB, Deloukas P, Franke L, Frayling TM, Groop LC, Hunter DJ, Kaplan RC, O'Connell JR, Qi L, Schlessinger D, Strachan DP, Thorsteinsdottir U, van Duijn CM, Willer CJ, Visscher PM, Yang J, Hirschhorn JN, Zillikens MC, McCarthy MI, Speliotes EK, North KE, Fox CS, Barroso I, Franks PW, Ingelsson E, Heid IM, Loos RJ, Cupples LA, Morris AP, Lindgren CM, Mohlke KL. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1401] [Cited by in RCA: 1181] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 37. | Bradfield JP, Taal HR, Timpson NJ, Scherag A, Lecoeur C, Warrington NM, Hypponen E, Holst C, Valcarcel B, Thiering E, Salem RM, Schumacher FR, Cousminer DL, Sleiman PM, Zhao J, Berkowitz RI, Vimaleswaran KS, Jarick I, Pennell CE, Evans DM, St Pourcain B, Berry DJ, Mook-Kanamori DO, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, van der Valk RJ, de Jongste JC, Postma DS, Boomsma DI, Gauderman WJ, Hassanein MT, Lindgren CM, Mägi R, Boreham CA, Neville CE, Moreno LA, Elliott P, Pouta A, Hartikainen AL, Li M, Raitakari O, Lehtimäki T, Eriksson JG, Palotie A, Dallongeville J, Das S, Deloukas P, McMahon G, Ring SM, Kemp JP, Buxton JL, Blakemore AI, Bustamante M, Guxens M, Hirschhorn JN, Gillman MW, Kreiner-Møller E, Bisgaard H, Gilliland FD, Heinrich J, Wheeler E, Barroso I, O'Rahilly S, Meirhaeghe A, Sørensen TI, Power C, Palmer LJ, Hinney A, Widen E, Farooqi IS, McCarthy MI, Froguel P, Meyre D, Hebebrand J, Jarvelin MR, Jaddoe VW, Smith GD, Hakonarson H, Grant SF; Early Growth Genetics Consortium. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. 2012;44:526-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 38. | Bonàs-Guarch S, Guindo-Martínez M, Miguel-Escalada I, Grarup N, Sebastian D, Rodriguez-Fos E, Sánchez F, Planas-Fèlix M, Cortes-Sánchez P, González S, Timshel P, Pers TH, Morgan CC, Moran I, Atla G, González JR, Puiggros M, Martí J, Andersson EA, Díaz C, Badia RM, Udler M, Leong A, Kaur V, Flannick J, Jørgensen T, Linneberg A, Jørgensen ME, Witte DR, Christensen C, Brandslund I, Appel EV, Scott RA, Luan J, Langenberg C, Wareham NJ, Pedersen O, Zorzano A, Florez JC, Hansen T, Ferrer J, Mercader JM, Torrents D. Publisher Correction: Re-analysis of public genetic data reveals a rare X-chromosomal variant associated with type 2 diabetes. Nat Commun. 2018;9:2162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, Mägi R, Strawbridge RJ, Rehnberg E, Gustafsson S, Kanoni S, Rasmussen-Torvik LJ, Yengo L, Lecoeur C, Shungin D, Sanna S, Sidore C, Johnson PC, Jukema JW, Johnson T, Mahajan A, Verweij N, Thorleifsson G, Hottenga JJ, Shah S, Smith AV, Sennblad B, Gieger C, Salo P, Perola M, Timpson NJ, Evans DM, Pourcain BS, Wu Y, Andrews JS, Hui J, Bielak LF, Zhao W, Horikoshi M, Navarro P, Isaacs A, O'Connell JR, Stirrups K, Vitart V, Hayward C, Esko T, Mihailov E, Fraser RM, Fall T, Voight BF, Raychaudhuri S, Chen H, Lindgren CM, Morris AP, Rayner NW, Robertson N, Rybin D, Liu CT, Beckmann JS, Willems SM, Chines PS, Jackson AU, Kang HM, Stringham HM, Song K, Tanaka T, Peden JF, Goel A, Hicks AA, An P, Müller-Nurasyid M, Franco-Cereceda A, Folkersen L, Marullo L, Jansen H, Oldehinkel AJ, Bruinenberg M, Pankow JS, North KE, Forouhi NG, Loos RJ, Edkins S, Varga TV, Hallmans G, Oksa H, Antonella M, Nagaraja R, Trompet S, Ford I, Bakker SJ, Kong A, Kumari M, Gigante B, Herder C, Munroe PB, Caulfield M, Antti J, Mangino M, Small K, Miljkovic I, Liu Y, Atalay M, Kiess W, James AL, Rivadeneira F, Uitterlinden AG, Palmer CN, Doney AS, Willemsen G, Smit JH, Campbell S, Polasek O, Bonnycastle LL, Hercberg S, Dimitriou M, Bolton JL, Fowkes GR, Kovacs P, Lindström J, Zemunik T, Bandinelli S, Wild SH, Basart HV, Rathmann W, Grallert H; DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium, Maerz W, Kleber ME, Boehm BO, Peters A, Pramstaller PP, Province MA, Borecki IB, Hastie ND, Rudan I, Campbell H, Watkins H, Farrall M, Stumvoll M, Ferrucci L, Waterworth DM, Bergman RN, Collins FS, Tuomilehto J, Watanabe RM, de Geus EJ, Penninx BW, Hofman A, Oostra BA, Psaty BM, Vollenweider P, Wilson JF, Wright AF, Hovingh GK, Metspalu A, Uusitupa M, Magnusson PK, Kyvik KO, Kaprio J, Price JF, Dedoussis GV, Deloukas P, Meneton P, Lind L, Boehnke M, Shuldiner AR, van Duijn CM, Morris AD, Toenjes A, Peyser PA, Beilby JP, Körner A, Kuusisto J, Laakso M, Bornstein SR, Schwarz PE, Lakka TA, Rauramaa R, Adair LS, Smith GD, Spector TD, Illig T, de Faire U, Hamsten A, Gudnason V, Kivimaki M, Hingorani A, Keinanen-Kiukaanniemi SM, Saaristo TE, Boomsma DI, Stefansson K, van der Harst P, Dupuis J, Pedersen NL, Sattar N, Harris TB, Cucca F, Ripatti S, Salomaa V, Mohlke KL, Balkau B, Froguel P, Pouta A, Jarvelin MR, Wareham NJ, Bouatia-Naji N, McCarthy MI, Franks PW, Meigs JB, Teslovich TM, Florez JC, Langenberg C, Ingelsson E, Prokopenko I, Barroso I. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 719] [Cited by in RCA: 657] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 40. | Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson Å, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PKE, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O'Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney ASF, Döring A, Elliott P, Epstein SE, Ingi Eyjolfsson G, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJP, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJF, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TVM, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YI, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PEH, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BHR, Ordovas JM, Boerwinkle E, Palmer CNA, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E, Abecasis GR; Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2340] [Cited by in RCA: 2324] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 41. | Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, Davila-Velderrain J, McGuire D, Tian C, Zhan X; 23andMe Research Team; HUNT All-In Psychiatry, Choquet H, Docherty AR, Faul JD, Foerster JR, Fritsche LG, Gabrielsen ME, Gordon SD, Haessler J, Hottenga JJ, Huang H, Jang SK, Jansen PR, Ling Y, Mägi R, Matoba N, McMahon G, Mulas A, Orrù V, Palviainen T, Pandit A, Reginsson GW, Skogholt AH, Smith JA, Taylor AE, Turman C, Willemsen G, Young H, Young KA, Zajac GJM, Zhao W, Zhou W, Bjornsdottir G, Boardman JD, Boehnke M, Boomsma DI, Chen C, Cucca F, Davies GE, Eaton CB, Ehringer MA, Esko T, Fiorillo E, Gillespie NA, Gudbjartsson DF, Haller T, Harris KM, Heath AC, Hewitt JK, Hickie IB, Hokanson JE, Hopfer CJ, Hunter DJ, Iacono WG, Johnson EO, Kamatani Y, Kardia SLR, Keller MC, Kellis M, Kooperberg C, Kraft P, Krauter KS, Laakso M, Lind PA, Loukola A, Lutz SM, Madden PAF, Martin NG, McGue M, McQueen MB, Medland SE, Metspalu A, Mohlke KL, Nielsen JB, Okada Y, Peters U, Polderman TJC, Posthuma D, Reiner AP, Rice JP, Rimm E, Rose RJ, Runarsdottir V, Stallings MC, Stančáková A, Stefansson H, Thai KK, Tindle HA, Tyrfingsson T, Wall TL, Weir DR, Weisner C, Whitfield JB, Winsvold BS, Yin J, Zuccolo L, Bierut LJ, Hveem K, Lee JJ, Munafò MR, Saccone NL, Willer CJ, Cornelis MC, David SP, Hinds DA, Jorgenson E, Kaprio J, Stitzel JA, Stefansson K, Thorgeirsson TE, Abecasis G, Liu DJ, Vrieze S. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1465] [Cited by in RCA: 1305] [Article Influence: 217.5] [Reference Citation Analysis (0)] |
| 42. | Zhong VW, Kuang A, Danning RD, Kraft P, van Dam RM, Chasman DI, Cornelis MC. A genome-wide association study of bitter and sweet beverage consumption. Hum Mol Genet. 2019;28:2449-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 43. | Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, Alexander GE, Chen Z, Going SB. Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes (Lond). 2018;42:1161-1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 269] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 44. | van de Vegte YJ, Said MA, Rienstra M, van der Harst P, Verweij N. Genome-wide association studies and Mendelian randomization analyses for leisure sedentary behaviours. Nat Commun. 2020;11:1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 45. | Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4747] [Cited by in RCA: 4854] [Article Influence: 693.4] [Reference Citation Analysis (0)] |
| 46. | Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6354] [Cited by in RCA: 7487] [Article Influence: 748.7] [Reference Citation Analysis (0)] |
| 47. | Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27:R195-R208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 794] [Cited by in RCA: 988] [Article Influence: 141.1] [Reference Citation Analysis (0)] |
| 48. | Relton CL, Davey Smith G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. 2012;41:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 49. | MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4557] [Cited by in RCA: 3235] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 50. | Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 2904] [Article Influence: 363.0] [Reference Citation Analysis (0)] |
| 51. | Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4015] [Cited by in RCA: 5755] [Article Influence: 639.4] [Reference Citation Analysis (0)] |
| 52. | Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 5398] [Article Influence: 771.1] [Reference Citation Analysis (0)] |
| 53. | Diehl AK, Schwesinger WH, Holleman DR Jr, Chapman JB, Kurtin WE. Clinical correlates of gallstone composition: distinguishing pigment from cholesterol stones. Am J Gastroenterol. 1995;90:967-972. [PubMed] |
| 54. | Wagener DK, McDonald M. Increased gallbladder-related mortality among Hispanics: does education play a role? Ethn Health. 1996;1:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Carey MC, Paigen B. Epidemiology of the American Indians' burden and its likely genetic origins. Hepatology. 2002;36:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Stokes CS, Krawczyk M, Lammert F. Gallstones: environment, lifestyle and genes. Dig Dis. 2011;29:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 57. | Lawrence EM. Why Do College Graduates Behave More Healthfully than Those Who Are Less Educated? J Health Soc Behav. 2017;58:291-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 58. | Knab LM, Boller AM, Mahvi DM. Cholecystitis. Surg Clin North Am. 2014;94:455-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 59. | Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, Kozaka K, Endo I, Deziel DJ, Miura F, Okamoto K, Hwang TL, Huang WS, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Noguchi Y, Shikata S, Ukai T, Higuchi R, Gabata T, Mori Y, Iwashita Y, Hibi T, Jagannath P, Jonas E, Liau KH, Dervenis C, Gouma DJ, Cherqui D, Belli G, Garden OJ, Giménez ME, de Santibañes E, Suzuki K, Umezawa A, Supe AN, Pitt HA, Singh H, Chan ACW, Lau WY, Teoh AYB, Honda G, Sugioka A, Asai K, Gomi H, Itoi T, Kiriyama S, Yoshida M, Mayumi T, Matsumura N, Tokumura H, Kitano S, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 689] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 60. | Banz V, Gsponer T, Candinas D, Güller U. Population-based analysis of 4113 patients with acute cholecystitis: defining the optimal time-point for laparoscopic cholecystectomy. Ann Surg. 2011;254:964-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Schwartz DA, Shah AA, Zogg CK, Nicholas LH, Velopulos CG, Efron DT, Schneider EB, Haider AH. Operative delay to laparoscopic cholecystectomy: Racking up the cost of health care. J Trauma Acute Care Surg. 2015;79:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Cafasso DE, Smith RR. Symptomatic cholelithiasis and functional disorders of the biliary tract. Surg Clin North Am. 2014;94:233-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Gahagan JV, Hanna MH, Whealon MD, Maximus S, Phelan MJ, Lekawa M, Barrios C, Bernal NP. Racial Disparities in Access and Outcomes of Cholecystectomy in the United States. Am Surg. 2016;82:921-925. [PubMed] |
| 64. | Braffett BH, Bebu I, El Ghormli L, Cowie CC, Sivitz WI, Pop-Busui R, Larkin ME, Gubitosi-Klug RA, Nathan DM, Lachin JM, Dagogo-Jack S; DCCT/EDIC Research Group. Cardiometabolic Risk Factors and Incident Cardiovascular Disease Events in Women vs Men With Type 1 Diabetes. JAMA Netw Open. 2022;5:e2230710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 65. | Liu J, Tse LA, Liu Z, Rangarajan S, Hu B, Yin L, Leong DP, Li W; PURE (Prospective Urban Rural Epidemiology) study in China. Predictive Values of Anthropometric Measurements for Cardiometabolic Risk Factors and Cardiovascular Diseases Among 44 048 Chinese. J Am Heart Assoc. 2019;8:e010870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 66. | Muzurović E, Peng CC, Belanger MJ, Sanoudou D, Mikhailidis DP, Mantzoros CS. Nonalcoholic Fatty Liver Disease and Cardiovascular Disease: a Review of Shared Cardiometabolic Risk Factors. Hypertension. 2022;79:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 67. | Pirola CJ, Sookoian S. NAFLD and Cardiometabolic Risk Factors: The Liver Fibrosis Trajectory Through the Lens of Biological Interactions. Hepatology. 2021;73:479-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Osborne MT, Shin LM, Mehta NN, Pitman RK, Fayad ZA, Tawakol A. Disentangling the Links Between Psychosocial Stress and Cardiovascular Disease. Circ Cardiovasc Imaging. 2020;13:e010931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 69. | Lambert EA, Straznicky NE, Dixon JB, Lambert GW. Should the sympathetic nervous system be a target to improve cardiometabolic risk in obesity? Am J Physiol Heart Circ Physiol. 2015;309:H244-H258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 70. | O'Kelly AC, Michos ED, Shufelt CL, Vermunt JV, Minissian MB, Quesada O, Smith GN, Rich-Edwards JW, Garovic VD, El Khoudary SR, Honigberg MC. Pregnancy and Reproductive Risk Factors for Cardiovascular Disease in Women. Circ Res. 2022;130:652-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 170] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 71. | Chen L, Yang H, Li H, He C, Yang L, Lv G. Insights into modifiable risk factors of cholelithiasis: A Mendelian randomization study. Hepatology. 2022;75:785-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 170] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 72. | Lim J, Wirth J, Wu K, Giovannucci E, Kraft P, Turman C, Song M, Jovani M, Chan AT, Joshi AD. Obesity, Adiposity, and Risk of Symptomatic Gallstone Disease According to Genetic Susceptibility. Clin Gastroenterol Hepatol. 2022;20:e1083-e1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 73. | Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 555] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 74. | Freedman DS, Thornton JC, Pi-Sunyer FX, Heymsfield SB, Wang J, Pierson RN Jr, Blanck HM, Gallagher D. The body adiposity index (hip circumference ÷ height(1.5)) is not a more accurate measure of adiposity than is BMI, waist circumference, or hip circumference. Obesity (Silver Spring). 2012;20:2438-2444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 75. | Taylor R. Type 2 diabetes: etiology and reversibility. Diabetes Care. 2013;36:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 76. | Wang J, Shen S, Wang B, Ni X, Liu H, Ni X, Yu R, Suo T, Liu H. Serum lipid levels are the risk factors of gallbladder stones: a population-based study in China. Lipids Health Dis. 2020;19:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 77. | Thijs C, Knipschild P, Brombacher P. Serum lipids and gallstones: a case-control study. Gastroenterology. 1990;99:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Lyons MA, Wittenburg H. Cholesterol gallstone susceptibility loci: a mouse map, candidate gene evaluation, and guide to human LITH genes. Gastroenterology. 2006;131:1943-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Gutt C, Schläfer S, Lammert F. The Treatment of Gallstone Disease. Dtsch Arztebl Int. 2020;117:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 80. | Zhang YP, Zhao YL, Sun YL, Zhu RT, Wang WJ, Li J. Physical Activity and the Risk of Gallstone Disease: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2017;51:857-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Bailey DP. Sedentary behaviour in the workplace: prevalence, health implications and interventions. Br Med Bull. 2021;137:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 926] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 83. | Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 2975] [Article Influence: 495.8] [Reference Citation Analysis (0)] |
| 84. | Ng R, Sutradhar R, Yao Z, Wodchis WP, Rosella LC. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int J Epidemiol. 2020;49:113-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 85. | Philipp E, Wilckens T, Friess E, Platte P, Pirke KM. Cholecystokinin, gastrin and stress hormone responses in marathon runners. Peptides. 1992;13:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Leitzmann MF, Giovannucci EL, Rimm EB, Stampfer MJ, Spiegelman D, Wing AL, Willett WC. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Katsika D, Grjibovski A, Einarsson C, Lammert F, Lichtenstein P, Marschall HU. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology. 2005;41:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |