Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4206
Revised: May 7, 2024
Accepted: May 29, 2024
Published online: July 16, 2024
Processing time: 112 Days and 15.7 Hours
Colorectal polyps are frequently observed in patients with type 2 diabetes mellitus (DM), posing a significant risk for colorectal cancer. Metformin, a widely prescribed biguanidine drug for type 2 DM, has been suggested to have potential chemoprophylactic effects against various cancers.
To explore the correlation between colorectal polyps and metformin use in type 2 DM patients.
Type 2 DM patients were categorized into polyp and non-polyp groups. Following this, all patients were categorized into the type 2 DM-metformin, type 2 DM-non-metformin, and non-type 2 DM groups. Based on the baseline colo
The rate of metformin use in patients with colorectal polyps was 0.502 times that of patients without colorectal polyps [odds ratio (OR) = 0.502, 95% confidence interval (CI): 0.365-0.689; P < 0.001]. The incidence of colorectal polyps did not differ significantly between the type 2 DM-metformin and non-type 2 DM groups (P > 0.05). Furthermore, the correlations between the duration of metformin use and the incidence of colorectal polyps and between the size and number of polyps and metformin use were not statistically significant (P > 0.05). Metformin use did not affect the incidence of colorectal polyps during interval colonoscopy (P > 0.05).
Metformin use and colorectal polyp incidence in type 2 DM patients showed a negative correlation, independent of the hypoglycemic effect of metformin.
Core Tip: Studies have shown that oral metformin can safely and effectively reduce the incidence of colorectal adenomas. There is also literature suggesting that metformin has potential efficacy in preventing the development of colonic polyps in patients with acromegaly.
- Citation: Wu XQ, Deng LH, Xue Q, Li X, Li MH, Wang JT. Metformin administration in prevention of colorectal polyps in type 2 diabetes mellitus patients. World J Clin Cases 2024; 12(20): 4206-4216
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4206.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4206
Colorectal cancer is the third most prevalent type of cancer and the fourth most common cause of cancer-related mortality in the world, following lung, liver, and gastric cancers, and it is responsible for nearly 700000 deaths annually[1]. The incidence and mortality of colorectal cancer have shown a consistent rise recently in China[2], partly due to the large number of people adopting a Western diet and lifestyle. According to the classical colorectal cancer formation model, colorectal polyps are precursors of most colorectal cancers[3]. This evolution is driven by the accumulation of DNA mutations and epigenetic alterations. Most colorectal cancers develop from a polyp with an aberrant crypt, evolving into an early adenoma, gradually progressing to an advanced adenoma, and finally developing into colorectal cancer[4]. Therefore, the early identification of colorectal polyps is crucial, especially for endoscopic evaluation[5]. However, the patient awareness of regular follow-up is often weak, which delays the optimal time for diagnosis and treatment of colorectal cancer. Therefore, there is an urgent need to develop effective preventive strategies, such as pharmacological prophylaxis. Metformin is a safe, widely used, cheap biguanidine drug, which has been in use for almost a century and is currently among the most commonly used medications for treating type 2 diabetes mellitus (DM)[6]. Different uses of metformin have been identified over time, and the most important of them is cancer prevention, including breast cancer, colorectal cancer, bone cancer, melanoma, and endometrial cancer[7]. However, evidence on whether metformin can prevent the occurrence of colorectal polyps remains unclear.
Studies have shown that oral metformin can reduce the incidence of colorectal adenomas safely and effectively[8-10]. Moreover, the literature suggests that metformin has potential efficacy in stopping the progression of colonic polyps in patients with acromegaly[11,12]. However, whether metformin can reduce colorectal polyp incidence in a larger population has not been elucidated[13-15].
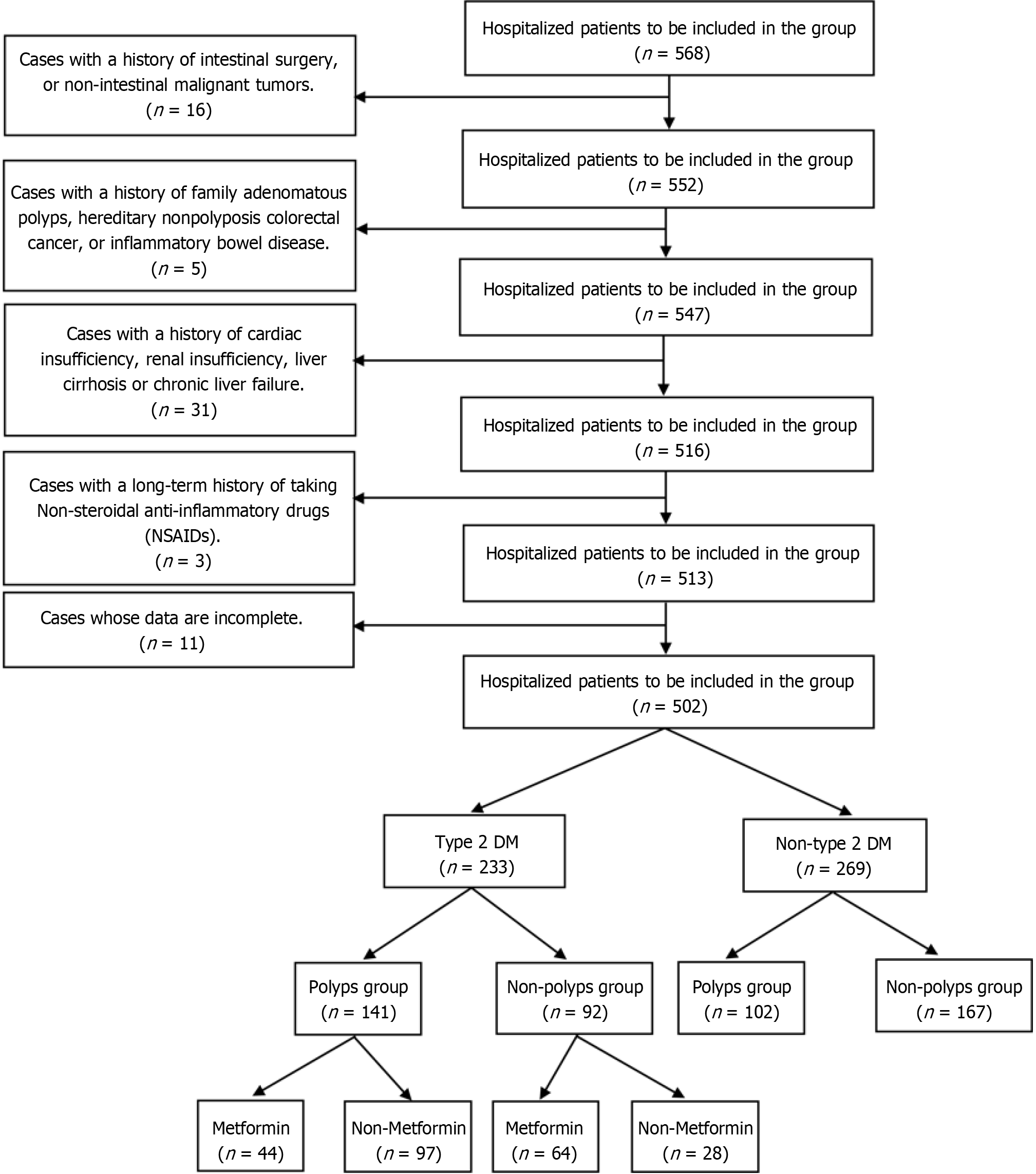
This single-center study was conducted at Peking University People's Hospital in Beijing. We enrolled patients hospitalized in the Geriatrics Department of Peking University from May 2018 to March 2022 who fulfilled the inclusion criteria. The type 2 DM group included patients aged > 18 years who were diagnosed with type 2 DM based on the Chinese Guideline for the Prevention and Treatment of type 2 Diabetes Mellitus (2020 edition)[16] and the American Diabetes Association 2020 criteria[17], those using standard doses of glucose-lowering agents for > 6 mo, and those who had undergone electronic colonoscopy with related pathological findings during hospitalization. The non-type 2 DM group included patients aged > 18 years who were hospitalized in the same period without previous history of type 2 or other types of DM and those who had undergone electronic colonoscopy with related pathological findings during hospitalization. We excluded patients with a history of intestinal surgery, family adenomatous polyps, inflammatory bowel disease, hereditary nonpolyposis colorectal cancer, or non-intestinal malignant tumors; those with a history of cardiac dysfunction, renal insufficiency, liver cirrhosis, or chronic liver failure; those with a long-term history of consuming non-steroidal anti-inflammatory drugs; and those with incomplete data. This study was reviewed and approved by the Ethics Review Committee of Peking University People's Hospital (Approval No. 2021B012-00). All study participants provide informed written consent prior to study registration.
Demographic data, specifically height, weight, age, and sex, were collected. Body mass index (BMI) was calculated as “weight (kg)/height2 (m2)”[18]. Body surface area was calculated as “0.0057 × height (cm) + 0.0121 × weight (kg) + 0.0082” for males and “0.0073 × height (cm) + 0.0127 × weight (kg) − 0.2106” for females[19]. Hypertension, coronary artery disease, fatty liver, atherosclerosis, aspirin use, statin use, alcohol consumption, and smoking were recorded according to the medical history.
We used an automatic biochemical analyzer to evaluate routine blood tests, blood glucose metabolism, and other biochemical indicators including liver enzymes, such as aspartate aminotransferase and alanine aminotransferase, and other markers, such as total serum protein, serum uric acid, serum albumin, serum creatinine (Cr), total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Simultaneously, we recorded the urinary microalbumin/creatinine ratio (urinary ACR), estimated glomerular filtration rate (eGFR), glycosylated hemoglobin (HbA1c), and C-reactive protein and calculated the Homeostatic Model Assess
We adopted various methods to clarify the metformin use in patients, including collecting data regarding history of present illness and past illness from the admission records, tracking the patient’s drug use from the outpatient prescription records in the Clinical Data Repository, and conducting telephone follow-up for some patients. Thus, we tried to ensure that the data of this retrospective case-control study was as authentic and reliable as possible.
Olympus electronic colonoscopy was conducted to detect colorectal polyps and exclude other intersectional lesions. Patients were required to refrain from eating high-cellulose vegetables and fruits 1–2 d before the examination and consume one packet of polyethylene glycol electrolyte powder (III) with 2000–3000 mL of lukewarm water within 2 hours at 19:00 the previous day and at 5:00 and 7:00 next day morning. We asked patients if they passed water-like stools and then decided whether to perform the drug enema. To ensure the quality of endoscopic examination, each endoscopic examination and operation was performed by an experienced gastroenterologist accompanied by at least one associate director physician or director physician, and every endoscopic report was written and reviewed by the two doctors. We consulted the endoscopic reports of patients and recorded in detail the presence or absence of polyps and their size, number, location, and pathological type. Usually, endoscopic doctors instruct patients with colorectal polyps to undergo re-examination after 1 year. We screened out patients who were re-examined at our hospital (median interval, 1 year) and analyzed the association between metformin use and the incidence of colorectal polyps during interval colonoscopy (n = 39).
In the first part of our study, the type 2 DM group patients were further categorized into the colorectal polyp group and non-colorectal polyp group based on the electronic colonoscopy observations. In the second part, the type 2 DM group patients were further categorized into the metformin group and non-metformin group based on the use of glucose-lowering agents (Figure 1).
Of 568 patients in our study, 16 had a history of intestinal surgery or non-intestinal malignant tumors; 5 had a history of family adenomatous polyps, hereditary nonpolyposis colorectal cancer, or inflammatory bowel disease; 31 had a history of cardiac dysfunction, renal insufficiency, liver cirrhosis, or chronic liver failure; 3 had a long-term history of taking non-steroidal anti-inflammatory drugs; and 11 had incomplete data. Finally, 233 patients were included in the type 2 DM group and 269 in the non-type 2 DM group.
Statistical Product and Service Solutions 26.0 software package (IBM) was used for data analyses. Normally distributed data from assays conducted in triplicate are presented as means ± SD. Analysis of variance or t-tests (independent sample) were applied for inter-group comparisons of the variables. Non-normally distributed data are presented as medians (P25–P75). These data were compared using a non-parametric test. Meanwhile, categorical variables were statistically analyzed using the chi-square test. Confounding variables were determined by employing univariate logistic regression. A binary logistic regression model (backward: Likelihood Ratio method) was employed to analyze the correlation between colorectal polyps and metformin use among type 2 DM patients. P-values < 0.05 were considered statistically significant.
After applying the inclusion and exclusion criteria, 502 patients were included, with 233 and 269 patients in the type 2 DM and non-type 2 DM groups, respectively (Figure 1). The proportion of men was greater in the polyp group than in the non-polyp group (63.1% vs 45.7%), with a statistically significant difference (P < 0.05). The mean age was significantly greater in the polyp group than in the non-polyp group (P < 0.05). Notably, the BMI did not vary significantly between the polyp and non-polyp groups (Table 1).
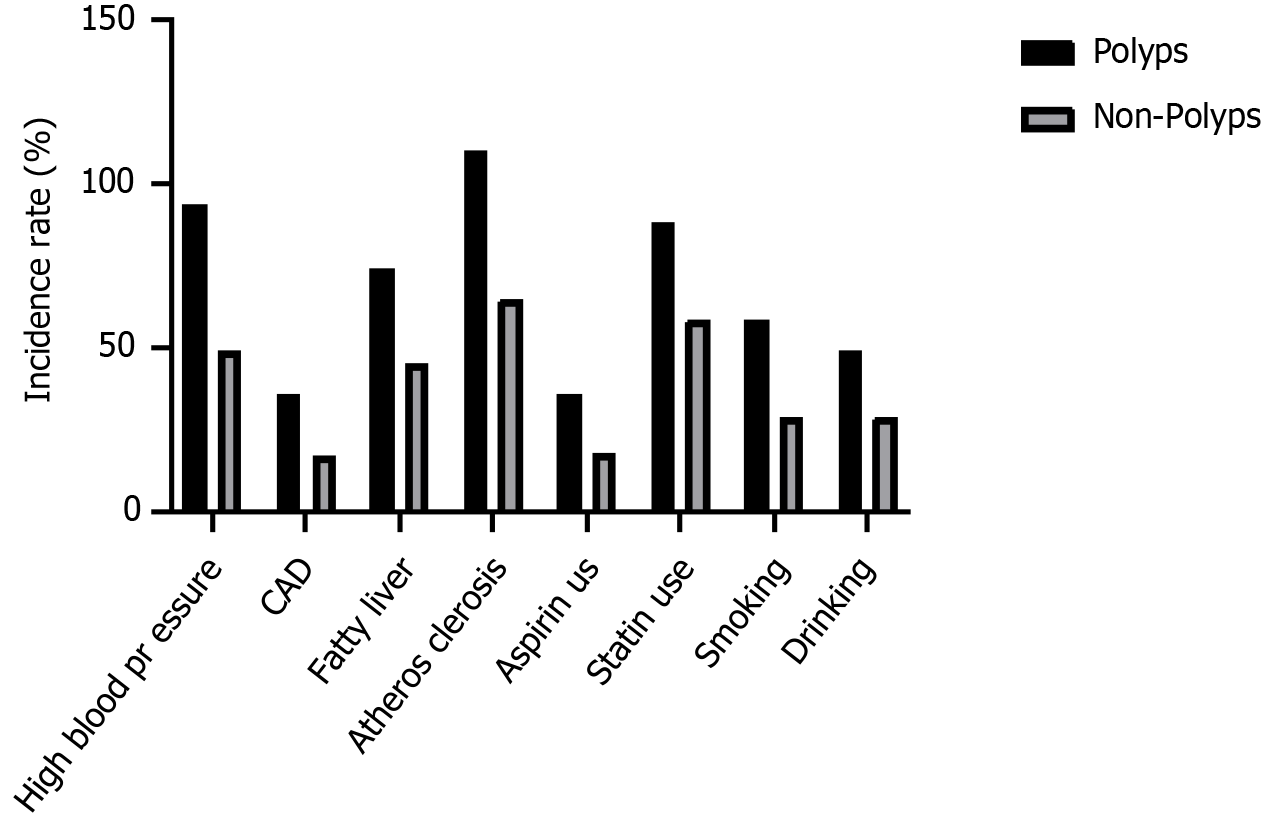
The proportion of patients with high blood pressure was higher in the polyp group than in the non-polyp group (66.67% vs 53.26%). Nevertheless, these differences were not statistically significant (P > 0.05). The incidence of atherosclerosis was significantly greater in the polyp group than in the non-polyp group (78.01% vs 70.65%; P < 0.01). The complications of coronary artery disease (P = 0.209) and fatty liver disease (P = 0.569) did not differ significantly between the two groups. Moreover, the use of aspirin and statins and the history of smoking and drinking did not differ significantly between the polyp and non-polyp groups (P > 0.05) (Table 2 and Figure 2).
| Parameter | Polyp group | Non-polyp group | χ2 value | P value |
| High blood pressure | 94 (66.67) | 49 (53.26) | 4.421 | 0.054 |
| CAD | 36 (25.53) | 17 (18.48) | 1.576 | 0.209 |
| Fatty liver | 74 (52.48) | 45 (48.91) | 0.324 | 0.569 |
| Atherosclerosis | 110 (78.01) | 65 (70.65) | 2.833 | 0.002a |
| Aspirin use | 36 (25.53) | 18 (19.57) | 1.113 | 0.291 |
| Statin use | 88 (62.41) | 59 (64.13) | 0.071 | 0.790 |
| Smoking | 59 (41.84) | 29 (31.52) | 2.524 | 0.112 |
| Drinking | 49 (34.75) | 29 (31.52) | 0.261 | 0.610 |
The Cr levels were significantly higher in the polyp group than in the non-polyp group (P = 0.033). Furthermore, the eGFR was significantly lower and the urinary ACR was significantly higher in the polyp group than in the non-polyp group (P = 0.002 and 0.016, respectively). Differences in other serological data were not statistically significant between the two groups (P > 0.05). Glucose metabolism markers did not differ significantly between the polyp and non-polyp groups (HbA1c, P = 0.328; HOMA-IR, P = 0.845). Additionally, the EF (P = 0.919) and E/A ratio (P > 0.614) did not differ significantly between the polyp and non-polyp groups (Table 3).
| Parameter | Polyp group | Non-polyp group | t/χ2/Z value | P value |
| ALT in U/L | 17.00 (12.25, 24.00) | 18.00 (13.00, 26.00) | −0.558 | 0.577 |
| AST in U/L | 18.00 (15.00, 22.00) | 18.00 (15.00, 22.00) | −0.009 | 0.993 |
| TP in g/L | 66.60 (61.75, 70.65) | 66.20 (63.40, 71.10) | −0.715 | 0.475 |
| Alb in g/L | 40.47 ± 4.75 | 41.15 ± 3.52 | 1.181 | 0.239 |
| UA in µmol/L | 359.88 ± 100.63 | 348.97 ± 90.37 | 0.843 | 0.400 |
| Cr in µmol/L | 71.50 (59.00, 82.75) | 65.00 (57.00, 76.00) | −2.132 | 0.033a |
| TC in mmol/L | 4.16 (3.51, 4.84) | 4.23 (3.54, 4.70) | −0.451 | 0.652 |
| TG in mmol/L | 1.56 (1.14, 2.09) | 1.52 (1.01, 2.22) | −0.004 | 0.997 |
| HDL-C in mmol/L | 1.09 ± 0.29 | 1.16 ± 0.25 | 1.869 | 0.063 |
| LDL-C in mmol/L | 2.63 ± 0.81 | 2.45 ± 0.76 | 1.682 | 0.094 |
| eGFR in mL/min × 1.73 m2 | 88.23 (75.79, 97.76) | 95.42 (81.07, 101.79) | −3.171 | 0.002a |
| Urinary ACR in mg/g | 7.48 (3.11, 21.47) | 5.52 (3.34, 11.79) | −2.410 | 0.016a |
| Ejection fraction in % | 68.40 (64.45, 72.50) | 68.30 (64.70, 73.45) | −0.102 | 0.919 |
| E/A ratio | 0.76 (0.64, 0.94) | 0.79 (0.65, 0.93) | −0.504 | 0.614 |
| CRP in mg/L | 0.80 (0.50, 1.87) | 0.70 (0.50, 1.90) | −0.394 | 0.693 |
| HbA1C in % | 6.80 (6.10, 7.50) | 6.80 (6.48, 7.40) | −0.978 | 0.328 |
| HOMA-IR score | 2.64 (1.53, 4.08) | 2.69 (1.70, 4.12) | −0.196 | 0.845 |
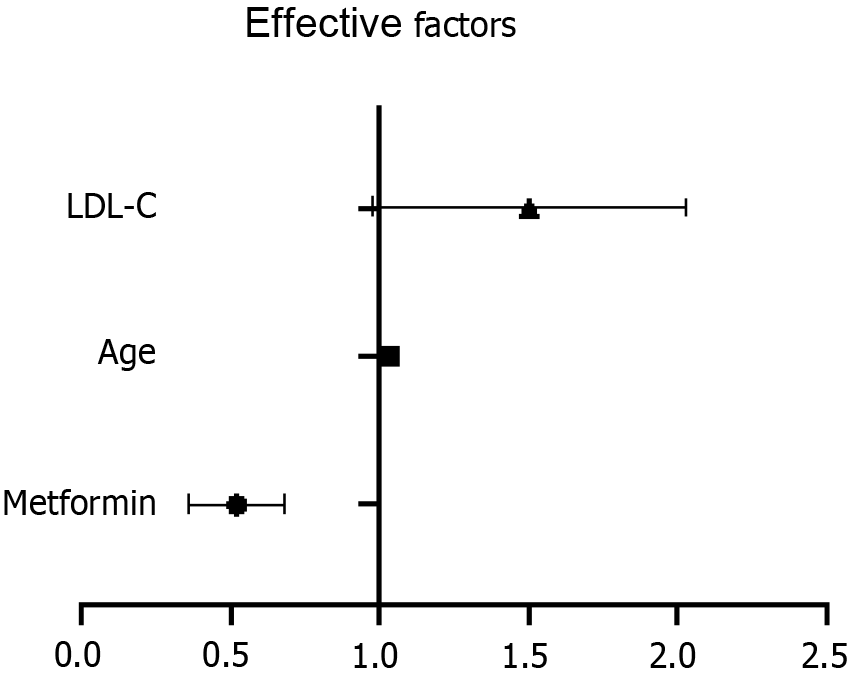
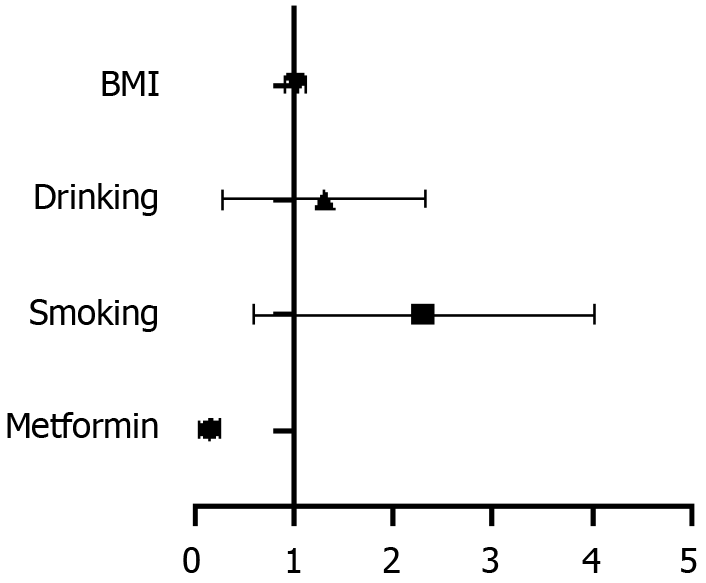
The factors with P-value < 0.1 were analyzed as confounding factors. Finally, sex, age, high blood pressure, atherosclerosis, Cr, HDL-C, LDL-C, eGFR, and urinary ACR were included as confounding factors in the multivariate logistic regression analysis. Metformin use remained independently correlated with colorectal polyps [odds ratio (OR) = 0.505, 95% confidence interval (CI): 0.367–0.694; P < 0.001] after adjusting for all confounding factors. Moreover, age (P < 0.05) and LDL-C (P < 0.05) independently predicted the risk for colorectal polyps (Table 4 and Figure 3).
Bonferroni correction was performed to assess and compare the incidence of colorectal polyps in the type 2 DM-metformin group, type 2 DM-non-metformin group, and non-type 2 DM group. The incidence of polyps was significantly lower in the type 2 DM-metformin group than in the type 2 DM-non-metformin group (P < 0.001). As expected, the incidence of colorectal polyps did not vary significantly between the type 2 DM-metformin and non-type 2 DM groups (P > 0.05) (Table 5).
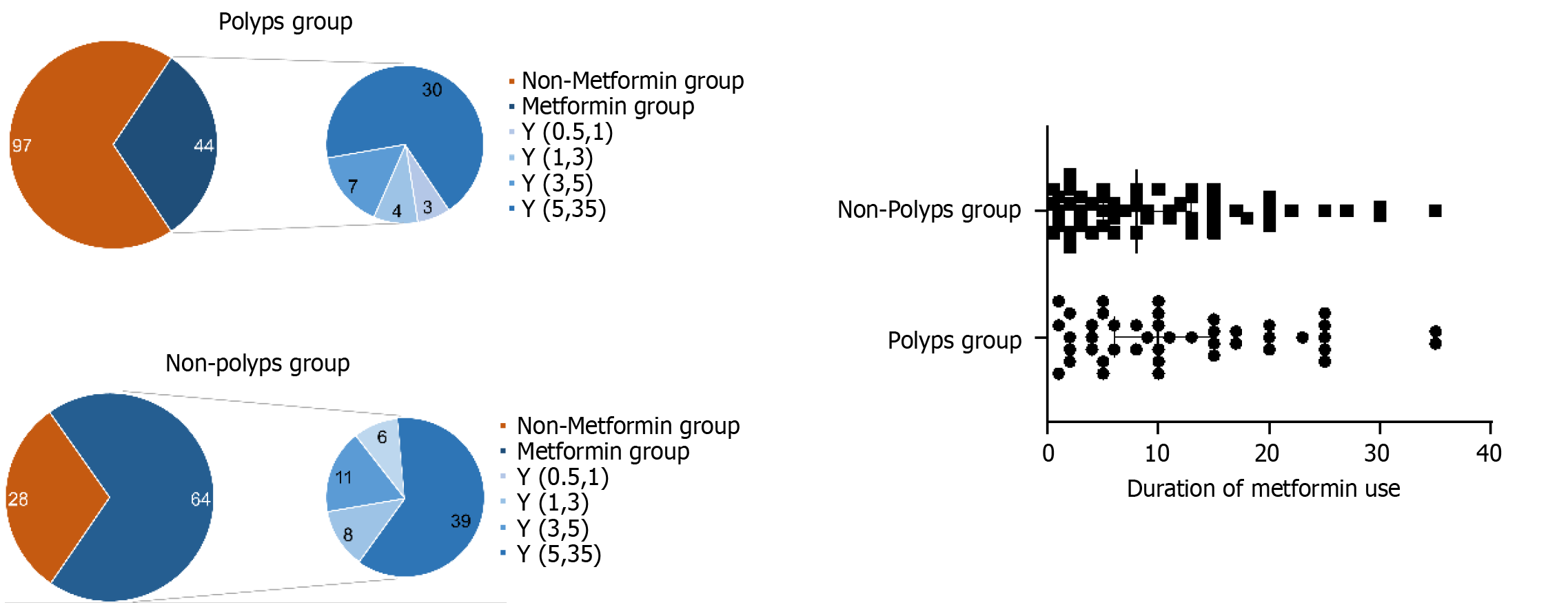
We used the Mann–Whitney rank-sum test to assess the correlation between the duration of metformin use and the incidence of colorectal polyps in patients with type 2 DM. No significant difference was noted between the duration of metformin use and colorectal polyps (Z = −1.075, P = 0.282; Figure 4).
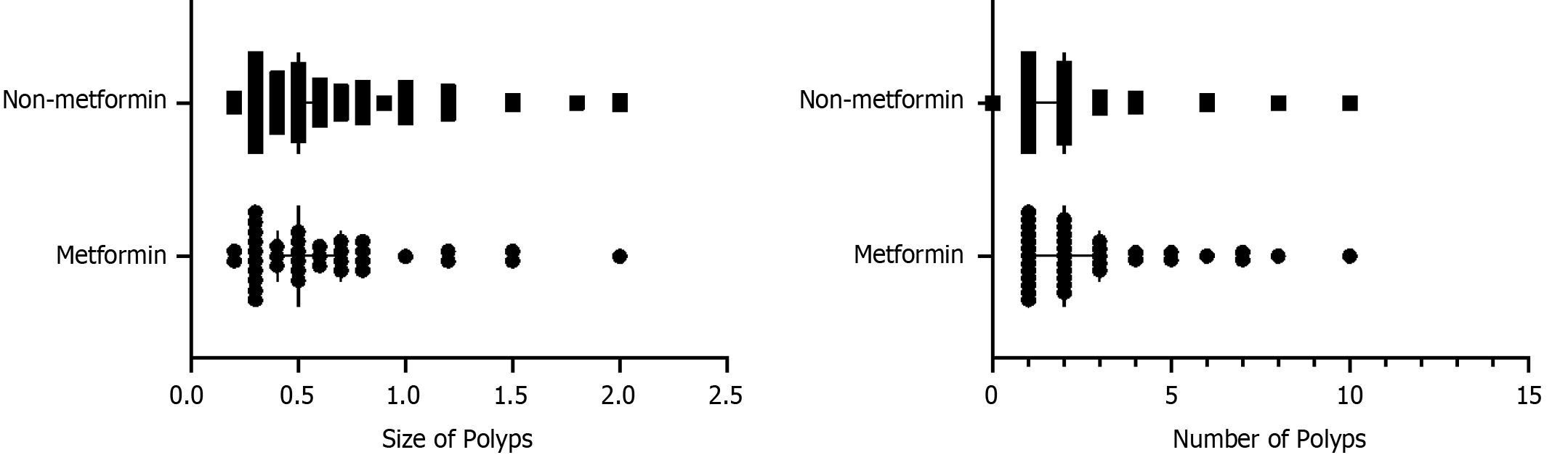
Additionally, we studied whether there were differences in the size and number of polyps between the metformin and non-metformin groups. Mann–Whitney rank-sum test was employed to analyze the statistical association between metformin use and polyp size in type 2 DM patients. No significant association was found between metformin use and polyp size (Z = −0.370, P = 0.711). The results of the correlation between metformin use and the number of polyps (Z = −0.768, P = 0.443) are shown in Figure 5.
Based on the endoscopic results collected, the patients with adenoma as the pathology type were screened. We compared the correlation between metformin use and the occurrence of adenoma, including risk factors for the occurrence of adenoma (Figure 6).
As mentioned previously, metformin use correlated negatively with the incidence of colorectal polyps during baseline colonoscopy. However, this association did not exist during interval colonoscopy (P > 0.05; Figure 7).
After adjusting for all confounding factors, we found that metformin use was independently correlated with the incidence of colorectal polyps in type 2 DM patients. Notably, the rate of metformin use in patients with colorectal polyps was 0.502 times that in patients without colorectal polyps (OR = 0.502, 95%CI: 0.365–0.689; P < 0.001). Moreover, the incidence of colorectal polyps in the type 2 DM-metformin and non-type 2 DM groups did not differ significantly (P > 0.05, Bonferroni correction). Particularly, the type 2 DM-non-metformin group excluded type 2 DM patients who did not use any glucose-lowering agents. Meanwhile, we included blood glucose-related indicators, glycosylated Hb1Ac, and HOMA-IR as confounding factors. Our results indicate that the negative correlation between colorectal polyps and metformin is independent of the hypoglycemic effect of metformin.
Cho et al[21] assessed the incidence of colonic polyps and adenomas in 3105 type 2 DM patients without a history of colorectal cancer and subjected to colonoscopy for the first time. The incidence of polyps and adenomas was significantly decreased in the metformin group, which corroborates the findings of this study. Krigel et al[22] reported a negative association between metformin use and the incidence of colorectal adenomas during baseline colonoscopy after a 4.5-year follow-up period. However, the history of the use of glucose-lowering agents in the non-metformin group in these studies was unclear, and they did not include patients without type 2 DM for comparison. Type 2 DM patients who did not use glucose-lowering agents (such as those who controlled blood glucose only by lifestyle adjustment) were excluded. Therefore, the effect of metformin on colorectal polyps, in addition to controlling blood glucose, can be further analyzed and compared with other glucose-lowering agents. Mansourian et al[14] included 11 studies (51991 patients) in a meta-analysis and found that metformin use correlated significantly and negatively with advanced adenoma risk (OR = 0.51; P < 0.001). However, total adenoma risk did not correlate significantly with metformin use (OR = 0.86; P = 0.274). Moreover, the risk of adenoma recurrence did not correlate significantly with metformin use (OR = 0.89, P = 0.137). The reason for the inconsistency between the findings of this meta-analysis and our study could be the different concerned groups and ages of the participants. For example, patients with type 2 DM were not categorized into those using metformin and other glucose-lowering agents.
Several published reports have demonstrated that type 2 DM patients are at great risk of colorectal polyps and co
However, the mechanism of metformin in preventing colorectal polyps remains unclear. Although metformin has been confirmed to reduce the risk of colorectal cancer, the results of different studies on whether metformin can prevent colorectal polyps remain inconsistent and controversial.
Nonetheless, this study also found that advanced age and enhanced LDL-C levels were risk factors for colorectal polyps. A case-control study by Xie et al[29] showed that increased LDL-C and TG levels correlated with the occurrence of polyps, which corroborates our results. However, the specific mechanisms of action need to be explored.
Few studies have elucidated the correlation between metformin use and the incidence of colorectal polyps in China. Comprehensive clinical indicators were considered in this study with a relatively large sample size of inpatients. Metformin use is independently associated with colorectal polyps in type 2 DM patients. This study explored the potential efficacy of metformin in preventing the development of colorectal polyps in patients, which is of clinical significance and requires prospective studies to explore and confirm the protective effect of metformin in a wider population. Additionally, this may open up the prospect of future chemoprophylaxis with metformin in patients at an increased risk of colorectal cancer. Early intervention to delay progression may help prevent colorectal cancer development from colorectal polyps.
With the increase in the diameter of polyps, the degree of intraepithelial neoplasia is aggravated, significantly increasing the risk of cancer development. Moreover, multiple polyps are more likely to become cancerous than solitary polyps, which is a high-risk factor for carcinogenesis[30]. Therefore, in patients with multiple polyps, we chose the maximum diameter as the size of the polyps. We then analyzed the relationship between the number and size of polyps and metformin use but did not find a significant difference.
Colorectal polyps are classified as conventional adenomas and serrated lesions[31]. Almost 50%–70% of colorectal cancers arise from adenomatous polyps. It is generally believed that inflammatory polyps and proliferative polyps do not become cancerous[32]. Therefore, it is necessary to screen adenomatous polyps from all pathological types of polyps and analyze the association between their incidence and metformin use. The results showed that metformin (OR = 0.136, 95%CI: 0.071– 0.261; P < 0.001) is an independent risk factor for colorectal adenomatous polyps.
Our study examined the association between metformin use and colorectal polyps during baseline colonoscopy and interval colonoscopy, which differed from previous Asian studies. However, no significant difference between metformin use and the incidence of polyps was observed during interval colonoscopy (P > 0.05). Corroborating our results, a retrospective analysis of an average-risk cohort by Krigel et al[22] found that this inverse association between metformin use and colorectal polyps did not persist during interval colonoscopy. They believed that the relatively short follow-up period influenced the results[33]. Meanwhile, patients with continued metformin exposure may have been less responsive to metformin.
This study has some limitations. First, we included inpatients from our hospital whose characteristics may differ from those of the general population to some extent; therefore, a multicenter study is necessary to increase the outreach of the results. Second, since this was a cross-sectional study, it mainly depended on medical records, and the causal relationship between metformin use and colorectal polyps could not be determined; therefore, further prospective cohort studies are warranted. Third, the mechanism of action of metformin in colorectal polyps is not clear. However, this study cannot interpret their relationship regarding pathophysiological mechanisms. Further basic experiments are required to overcome the limitations of this study.
Metformin can decrease the risk of colorectal polyps in type 2 DM patients, independent of its hypoglycemic effect. Therefore, the correct early administration of metformin in these patients could be crucial in preventing the occurrence and development of colorectal polyps. Furthermore, a large-scale, prospective, randomized study including various confounding factors is warranted to prove the effectiveness of metformin in preventing colorectal polyps.
| 1. | Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 948] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 2. | Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: A need for sustainable actions. Cancer Commun (Lond). 2020;40:205-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 3. | Crockett SD, Nagtegaal ID. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. 2019;157:949-966.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 4. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3029] [Article Influence: 504.8] [Reference Citation Analysis (3)] |
| 5. | Shaukat A, Kaltenbach T, Dominitz JA, Robertson DJ, Anderson JC, Cruise M, Burke CA, Gupta S, Lieberman D, Syngal S, Rex DK. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2020;115:1751-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60:1566-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 635] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 7. | Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:2184-2195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 332] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 8. | Jung YS, Park CH, Eun CS, Park DI, Han DS. Metformin use and the risk of colorectal adenoma: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, Uchiyama T, Taniguchi L, Hata Y, Uchiyama S, Hattori A, Nagase H, Kessoku T, Arimoto J, Matsuhashi N, Inayama Y, Yamanaka S, Taguri M, Nakajima A. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 10. | Higurashi T, Arimoto J, Ashikari K, Takatsu T, Misawa N, Yoshihara T, Matsuura T, Fuyuki A, Ohkubo H, Nakajima A. The efficacy of aspirin and metformin combination therapy in patients with rectal aberrant crypt foci: a double-blinded randomized controlled trial. BMC Cancer. 2020;20:1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Niculescu DA. Metformin and colonic polyps in acromegaly: is the solution closer than we think? Eur J Endocrinol. 2021;184:C9-C11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Albertelli M, Nazzari E, Dotto A, Grasso LF, Sciallero S, Pirchio R, Rebora A, Boschetti M, Pivonello R, Ricci Bitti S, Colao AAL, Ferone D. Possible protective role of metformin therapy on colonic polyps in acromegaly: an exploratory cross-sectional study. Eur J Endocrinol. 2021;184:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Suh S, Kang M, Kim MY, Chung HS, Kim SK, Hur KY, Kim JH, Lee MS, Lee MK, Kim KW. Korean type 2 diabetes patients have multiple adenomatous polyps compared to non-diabetic controls. J Korean Med Sci. 2011;26:1196-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Mansourian M, Karimi R, Vaseghi G. Different effects of metformin and insulin on primary and secondary chemoprevention of colorectal adenoma in diabetes type 2: Traditional and Bayesian meta-analysis. EXCLI J. 2018;17:45-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Park JJ, Kim BC, Hong SP, Seo Y, Lee HS, Park YS, Na SY, Park SC, Park J, Kim JH, Moon CM, Huh KC, Park SJ, Cheon JH, Kim WH, Kim TI. The Effect of Metformin in Treatment of Adenomas in Patients with Familial Adenomatous Polyposis. Cancer Prev Res (Phila). 2021;14:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 16. | [Diabetes Branch of Chinese Medical Association Chinese Guidelines for the Prevention and Treatment of Type 2 diabetes (2020 Edition)]. Zhongguo Tangniaobing Zazhi. 2021;13:95. [DOI] [Full Text] |
| 17. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2132] [Article Influence: 426.4] [Reference Citation Analysis (0)] |
| 18. | Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond). 2008;32 Suppl 3:S56-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 755] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 19. | Yu CY, Lo YH, Chiou WK. The 3D scanner for measuring body surface area: a simplified calculation in the Chinese adult. Appl Ergon. 2003;34:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24519] [Article Influence: 613.0] [Reference Citation Analysis (1)] |
| 21. | Cho YH, Ko BM, Kim SH, Myung YS, Choi JH, Han JP, Hong SJ, Jeon SR, Kim HG, Kim JO, Lee MS. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intest Res. 2014;12:139-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Krigel A, Nguyen STT, Talukder N, Huang CH, Buitrago C, Karkenny G, Lebwohl B, Abrams JA, Araujo JL. Metformin Use Is Inversely Associated with Prevalent, but Not Incident Colorectal Adenomas. Dig Dis Sci. 2022;67:4886-4894. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Onitilo AA, Engel JM, Glurich I, Stankowski RV, Williams GM, Doi SA. Diabetes and cancer II: role of diabetes medications and influence of shared risk factors. Cancer Causes Control. 2012;23:991-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804-10812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 25. | Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1231] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 26. | Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1301] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 27. | Garrett CR, Hassabo HM, Bhadkamkar NA, Wen S, Baladandayuthapani V, Kee BK, Eng C, Hassan MM. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer. 2012;106:1374-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol. 2012;29:1314-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 29. | Xie C, Wen P, Su J, Li Q, Ren Y, Liu Y, Shen R, Ren J. Elevated serum triglyceride and low-density lipoprotein cholesterol promotes the formation of colorectal polyps. BMC Gastroenterol. 2019;19:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Risio M. The natural history of colorectal adenomas and early cancer. Pathologe. 2012;33 Suppl 2:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150:64-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 641] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 32. | Jass JR. Colorectal cancer: a multipathway disease. Crit Rev Oncog. 2006;12:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Chen CH, Lin CL, Hsu CY, Kao CH. Insulin enhances and metformin reduces risk of colorectal carcinoma in type-2 diabetes. QJM. 2020;113:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |