Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4146
Revised: May 13, 2024
Accepted: May 20, 2024
Published online: July 16, 2024
Processing time: 83 Days and 15.9 Hours
Cervical intraepithelial neoplasia (CIN) is an important precursor of cervical cancer. Early detection and treatment can reduce the incidence of cervical cancer.
To investigate the detection rate of human papillomavirus (HPV) E6/E7 mRNA in cervical tissue of patients with different types of epithelial cell neoplasia (CIN) and its relationship with CIN progression and diagnosis.
One hundred women with HPV infection detected by cervical exfoliation cytology between January 2022 and January 2023 were retrospectively selected. These patients were graded CIN based on colposcopy and cervical pathology. The positive expression rates of HPV E6/E7 mRNA and HPV [polymerase chain reaction (PCR)-reverse dot crossing] were compared among all groups. Patients with HPV E6/E7 mRNA expression in the grade 1 CIN group were followed up for 1 yr. The relationship between atypical squamous epithelium and high malig
The diagnostic sensitivity, specificity, and sensitivity of PCR-reverse point hybridization technology for secondary CIN were 70.41%, 70.66%, and 0.714, respectively. Sensitivity and specificity for secondary CIN were 752% and 7853%, respectively, the area under the curve value was 0.789. Logistic Multifactorial model analysis revealed that the HPV positive rates and the HPV E6/E7 mRNA positive rates were independent risk factors of CIN grade I (P < 0.05). In CIN grade I patients with positive for HPV E6/E7 mRNA, in its orientation to grade CIN patients, in its orientation to grade CIN patients, at 69.2%, compared with patients negative for HPV E6/E7 mRNA (30.8%), significant difference (P < 0.05).
HPV E6/E7 mRNA and HPV (PCR-reverse dot hybrid) positive expression have a close relationship with CIN-grade disease progression and is an independent risk factor for high-grade CIN lesions.
Core Tip: In this study, cervical cancer is a common gynecological malignancy, its incidence is increasing year by year, and human papillomavirus (HPV) infection is closely related to the occurrence and development of cervical cancer. Cervical intraepithelial neoplasia (CIN) is an important precursor to cervical cancer development, and early detection and treatment can reduce the incidence of cervical cancer. We plan to use RNA-seq technology to detect the expression level of HPV E6/E7 mRNA in cervical cancer tissues of CIN patients, and for correlation with the diagnosis, prognosis, recurrence, and other factors of CIN patients, to provide new ideas for the follow-up and treatment of CIN patients.
- Citation: Zhang LL, Du MY, Du X, Duan J, Yao DM, Jing J, Feng C, Song L. Correlation analysis of human papillomavirus E6/E7 mRNA detection with diagnosis, prognosis and recurrence risk in patients with cervical epithelioma. World J Clin Cases 2024; 12(20): 4146-4153
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4146.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4146
Cervical cancer is a common gynecological malignancy, its incidence is increasing year by year, human papillomavirus (HPV) infection is closely related to the occurrence and development of cervical cancer[1]. HPV is a DNA virus encoded by eight genes. Can cause gastric sinus cancer, reproductive tract cancer, upper respiratory tract cancer, and digestive tract cancer disease[2]. Cervical intraepithelial neoplasia (CIN) is an important precursor to cervical cancer development, and early detection and treatment can reduce the incidence of cervical cancer[3,4]. With the increasing maturity of clinical screening for cervical cancer, the detection rate of CIN in clinics is also increasing. Patients with CIN must undergo multiple cervical cytology and HPV tests, which will undoubtedly increase the economic burden of patients and also cause a waste of medical resources[5]. Therefore, it is urgent to find effective markers to evaluate CIN clinically. Persistent infection with HPV can lead to CIN development, and the tumorigenic protein encoded by the HPV E6/E7 gene is closely related to CIN[6-8]. High-risk HPV E6/E7 proteins can affect cell adhesion, polarity, and transcription, and then affect the recognition of HPV[9-11]. When the HPV E6/E7 gene expresses the oncoprotein, its DNA is activated, under
In recent years, with the development of immunohistochemistry technology, transcriptome sequencing (RNA-Seq) has been widely used in the biomedical field[13,14]. E6 and E7 have important cancer-promoting functions in cervical cancer, but the relationship between their expression in cervical cancer and patient prognosis and recurrence is not clear, and high-throughput sequencing technology is expected to solve the above problems. Therefore, we plan to use RNA-seq technology to detect the expression level of HPV E6/E7 mRNA in cervical cancer tissues of CIN patients, and for correlation with the diagnosis, prognosis, recurrence, and other factors of CIN patients, to provide new ideas for the follow-up and treatment of CIN patients.
Retrospectively, 100 women from January 2022 to the gynecological clinic in January 2023 were selected as the study subjects, and this study strictly complied with the ethical research requirements of our hospital.
Inclusion criteria: (1) The mother's age between 22 and 59 yr; (2) Cervical cancer examination; (3) The diagnosis of atypical squamous epithelial cells according to TBS (TBS) guidelines; (4) Sex over 3 yr; (5) Advanced epithelial neoplasia by grade or above CIN (including CIN and CIN); and (6) The diagnostic criteria of obstetrics and gynecology[15].
Exclusion criteria were: (1) History of cervical surgery; (2) History of radiotherapy and immunotherapy; (3) Presence of cancer elsewhere; (4) Recent antiviral use; and (5) History of psychiatric conditions.
CIN grades the criteria set by the American Society of Colposcopy and Cervical Pathology and American Society of Pathology (Japanese CIN): CIN I: equivalent to very minor, minor dysplasia; Grade CIN II: equivalent to moderate dysplasia; Grade CIN III: similar to severe dysplasia, carcinoma in situ, squamous cell carcinoma, adenoid carcinoma, etc.
The time of specimen collection is within 7 d after the end of menstruation, and the same sex is prohibited for 48 h before testing. Doctors use a vaginal speculum on the cervix, with dry cotton ball cervix secretions wipe clean, sampling brush placed in the cervix, and close to the cervix for 3-5 wk, and then take a bottle containing holding liquid to retain the collected samples, make smear, and pathological examination, finally by two senior cell pathology doctor to complete the diagnosis, in the case of the two sides disagree, also need to be by the third person to judge.
Samples were collected using the cell test collection method described above. After processing the samples, the automated hybridization device of Taipu Biotechnology (China) Co., Ltd.
The PANTHER system was used for testing, and the AssayReagent was removed from the 4°C refrigerator and reduced to room temperature, Reagent corresponds to Reconstitution Solution (A-AR, E-ER, P-PR, IC-TCR), and marked the preparation date and manufacturer. Mix the Thinprep cell preservation solution well, open the lid and remove 1 mL of the specimen, put it in the corresponding STU tube, and board the machine. This project intends to clinically diagnose 1000 female patients selected for ASC-US by using the APTIMA HPV test tool. The key indicators used to screen for key indicators are the detection of sensitivity greater than CIN 2 in the ASC-US population and the maintenance of its clinically acceptable specificity. The APTIMA HPV threshold for the detection kit was set to 0.50 S / CO as described above.
The positive expression rate of HPV E6/E7 mRNA and HPV E6/E7 genes [polymerase chain reaction (PCR) Receptor Predictive Polymorphism, PCR)] between the groups; our previous study found that HPV E6/E7 mRNA varied in the CIN primary group, but it showed no significant progress within 1 yr.
The data is processed using professional SPSS21.0 software, through the normal distribution of these data, all meet approximate normal or normal distribution and are expressed by mean ± SD; the count data is expressed in percentage and χ2 test for comparison; receiver operating characteristic (ROC) curve for diagnostic analysis and Kaplan-Meier method for survival analysis; significant difference at P < 0.05.
The patients were 48.24 ± 11.44 yr, including 39 patients > 45 yr and 61 patients < 45 yr; 49 pregnant > 2 and 51 pregnant < 2 times; 39 births > 2 and 61 births < 2 times.
According to Table 1, the positive expression rate of HPV (PCR-reverse point hybridization) and HPV E6/E7 mRNA of patients in grade C IN I group were lower than those of CIN, CIN, CIN, invasive squamous cell carcinoma and adenocarcinoma, respectively (P < 0.05); the positive expression rate of HPV (PCR-reverse point hybrid) and HPV E6/E7 mRNA were not different in CIN, CIN, invasive squamous cell carcinoma and adenocarcinoma group (P > 0.05).
| Group | Example number | HPV E6/E7 mRNA | HPV (PCR-reverse point hybridization) | ||
| Positive | Negative | Positive | Negative | ||
| CINI level | 48 | 13 (27.1) | 35 (72.9) | 15 (31.3) | 33 (68.7) |
| CIN II level | 31 | 24 (77.4) | 7 (22.6) | 21 (67.7) | 10 (32.3) |
| CIN III level | 16 | 12 (75.0) | 4 (25.0) | 11 (68.7) | 5 (31.3) |
| Squamous or adenocarcinoma | 5 | 3 (18.8) | 2 (12.5) | 4 (25.0) | 1 (6.3) |
| χ2 | 23.483 | 14.669 | |||
| P value | < 0.001 | 0.002 | |||
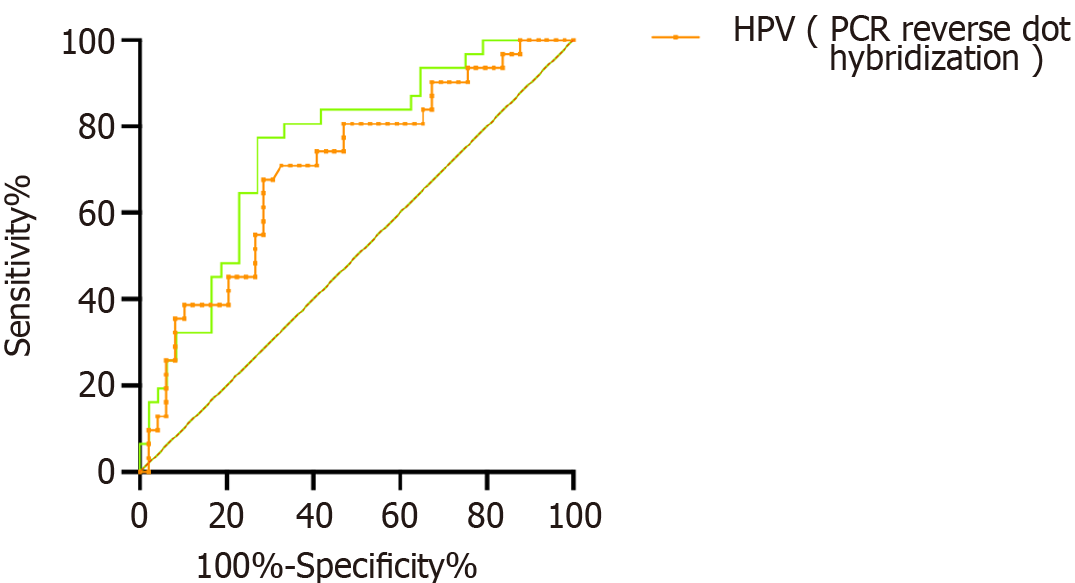
As shown in Figure 1, using the test results of HPV (PCR-reverse dot hybrid), HPV E6/E7 mRNA as the standard, Drawing the ROC curve, the results show that, The sensitivity of HPV (PCR-reverse point hybridization) for diagnostic grade CIN II (high-grade lesions) was 70.41%, the specificity was 70.66%, and the area under the curve (AUC) value of 0.714; For the CIN II level (high level), The sensitivity of HPV E6/E7 mRNA was 75.20%, The specificity was 78.53%, The AUC value was 0.789.
As shown in Table 2, there were no significant differences in CIN II and CIN I HPV patients at age, pregnancy, delivery, and age (P > 0.05); the proportion of HPV (PCR-reverse point) and HPV E6/E7 mRNA patients above CIN I was signi
| Project | CIN grade (n = 52) | CIN grade I (n = 48) | χ2 | P value |
| Age | 0.495 | 0.482 | ||
| ≥ 45 | 15 (28.8) | 17 (35.4) | ||
| < 45 | 37 (71.2) | 31 (64.6) | ||
| Gravidity | 0.031 | 0.861 | ||
| ≥ 2 | 16 (30.8) | 14 (29.2) | ||
| < 2 | 36 (69.2) | 34 (70.8) | ||
| Parity | 0.495 | 0.482 | ||
| ≥ 2 | 15 (28.8) | 17 (35.4) | ||
| < 2 | 37 (71.2) | 31 (64.6) | ||
| HPV (PCR reverse-dot hybridization) | 14.408 | < 0.001 | ||
| Positive | 36 (69.2) | 15 (31.3) | ||
| Negative | 16 (30.8) | 33 (68.7) | ||
| HPV E6/E7 mRNA | 22.960 | < 0.001 | ||
| Positive | 39 (75.0) | 13 (27.1) | ||
| Negative | 13 (25.0) | 35 (72.9) |
As shown in Table 3, the Logistic multivariate model was constructed with> CIN II I and CIN I results as dependent variables, qualitative results of HPV (PCR-reverse point hybrid), and qualitative results of HPV E6/E7 mRNA. The independent risk factor of HPV (PCR-reverse point hybrid) and HPVE6/E7 mRNA as grade CIN II (high grade) (P < 0.05).
| Metric | β | SE | Walds | P value | OR | 95%CI |
| HPV (PCR reverse-dot hybridization) | -0.163 | 0.511 | 0.102 | 0.007 | 1.850 | 1.312-2.312 |
| HPV E6/E7 mRNA | 0.630 | 0.510 | 0.523 | 0.022 | 1.877 | 1.690-5.120 |
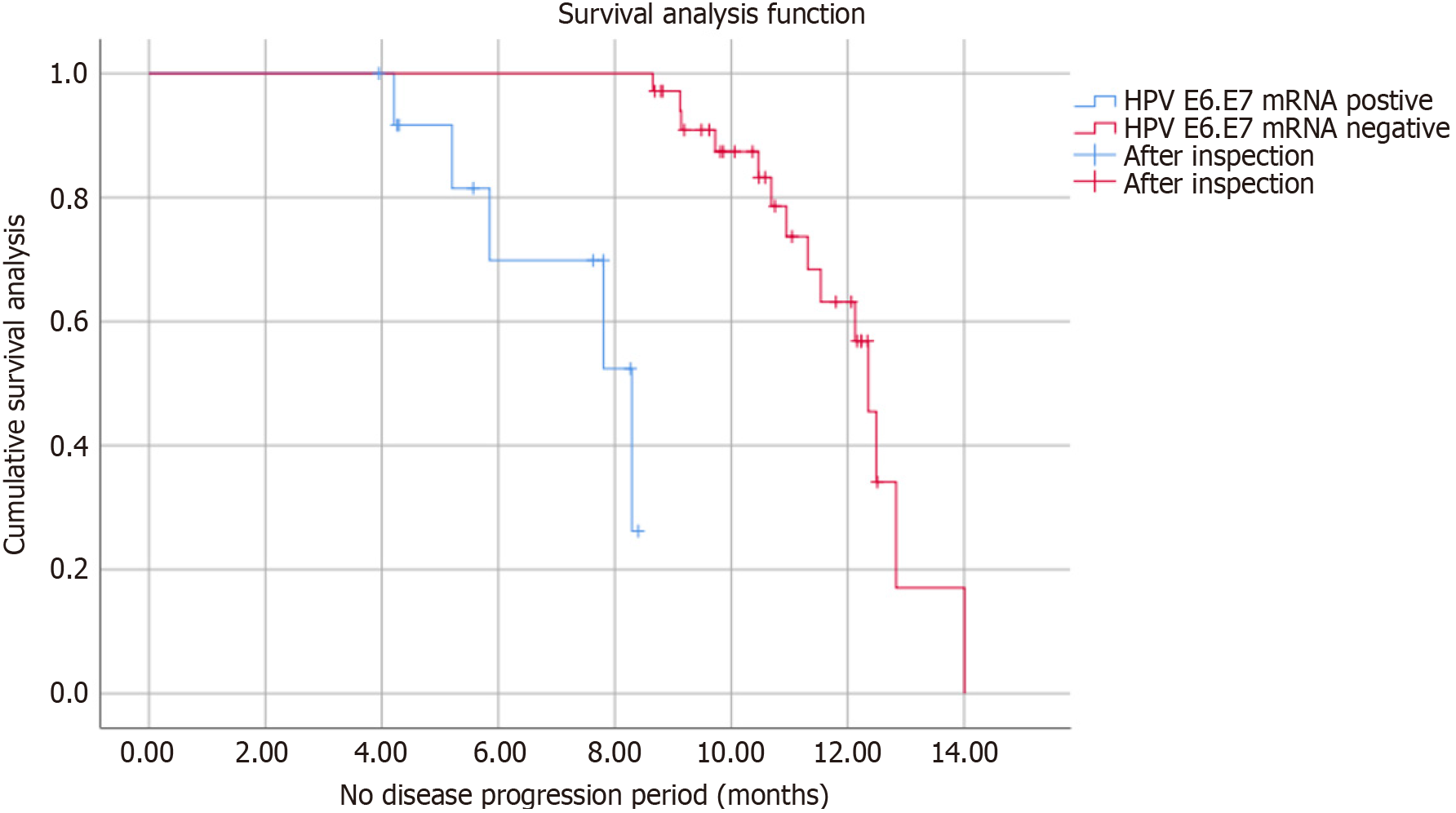
As shown in Table 4 and Figure 2, through our one-year follow-up of 48 patients with CIN I HPV, patients found to be positive for HPV E6/E7 mRNA. The chance of conversion to type CIN II was higher than that of HPV E6/E7 mRNA negative patients (P < 0.05); among, the median time to CINI to CIN II for HPV E6/E7 mRNA positive was significantly shorter than the median time to CINI to CIN II for HPV E6/E7 mRNA negative Log Rank (Mantel-Cox) = 11.828, P < 0.05].
| HPV E6/E7 mRNA | Example number | CIN I level | CIN II level |
| Positive | 13 | 4 (30.8) | 9 (69.2) |
| Negative | 35 | 25 (71.4) | 10 (28.6) |
| χ2 | 6.553 | ||
| P value | 0.010 | ||
Studies have found that E6/E7 gene in HPV virus is the main pathogenic gene of cervical cancer[16,17]. E6 gene can affect the apoptosis of cells by inhibiting the expression pathway of the host P53 gene, and then affect the relevant anticancer cells[18,19] in vivo. However, the E7 gene will have an impact on the expression process of the pRB gene, and then on the cell cycle, leading to the malignant proliferation of a large number of inflammatory cells, and then the cancerous[20,21]. The HPV E6/E7 thus plays an important role in the process of the HPV E6/E7 infection and tumorigenesis[22]. Both CIN and cervical cancer are known to be associated with HPV infection, which can be considered as the early lesion of cervical cancer[23,24]. The transformation of CIN to cervical cancer requires a process, but not all CIN will transition to high-risk areas; some CIN patients can return to normal state[25,26] through autoimmune regulation without surgical treatment. The clinical morphological evaluation method is commonly used for the clinical morphological evaluation method of cervical lesions[27,28].
HPV (PCR) and HPV (E6/E7) were significantly lower than those in CIN and CIN groups and invasive squamous carcinoma and adenocarcinoma groups; there was no significant difference in mRNA (PCR reverse point hybrid) and HPV E6/E7 mRNA expression of HPV in CIN, group and invasive squamous and adenocarcinoma groups. The main reasons for this are as follows: (1) HPV infection has transient characteristics; (2) In malignant tumors, HPV-DNA mostly binds to host cells, which easily causes the high expression of E6/E7 protein in the body, suppresses the expression process of P53 and other tumor suppressor genes, and then affects the biological cycle of cells, affects the apoptosis and immune function of cells, and finally causes lesions, and causes the occurrence of cancer; (3) The type and degree of HPV infection are highly correlated with the type of infection; and (4) On this basis, combined with HPV E6/E7 mRNA detection technology, the malignant activity of cervical cells after HPV infection can be accurately detected, to better screen patients with high-grade CIN and cervical invasive cancer, providing valuable time for effective screening and treatment of precancerous lesions.
The previous work of our research group found that HPV E6/E7 mRNA is expressed at higher levels and more specific in grade 11 CIN compared with PCR PCR technology, suggesting that HPV mRNA is more accurate in grade 11 CIN. Two oncogenes, E6 and E7 are known oncogenes in the HPV integrated genome. They can inhibit p53 activity, leading to the malignant transformation of the host cells. Conversely, if E6 and E7 do not appear, the cells will not become cancerous; if the host may have been infected with HPV, the test will be positive. Therefore, during CIN screening, HPV E6/E7 mRNA can be tested. The HPV E6/E7 mRNA level is an important indicator for predicting postoperative recurrence of cervical cancer.
Our logistic analysis found that HPV positivity and HPV E6/E7 mRNA were independent risk factors of grade CIN II; HPV E6/E7 mRNA positive expression was more likely to shift from CINI to type CIN II. After the fusion of HPV DNA to the host cell DNA, its overexpression forms the HPV E6/E7 oncoprotein. Previous studies have shown that HPV E6/E7 mRNA can be used as a molecular marker of HPV E6/E7 and as a molecular marker of HPV E6/E7[29,30]. In the process of cervical epithelial cell cancer, due to the stimulation of external carcinogens, cervical epithelial cells will be activated, and transcription to E6/E7 mRNA, and then combined with P53, PRB, and other tumor suppressor protein, thus inhibiting the activity of P53, PRB, so the transcription and translation of E6/E7 is a key pathway lead to HPV infection, HPV E6/E7 mRNA detection can improve the specificity of cervical cancer screening, is expected to accurate diagnosis of type CIN II lesions.
Our previous study found that the time to progression to type II was significantly reduced in HPV E6/E7 mRNA (E6/E7 mRNA) positive CIN I patients. Our previous study found that the HPV E6/E7 mRNA expression level was closely related to the development of CIN, while the HPV E6/E7 mRNA expression level was closely related to the development of CIN. Our previous work showed that HPV E6/E7 mRNA expression is significantly higher than HPV (PCR) in cervical cancer tissues, and it is accompanied by increased expression levels of HPV E6/E7 mRNA in cervical cancer tissues. Therefore, we plan to use high-throughput sequencing technology to detect HPV E6/E7 mRNA and analyze its expression in CIN to provide the basis for the diagnosis and prognosis of CIN. The disadvantage of this paper is that it has been affected by some objective factors in the allocation process of selected patients, which cannot be completely excluded, and further research is needed.
In conclusion, the positive expression of HPV E6/E7 mRNA and HPV (PCR-reverse point hybrid) has a close relationship with the disease progression of CIN grade, and are independent risk factors for high-grade lesions of CIN.
Authors thank to the assistance of all colleagues and partners.
| 1. | Kong TW, Kim M, Kim YH, Kim YB, Kim J, Kim JW, Park MH, Park JH, Rhee JH, Lim MC, Hong JS; Korean Society of Obstetrics and Gynecology developed by Position Statement Writing Committee. High-risk human papillomavirus testing as a primary screening for cervical cancer: position statement by the Korean Society of Obstetrics and Gynecology and the Korean Society of Gynecologic Oncology. Obstet Gynecol Sci. 2020;63:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Dunne EF, Park IU. HPV and HPV-associated diseases. Infect Dis Clin North Am. 2013;27:765-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Winer RL, Lin J, Tiro JA, Miglioretti DL, Beatty T, Gao H, Kimbel K, Thayer C, Buist DSM. Effect of Mailed Human Papillomavirus Test Kits vs Usual Care Reminders on Cervical Cancer Screening Uptake, Precancer Detection, and Treatment: A Randomized Clinical Trial. JAMA Netw Open. 2019;2:e1914729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Yanatatsaneejit P, Chalertpet K, Sukbhattee J, Nuchcharoen I, Phumcharoen P, Mutirangura A. Promoter methylation of tumor suppressor genes induced by human papillomavirus in cervical cancer. Oncol Lett. 2020;20:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Arroyo Mühr LS, Lagheden C, Lei J, Eklund C, Nordqvist Kleppe S, Sparén P, Sundström K, Dillner J. Deep sequencing detects human papillomavirus (HPV) in cervical cancers negative for HPV by PCR. Br J Cancer. 2020;123:1790-1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Abdul Rahman SF, Xiang Lian BS, Mohana-Kumaran N. Targeting the B-cell lymphoma 2 anti-apoptotic proteins for cervical cancer treatment. Future Oncol. 2020;16:2235-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Bule P, Aguiar SI, Aires-Da-Silva F, Dias JNR. Chemokine-Directed Tumor Microenvironment Modulation in Cancer Immunotherapy. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 8. | Beneduce E, Matte A, De Falco L, Mbiandjeu S, Chiabrando D, Tolosano E, Federti E, Petrillo S, Mohandas N, Siciliano A, Babu W, Menon V, Ghaffari S, Iolascon A, De Franceschi L. Fyn kinase is a novel modulator of erythropoietin signaling and stress erythropoiesis. Am J Hematol. 2019;94:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Goïta AA, Guenot D. Colorectal Cancer: The Contribution of CXCL12 and Its Receptors CXCR4 and CXCR7. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Pal A, Kundu R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front Microbiol. 2019;10:3116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 349] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 11. | Cho HW, Hong JH, Lee JK. Human papillomavirus testing as a primary screening tool for cervical cancer. J Gynecol Oncol. 2021;32:e56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Mchome BL, Kjaer SK, Manongi R, Swai P, Waldstroem M, Iftner T, Wu C, Mwaiselage J, Rasch V. HPV types, cervical high-grade lesions and risk factors for oncogenic human papillomavirus infection among 3416 Tanzanian women. Sex Transm Infect. 2021;97:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Inoue M, Shimizu Y, Ishikawa M, Abiko S, Shimoda Y, Tanaka I, Kinowaki S, Ono M, Yamamoto K, Ono S, Sakamoto N. Relationships of early esophageal cancer with human papillomavirus and alcohol metabolism. World J Gastroenterol. 2020;26:6047-6056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Acevedo-Sánchez V, Rodríguez-Hernández RM, Aguilar-Ruíz SR, Torres-Aguilar H, Romero-Tlalolini MLA. Extracellular Vesicles in Cervical Cancer and HPV Infection. Membranes (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Yuan Y, Cai X, Shen F, Ma F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021;497:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 16. | Aggarwal C, Cohen RB, Morrow MP, Kraynyak KA, Sylvester AJ, Knoblock DM, Bauml JM, Weinstein GS, Lin A, Boyer J, Sakata L, Tan S, Anton A, Dickerson K, Mangrolia D, Vang R, Dallas M, Oyola S, Duff S, Esser M, Kumar R, Weiner D, Csiki I, Bagarazzi ML. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin Cancer Res. 2019;25:110-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Attademo L, Tuninetti V, Pisano C, Cecere SC, Di Napoli M, Tambaro R, Valabrega G, Musacchio L, Setola SV, Piccirillo P, Califano D, Spina A, Losito S, Greggi S, Pignata S. Immunotherapy in cervix cancer. Cancer Treat Rev. 2020;90:102088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Britto AMA, Goes LR, Sivro A, Policarpo C, Meirelles ÂR, Furtado Y, Almeida G, Arthos J, Cicala C, Soares MA, Machado ES, Giannini ALM. HPV Induces Changes in Innate Immune and Adhesion Molecule Markers in Cervical Mucosa With Potential Impact on HIV Infection. Front Immunol. 2020;11:2078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Bruand M, Barras D, Mina M, Ghisoni E, Morotti M, Lanitis E, Fahr N, Desbuisson M, Grimm A, Zhang H, Chong C, Dagher J, Chee S, Tsianou T, Dorier J, Stevenson BJ, Iseli C, Ronet C, Bobisse S, Genolet R, Walton J, Bassani-Sternberg M, Kandalaft LE, Ren B, McNeish I, Swisher E, Harari A, Delorenzi M, Ciriello G, Irving M, Rusakiewicz S, Foukas PG, Martinon F, Dangaj Laniti D, Coukos G. Cell-autonomous inflammation of BRCA1-deficient ovarian cancers drives both tumor-intrinsic immunoreactivity and immune resistance via STING. Cell Rep. 2021;36:109412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 20. | Inturi R, Jemth P. CRISPR/Cas9-based inactivation of human papillomavirus oncogenes E6 or E7 induces senescence in cervical cancer cells. Virology. 2021;562:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Lu Y, Xu X, Nong XH, Yao DS. Detection of high-risk human papillomavirus DNA in sentinel lymph nodes of patients with cervical cancer. Oncol Lett. 2020;19:2317-2325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Chalertpet K, Pakdeechaidan W, Patel V, Mutirangura A, Yanatatsaneejit P. Human papillomavirus type 16 E7 oncoprotein mediates CCNA1 promoter methylation. Cancer Sci. 2015;106:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Yang YM, Wang SJ, Wang FY, Chen R, Xiao Q, Kang N, Liao QP. Preliminary study of the use of E6/E7mRNA detection in screening and triage management of HR-HPV infection during pregnancy. Ann Transl Med. 2021;9:1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Argyri E, Tsimplaki E, Daskalopoulou D, Stravopodis DJ, Kouikoglou O, Terzakis E, Panotopoulou E. E6/E7 mRNA expression of high-risk HPV types in 849 Greek women. Anticancer Res. 2013;33:4007-4011. [PubMed] |
| 25. | Zhang M, Yang W, Wang P, Deng Y, Dong YT, Liu FF, Huang R, Zhang P, Duan YQ, Liu XD, Lin D, Chu Q, Zhong B. CCL7 recruits cDC1 to promote antitumor immunity and facilitate checkpoint immunotherapy to non-small cell lung cancer. Nat Commun. 2020;11:6119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Wang F, Tao R, Zhao L, Hao XH, Zou Y, Lin Q, Liu MM, Goldman G, Luo D, Chen S. Differential lncRNA/mRNA Expression Profiling and Functional Network Analyses in Bmp2 Deletion of Mouse Dental Papilla Cells. Front Genet. 2021;12:702540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Wang H, Wang Z, Li Y, Lu T, Hu G. Silencing Snail Reverses Epithelial-Mesenchymal Transition and Increases Radiosensitivity in Hypopharyngeal Carcinoma. Onco Targets Ther. 2020;13:497-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Zhu S, Li Y, Bennett S, Chen J, Weng IZ, Huang L, Xu H, Xu J. The role of glial cell line-derived neurotrophic factor family member artemin in neurological disorders and cancers. Cell Prolif. 2020;53:e12860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Marotta G, Basagni F, Rosini M, Minarini A. Role of Fyn Kinase Inhibitors in Switching Neuroinflammatory Pathways. Curr Med Chem. 2022;29:4738-4755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | von Lersner A, Droesen L, Zijlstra A. Modulation of cell adhesion and migration through regulation of the immunoglobulin superfamily member ALCAM/CD166. Clin Exp Metastasis. 2019;36:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |