Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.374
Peer-review started: September 12, 2023
First decision: November 30, 2023
Revised: December 12, 2023
Accepted: December 27, 2023
Article in press: December 27, 2023
Published online: January 16, 2024
Processing time: 121 Days and 0.8 Hours
Primary central nervous system lymphoma (PCNSL) is a non-Hodgkin lymphoma that originates in the central nervous system (CNS) and is exclusively limited to the CNS. Although most PCNSLs are diffuse large B-cell lymphomas, primary CNS T-cell lymphomas (PCNSTLs) are rare. PCNSTLs typically demonstrate some degree of enhancement on contrast-enhanced magnetic resonance imaging (MRI). To the best of our knowledge, non-enhancing PCNSTL has not been reported previously.
A 69-year-old male presented to the neurology department with complaints of mild cognitive impairment and gradual onset of left lower leg weakness over a span of two weeks. Initial MRI showed asymmetric T2-hyperintense lesions within the brain. No enhancement was observed on the contrast-enhanced T1 image. The initial diagnosis was neuro-Behçet’s disease. Despite high-dose steroid therapy, no alterations in the lesions were identified on initial MRI. The patient’s symptoms deteriorated further. An MRI performed one month after the initial scan revealed an increased lesion extent. Subsequently, brain biopsy confirmed the diagnosis of PCNSTL. The patient underwent definitive combined chemo-radiotherapy. However, the patient developed bacteremia and died of septic shock approximately three months after diagnosis.
The absence of enhancement in the lesion did not rule out PCNSTL. A biopsy approach is advisable for pathological confirmation.
Core Tip: The characteristic features of primary central nervous system T-cell lymphoma (PCNSTL) are not widely recognized owing to its low incidence rate. However, most malignant tumors demonstrate enhancement on gadolinium-enhanced magnetic resonance imaging (MRI). Consequently, a lesion without enhancement on gadolinium-enhanced MRI can easily be misinterpreted, potentially underestimating its malignant potential. Given the significant differences in the management of malignant and benign lesions, an accurate diagnosis is imperative for appropriate intervention. We present a case of PCNSTL without MRI enhancement. In instances of non-enhancing lesions with a clinical suspicion of malignancy, a more aggressive diagnostic approach should be adopted.
- Citation: Kim CS, Choi CH, Yi KS, Kim Y, Lee J, Woo CG, Jeon YH. Absence of enhancement in a lesion does not preclude primary central nervous system T-cell lymphoma: A case report. World J Clin Cases 2024; 12(2): 374-382
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/374.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.374
Primary central nervous system lymphoma (PCNSL) is a non-Hodgkin lymphoma that originates within the central nervous system (CNS) and remains largely confined to the CNS. Approximately 85% of PCNSL cases are restricted within the CNS[1,2]. It is an aggressive tumor with a poorer prognosis than lymphomas outside the CNS, with a median survival of less than two years[3,4].
PCNSL affects both immunocompromised and immunocompetent patients. It is rare among immunocompetent patients, accounting for 2%-4% of all newly diagnosed intracranial tumors[5]. While most PCNSL cases are diffuse large B-cell lymphomas, primary CNS T-cell lymphoma (PCNSTL) is notably rare[2,3]. This rarity contributes to a limited understanding of the radiologic characteristics.
PCNSTLs typically exhibit some degree of enhancement on T1-weighted MRI after contrast administration, and most lesions show diffusion restriction on diffusion-weighted imaging[6]. Here, we present a case of a 69-year-old male with PCNSTL in which the diagnosis was delayed because of the absence of the expected enhancement.
A 69-year-old male presented to the neurology department complaining of cognitive impairment and gradual onset of left lower leg weakness over the past two weeks.
Two years earlier, the patient had been treated for early-stage gastric cancer using endoscopic mucosal dissection. One month before the neurology consultation, he visited the ophthalmology department with right visual discomfort and was diagnosed with anterior uveitis. This diagnosis was confirmed by positive results for the Epstein-Barr virus (EBV) polymerase chain reaction (PCR) and Toxocara canis antibody IgG obtained from anterior chamber aspiration. He also reported a history of recurrent oral ulcers; however, no other significant illnesses were noted.
Two years earlier, the patient had been treated for early-stage gastric cancer using endoscopic mucosal dissection. One month before the neurology consultation, he visited the ophthalmology department with right visual discomfort and was diagnosed with anterior uveitis. This diagnosis was confirmed by positive results for the EBV PCR and Toxocara canis antibody IgG obtained from anterior chamber aspiration. He also reported a history of recurrent oral ulcers; however, no other significant illnesses were noted.
The patient denied any family history of malignant neoplasms.
Vital signs on presentation were body temperature, 36.1 °C; blood pressure, 127/67 mmHg; heart rate, 65 beats per minute; and respiratory rate, 20 breaths per minute. Neurological examination revealed Medical Research Council (MRC) grade 3 weakness in the left leg, with no other notable neurological abnormalities.
Serum white blood cell (WBC) levels were within the normal range (7680/103 μL), but the WBC count in the cerebrospinal fluid (CSF) showed a slight elevation (16/μL). Serum tests for human immunodeficiency virus and CSF assays for both EBV and John Cunningham virus were also negative.
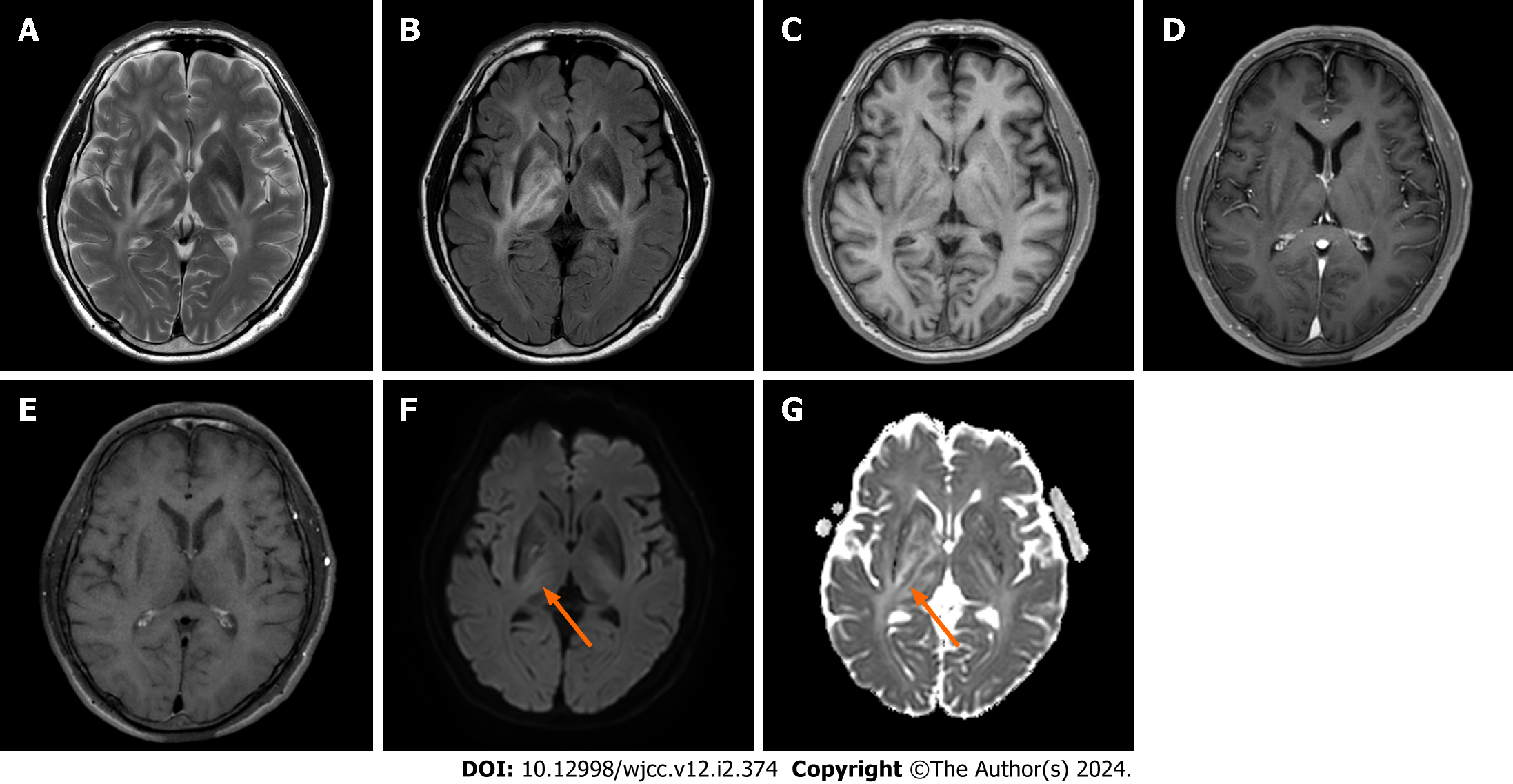
The initial MRI scan showed asymmetric T2-hyperintense lesions in the bilateral periventricular white matter, basal ganglia, thalamus, right cerebral peduncle, and right middle cerebellar peduncle. Mild diffusion restriction was observed in the right posterior limb of the internal capsule. Contrast-enhanced T1 imaging showed no notable enhancement, and the magnetic resonance angiography results were unremarkable (Figure 1).
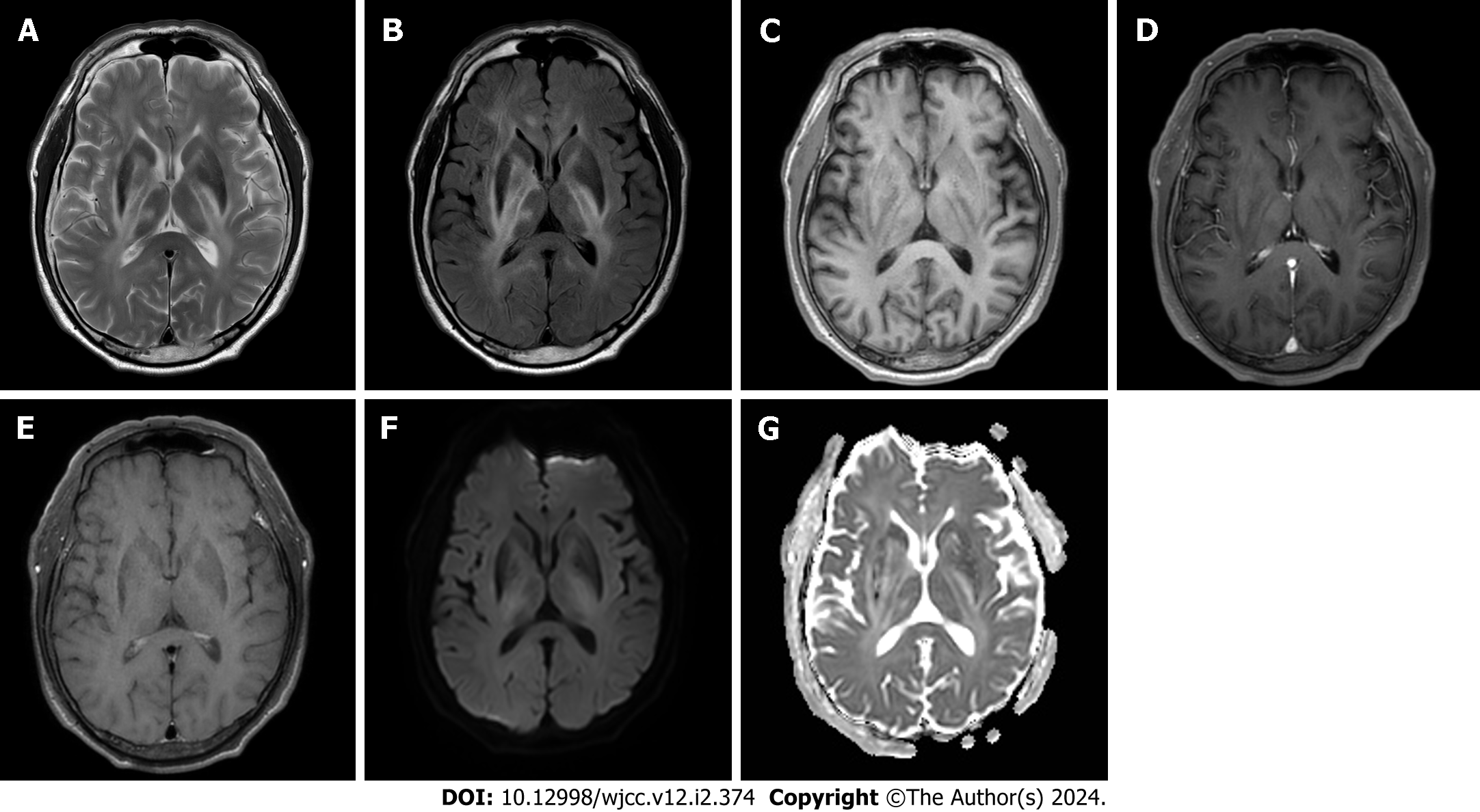
Based on the patient's history of anterior uveitis, an initial diagnosis of neuro-Behçet’s disease was considered, although the imaging findings were inconsistent. Given the absence of enhancement and significant diffusion restriction, a tumor lesion was initially considered less probable. Considering the patient’s age, a preliminary diagnosis of neuro-Behçet’s disease was made without further investigation. The patient was administered a high-dose steroid regimen, specifically prednisolone at 1 mg/d for five days. The patient reported an improvement in his subjective symptoms. However, on neurological examination, left leg weakness remained at MRC grade 3, and a follow-up MRI performed five days post-steroid initiation revealed no change in the previously identified lesions, an unanticipated result (Figure 2). The patient was discharged at his request, against medical advice.
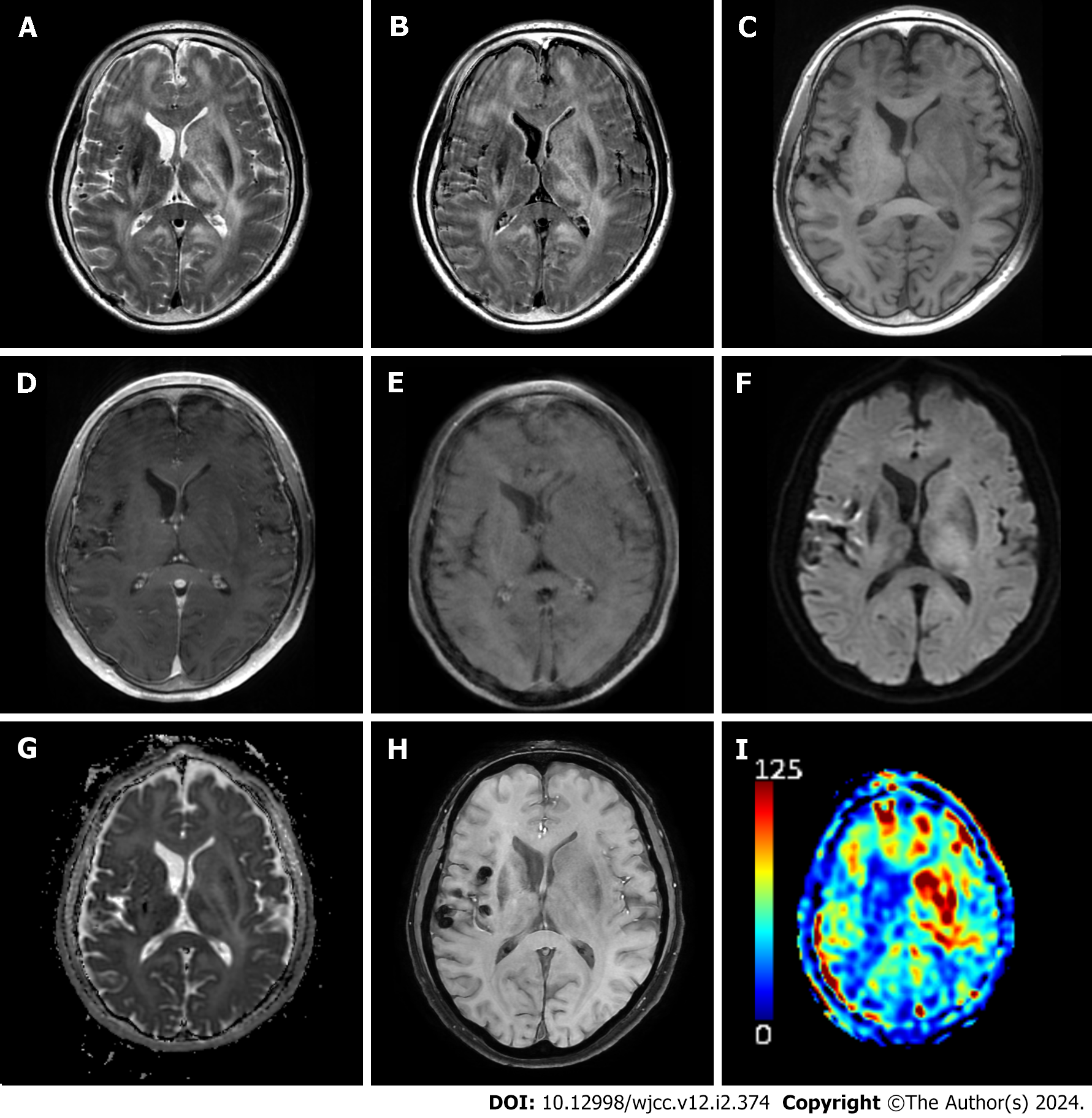
Over the subsequent weeks, the patient’s mental status became stuporous, and he was soon comatose. The patient was hospitalized in the emergency room. MRI conducted one month after the initial imaging illustrated a reduction in the extent of the lesion in the right basal ganglia. However, the other lesions had expanded, causing a mass effect on the left lateral ventricle. New subarachnoid space multifocal hemorrhages were identified, and arterial spin labeling-perfusion MRI revealed increased cerebral blood flow (Figure 3). The patient’s symptoms, MRI findings, and diagnoses are summarized in Table 1. Based on these findings, conditions such as glioblastoma, lymphoma, and metastasis were considered. Chest and abdominal computed tomography (CT) and positron emission tomography-CT were performed to rule out metastases. Brain biopsy was performed.
| Time | Initial | 5 d after | 1 month after |
| Neurologic symptom | Mild cognitive impairments | No significant change in MRC grade 3 left lower leg weakness | Changed mental status to comatose |
| MRC grade 3 left lower leg weakness | |||
| MRI | Asymmetric hyperintensities at the bilateral periventricular white matters, basal ganglia, thalamus, cerebral and cerebellar peduncles | No significant change in extent of T2 hyperintense lesion | Reduced extent of T2 hyperintense lesion in right basal ganglia, but increased extent in left hemisphere compressing left lateral ventricle |
| Newly appeared SAH | |||
| Laboratory exams | Slight elevation of WBC in CSF | Nonspecific | Nonspecific |
| Considered diagnoses | Neuro-Behçet’s disease | Neuro-Behçet’s disease | Glioblastoma |
| Lymphoma | |||
| Metastasis | |||
| Treatment | Prednisolone at 1 mg/d for 5 d | None | Supportive care for bacteremia and sepsis |
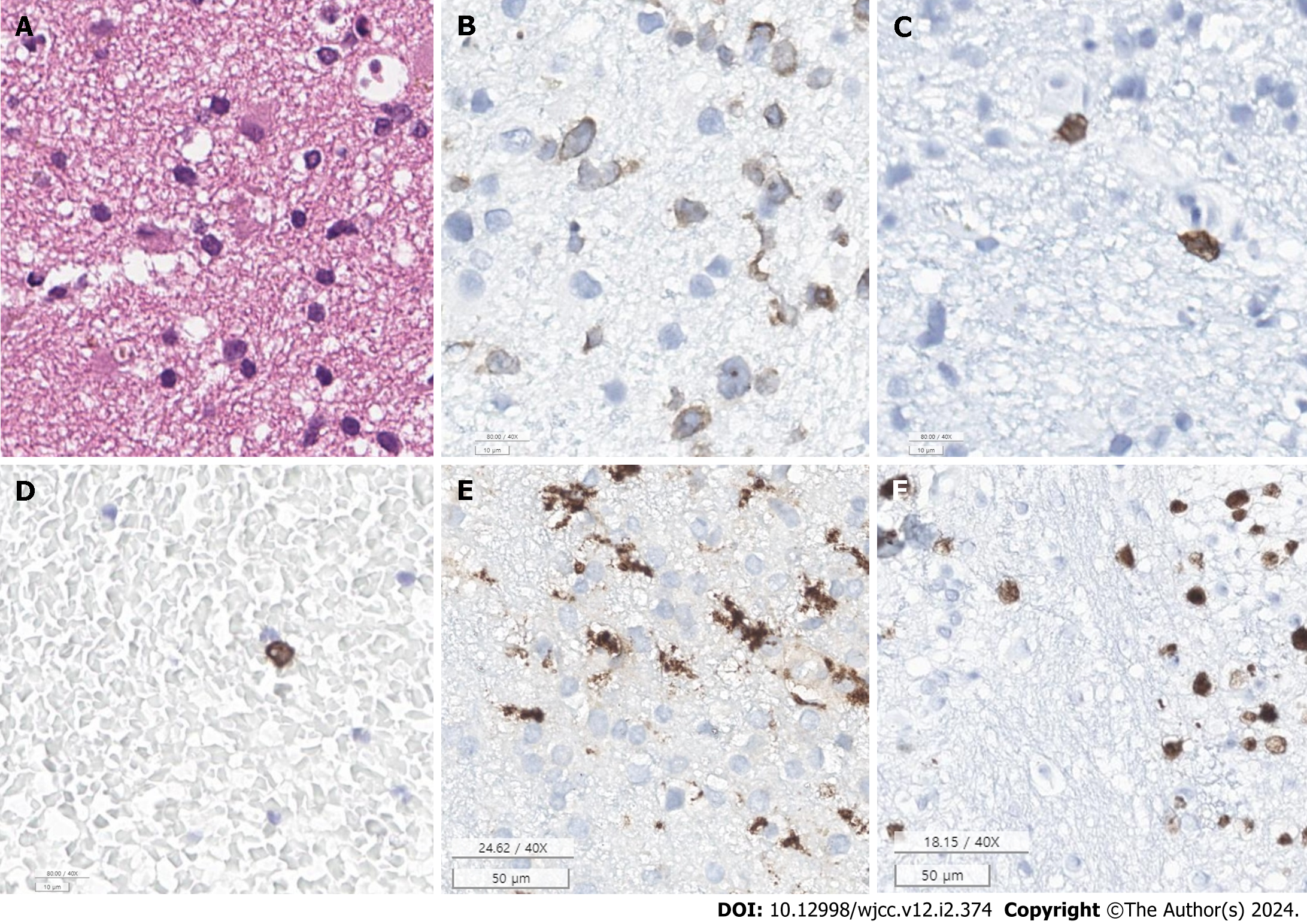
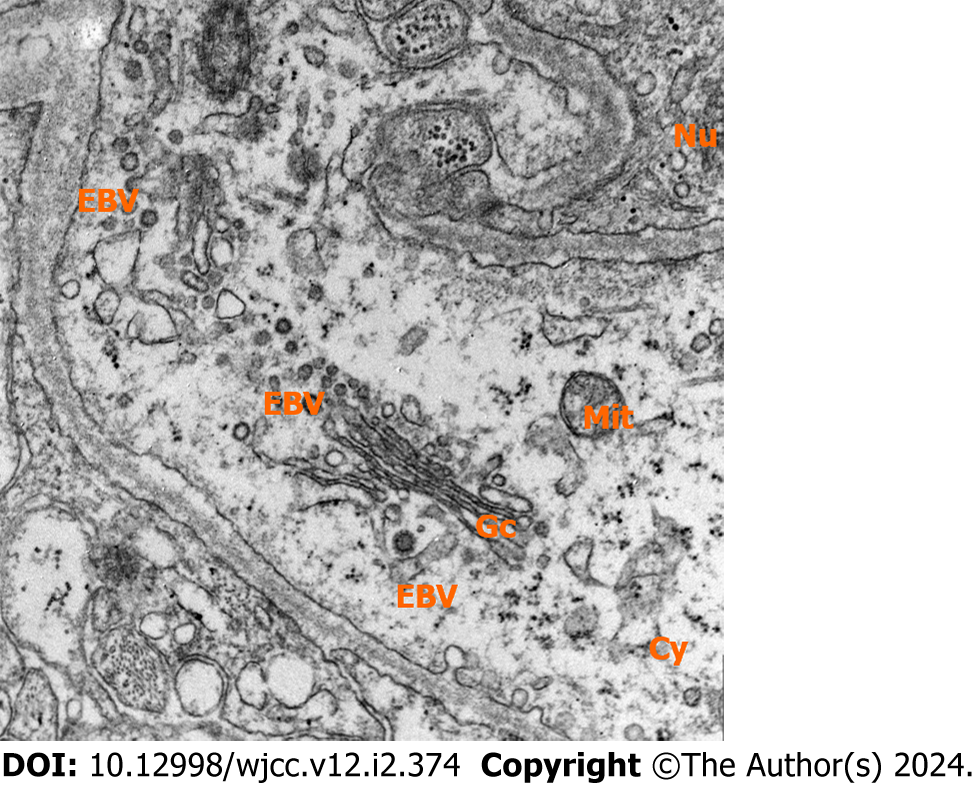
A stereotactic biopsy of the left putamen was performed. Immunohistochemical analysis revealed cluster of differentiation (CD)3 and CD8 positivity, indicating T-cell infiltration. CD68-positive microglia were also observed, suggesting a response to brain injury or pathology. Positive P53 staining was observed, which might indicate disrupted cell cycle regulation, often associated with malignant tumors. Moreover, Antigen Kiel-67 positivity in approximately 40% of the cells indicated a high proliferation rate, which is typical of aggressive neoplasms (Table 2, Figure 4). Electron microscopy corroborated a lymphoid neoplasm diagnosis, describing neoplastic lymphoid cells and virus particles indicative of a possible associated viral infection, like EBV, which has been linked to certain lymphomas. This evidence pointed towards a possible diagnosis of PCNSTL (Figure 5).
| Immunohistochemical | P53: Positive |
| CD68: Positive in the microglia | |
| Glial Fibrillary Acidic Protein: Positive in the reactive astrocytes | |
| Myeloperoxidase: Negative | |
| K27M: Negative | |
| Vimentin: Focal positive in the lymphocytes and reactive astrocytes | |
| CD56: Nonspecifically positive | |
| Phospho-Histone H3: 23/10 HPFs | |
| LFB: Positive in the myelin sheath (no loss of myelin sheath) | |
| Isocitrate Dehydrogenase 1: Negative (no mutation) | |
| EZHIP: Negative | |
| Olig2: Focal positive | |
| TMHH3 (Lys27): Positive (no mutation) | |
| CD3: Some CD3-positive T-cells infiltration | |
| CD20: A few positive cells in a perivascular area | |
| IBA-1: Positive in the microglia | |
| Ki-67: Positive in 40.0% | |
| CD8: A few positive cells | |
| Electronic microscopy | Ultrathin sections show sheets of round to oval-shaped neoplastic lymphoid cells. The nuclei of tumor cells are round to oval with heterochromatin and prominent nucleoli. The cytoplasm is scanty and contains eccentrically located a few mitochondria and electron-dense granules |
| Diagnosis | Brain, putamen, left, stereotactic biopsy: Scattered proliferative T-cell infiltration, consistent with T-cell lymphoma |
Considering both the pathological findings and confinement of the lesion within the brain, the definitive diagnosis was PCNSTL.
The patient was slated for combined chemoradiotherapy. However, this was postponed due to the onset of bacteremia during hospitalization.
The patient died of septic shock approximately three months post-diagnosis.
PCNSL in immunocompetent patients is predominantly a diffuse large B-cell lymphoma, comprising up to 90% of all cases. Other types, including PCNSTL, constitute the remainder. The prevalence of PCNSTL among all cases of PCNSL is 2% in Western countries, 8.5%-14.3% in Japan, and 16.7% in Korea[4,7]. However, these elevated rates in East Asian countries may be biased due to small sample sizes. Despite its rarity, the clinical features and prognosis of PCNSTL resemble those of other types of PCNSL[8].
The present case posed a diagnostic challenge owing to the atypical imaging characteristics, which, to our knowledge, have not been previously reported. Given the low incidence of PCNSTL, limited imaging findings have been reported, and its diagnosis is histopathologically challenging because of the frequently absent atypical cytological features of most T-cell lymphomas[7,9]. Additionally, no specific immunohistochemical markers for neoplastic T-cells exist[10].
Previous imaging studies of PCNSTL in immunocompetent individuals have reported features such as heterogeneous or rim enhancement, which are uncharacteristic of PCNSL, given that typical primary CNS B-cell lymphoma (PCNSBL) usually presents with homogenous enhancement[11]. Moreover, PCNSTL exhibits hemorrhage or cystic changes more frequently than PCNSBL. In East Asian countries, PCNSTL typically manifests in the supratentorial or subcortical areas without leptomeningeal involvement[11]. Other studies have described nodular, patchy, homogenously enhancing lesions[12] and multifocal mass-like lesions with heterogeneous enhancement and mass effects[13]. In contrast, our patient had asymmetric T2 high signal intense lesions in the pons and basal ganglia with minimal diffusion restriction and no enhancement. These features were not previously associated with PCNSTL.
Histologically, PCNSTL predominantly presents with small cells, occasionally interspersed with medium-sized cells. These cells do not typically form a solid mass or aggregate but demonstrate notable perivascular infiltration, a significantly different histopathologic growth pattern from that of PCNSBL[7]. Our patient exhibited scattered proliferative T cells, leading to a diagnosis of PCNSTL. This histopathologic pattern might explain the observed lack of enhancement, suggesting that there was no solid mass-like lesion to enhance, but rather relatively sparse proliferative T-cells.
The lesion distribution was also distinctive and localized in deeper brain regions, such as the basal ganglia and pons, which are uncommon sites for PCNSL. Previous studies have indicated that low-grade PCNSLs have different imaging features from their high-grade counterparts[9]. Given that PCNSTL more often presents with low-grade histology than B-cell-origin lymphomas, this case might represent a low-grade PCNSTL with a deep brain location and no enhancement[2,7]. Unfortunately, further grading and subtyping were not possible.
The patient was initially considered to have potential neuro-Behçet’s disease, given that the clinical history and imaging findings aligned with the neurological manifestations of Behçet’s disease[14]. High-dose corticosteroids, the first-line treatment for neuro-Behçet’s disease[15], were administered. However, corticosteroids induce T-cell apoptosis, which complicates the diagnosis of PCNSTL[10]. Some CNS lymphomas respond to corticosteroids, resulting in symptomatic and radiological improvements that mimic vasculitis. Over time, the growth of corticosteroid-resistant clones may render the lymphoma resistant to corticosteroid treatment[9,10]. Consequently, corticosteroid therapy before stereotactic brain biopsy is discouraged as it may delay an accurate diagnosis. Given the significant differences in treatment strategies for vasculitis (such as neuro-Behçet’s disease) and PCNSTL, adopting a more proactive biopsy approach could potentially enhance the diagnosis and prognosis of PCNSTL, especially in cases presenting with atypical radiological features, such as the absence of enhancement.
Herein, we present a unique case of PCNSTL without enhancement. Given its rarity, known imaging features of PCNSTL are limited. To the best of our knowledge, the absence of enhancement, as observed in the present case, has not been previously documented. This case underscores the importance of not ruling out PCNSTL in the differential diagnosis, even without enhancement. Considering the stark differences in the management of PCNSTL and other benign conditions, biopsy for definitive pathological confirmation is strongly recommended.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Yoshida N, Japan S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Plasswilm L, Herrlinger U, Korfel A, Weller M, Küker W, Kanz L, Thiel E, Bamberg M. Primary central nervous system (CNS) lymphoma in immunocompetent patients. Ann Hematol. 2002;81:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Jahnke K, Thiel E, Schilling A, Herrlinger U, Weller M, Coupland SE, Krümpelmann U, Stein H, Korfel A. Low-grade primary central nervous system lymphoma in immunocompetent patients. Br J Haematol. 2005;128:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol. 2017;35:2410-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 406] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 4. | Menon MP, Nicolae A, Meeker H, Raffeld M, Xi L, Jegalian AG, Miller DC, Pittaluga S, Jaffe ES. Primary CNS T-cell Lymphomas: A Clinical, Morphologic, Immunophenotypic, and Molecular Analysis. Am J Surg Pathol. 2015;39:1719-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Song KW, Issa S, Batchelor T. Primary central nervous system lymphoma: epidemiology and clinical presentation. Ann Lymphoma. 2021;5:16. [DOI] [Full Text] |
| 6. | Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 306] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 7. | Choi JS, Nam DH, Ko YH, Seo JW, Choi YL, Suh YL, Ree HJ. Primary central nervous system lymphoma in Korea: comparison of B- and T-cell lymphomas. Am J Surg Pathol. 2003;27:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Ferreri AJ. How I treat primary CNS lymphoma. Blood. 2011;118:510-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Dulai MS, Park CY, Howell WD, Smyth LT, Desai M, Carter DM, Vogel H. CNS T-cell lymphoma: an under-recognized entity? Acta Neuropathol. 2008;115:345-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Fraser E, Gruenberg K, Rubenstein JL. New approaches in primary central nervous system lymphoma. Chin Clin Oncol. 2015;4:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 11. | Kim EY, Kim SS. Magnetic resonance findings of primary central nervous system T-cell lymphoma in immunocompetent patients. Acta Radiol. 2005;46:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Byun SH, Kim DM, Lee IH, Song CJ, Kim KH, Choi SY. Primary Central Nervous System Involvement in Peripheral T-Cell Lymphoma: A Case Report. Taehan Yongsang Uihakhoe Chi. 2021;82:255-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Manenti G, Di Giuliano F, Bindi A, Liberto V, Funel V, Garaci FG, Floris R, Simonetti G. A Case of Primary T-Cell Central Nervous System Lymphoma: MR Imaging and MR Spectroscopy Assessment. Case Rep Radiol. 2013;2013:916348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Kim SW, Kim TG, Oh J, Kim DY, Choi YC, Kim SM, Shin HY, Bang D. Clinical and Radiographic Characteristics of Neuro-Behçet's Disease in South Korea. J Clin Neurol. 2019;15:429-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Alpsoy E, Leccese P, Emmi G, Ohno S. Treatment of Behçet's Disease: An Algorithmic Multidisciplinary Approach. Front Med (Lausanne). 2021;8:624795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |