Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.367
Peer-review started: September 7, 2023
First decision: November 20, 2023
Revised: November 28, 2023
Accepted: December 26, 2023
Article in press: December 26, 2023
Published online: January 16, 2024
Processing time: 125 Days and 21.5 Hours
Intraductal papillary neoplasms of the bile duct (IPNBs) are rare and characterized by papillary growth within the bile duct lumen. IPNB is similar to obstructive biliary pathology. In this report, we present an unexpected case of asymptomatic IPNB and consolidate our findings with the relevant literature to augment our understanding of this condition. Integrating relevant literature contributes to a more comprehensive understanding of the disease.
A 66-year-old Chinese male patient was admitted to our hospital for surgical intervention after gallstones were discovered during a routine physical examination. Preoperative imaging revealed a lesion on the left side of the liver, which raised the suspicion of IPNB. A laparoscopic left hemihepatectomy was performed, and subsequent histopathological examination confirmed the diagnosis of IPNB. At the 3-mo postoperative follow-up, the patient reported good recovery and no metastasis. IPNB can manifest both latently and asymptomatically. Radical surgical resection is the most effective treatment for IPNB.
Hepatic and biliary masses, should be considered to diagnose IPNB. Prompt surgery and vigilant follow-up are crucial in determining prognosis.
Core Tip: Intraductal papillary neoplasms are relatively uncommon in clinical practice, often eluding detection by clinicians owing to the inadequacy of conventional imaging tests such as abdominal ultrasound and computed tomography, to identify and diagnose tumors. In the present case, the patient was asymptomatic. However, if the tumor was not detected and resected promptly, the patient could have lost the opportunity for surgical intervention when developing relevant biliary symptoms. Early detection and surgical intervention hold promise for a positive prognosis. Clinicians must further enhance their understanding of the clinical attributes and imaging indications to prevent missed diagnoses.
- Citation: Zhu SZ, Gao ZF, Liu XR, Wang XG, Chen F. Surgically treating a rare and asymptomatic intraductal papillary neoplasm of the bile duct: A case report. World J Clin Cases 2024; 12(2): 367-373
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/367.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.367
Intraductal papillary neoplasms of the bile duct (IPNBs) are rare tumors that predominantly affect middle-aged and elderly individuals. It exhibits a slightly higher prevalence in men than in women and has significant potential for malignancy. Most patients present with symptoms, such as epigastric pain, acute cholangitis, and jaundice, accompanied by elevated liver enzyme levels. However, asymptomatic cases are less frequent. Here, we report a case of asymptomatic IPNB and conduct a comprehensive review of the relevant literature to enhance our understanding of this disease.
A 66-year-old male patient was admitted to our hospital with a 7-year history of gallbladder stones, detected during a physical examination, leading to a proposed laparoscopic cholecystectomy.
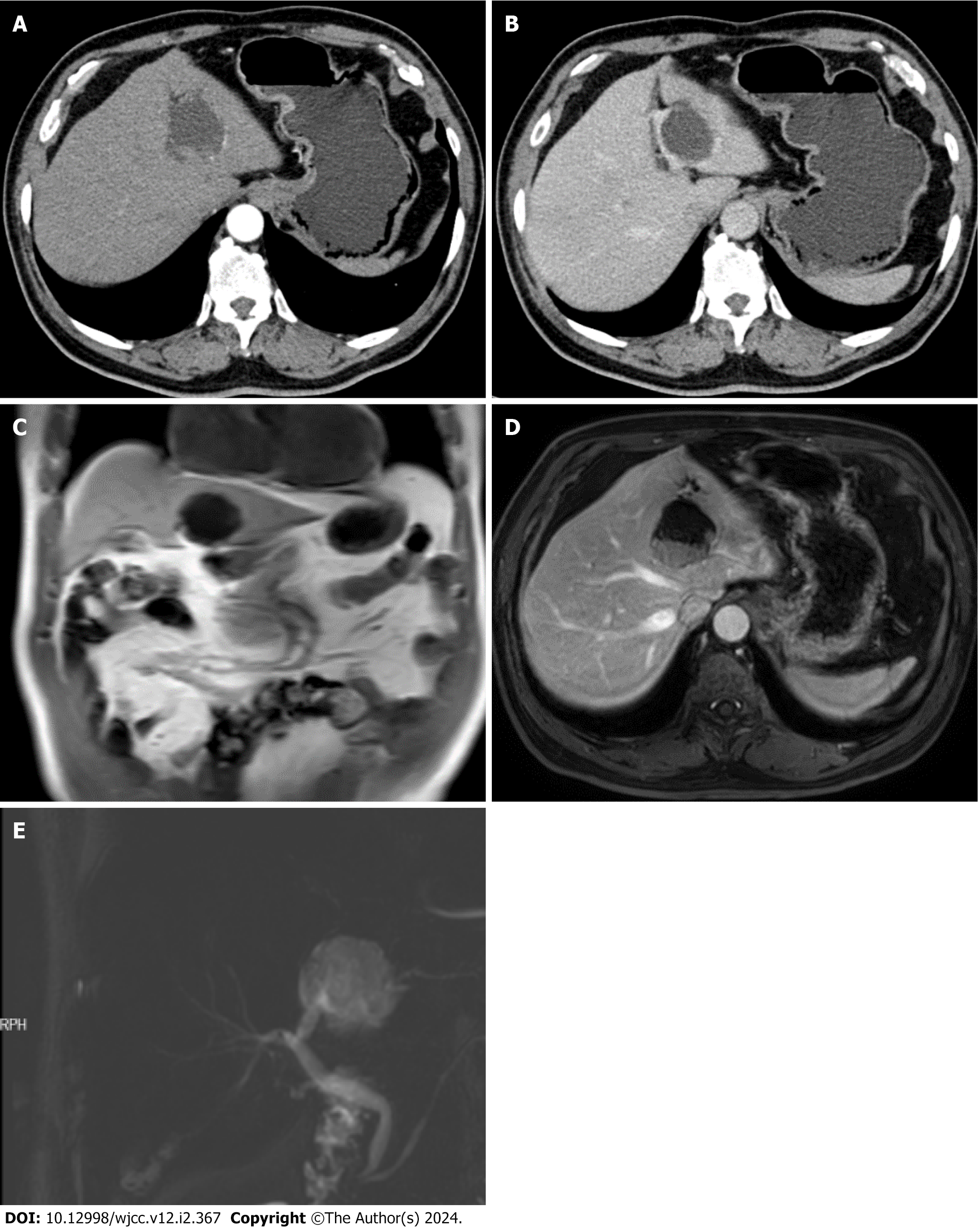
Preoperative abdominal ultrasonography revealed multiple gallstones and cysts in the left lobe of the liver. Plain computed tomography (CT) of the chest and abdomen revealed low-density lesions in the left hepatic lobe, prompting the recommendation for an enhanced abdominal CT examination.
The patient had no prior history of surgery but had a history of multiple chronic conditions, including hypertension, diabetes, hyperlipidemia, and atrial fibrillation. The patient consistently adhered to the medication regimen and reported satisfactory management.
The patient denied any family history of tumors.
The patient was alert and in good condition. No abnormal symptoms such as jaundice, nausea, vomiting, or acid reflux were noted. An abdominal examination revealed no tenderness, rebound tenderness, or muscle tension. No abnormal masses were palpable, and the Murphy's sign was negative.
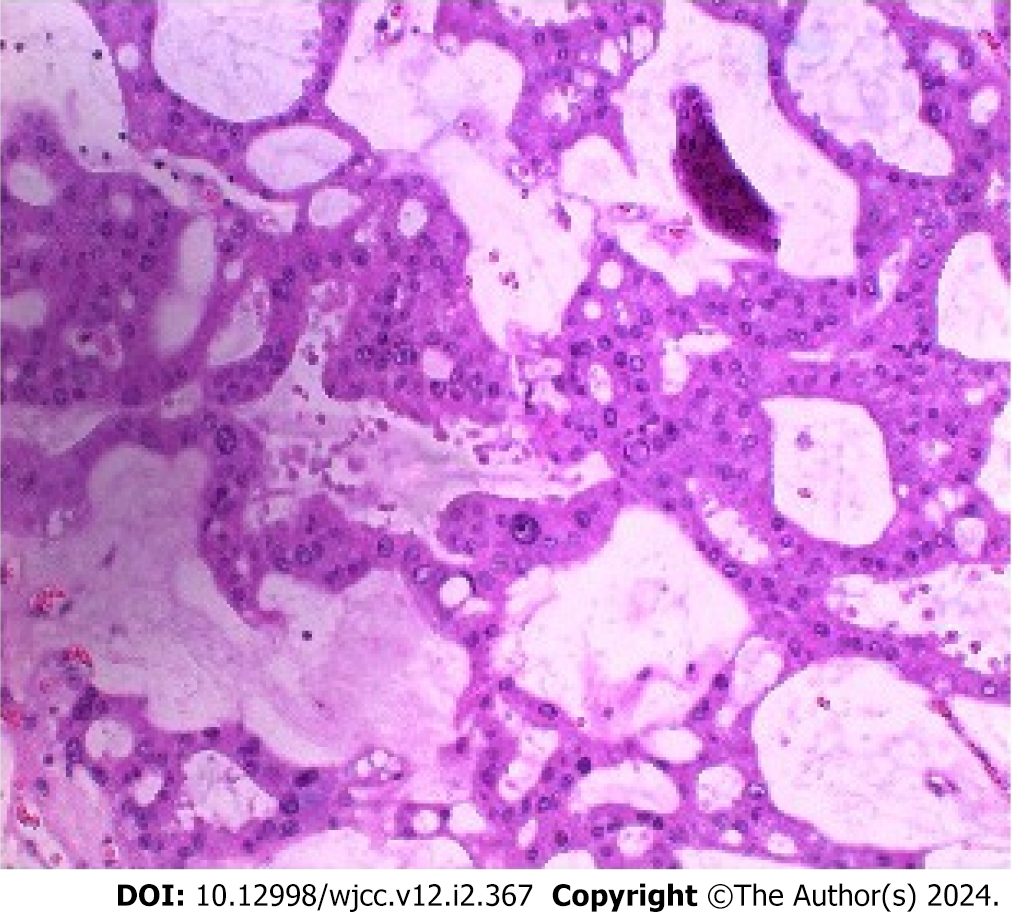
Total bilirubin, 20.6 μmol/L (reference range: 0-23 μmol/L); direct bilirubin, 7 μmol/L (reference range: 0-4 μmol/L); indirect bilirubin, 13.6 μmol/L (reference range: 0-17 μmol/L); alanine transaminase (ALT), 13 U/L (reference range: 9-50 U/L); aspartate transaminase (AST), 19 U/L (reference range: 15-40 U/L); carcinoembryonic antigen (CEA), 5.46 ng/mL (reference range: 0-5 ng/mL); alpha fetoprotein, 2.61 ng/mL (reference range: 0-20 ng/mL); carbohydrate antigen 199 (CA199), 29.88 U/mL (reference range: 0-37 U/mL). The rest of the laboratory results were normal. Pathological examination of the lesion revealed an IPNB with severe epithelial dysplasia or high-grade intraepithelial neoplasia (Figure 1).
Abdominal enhanced CT arterial phase (Figure 2A), abdominal enhanced CT venous phase (Figure 2B), and an initial diagnosis of a cystic, solid lesion in the left lobe of the liver with dilatation of the distal intrahepatic bile ducts were made; cholangiocarpal cystadenoma was ruled out, and magnetic resonance (MR) enhancement examination was recom
The patient was diagnosed with IPNB, accompanied by severe epithelial dysplasia and high-grade intraepithelial neoplasia.
The patient underwent a laparoscopic left hemihepatectomy and cholecystectomy. No additional masses were identified perioperatively, and no subsequent treatment was required.
The patient exhibited favorable postoperative recovery and underwent regular follow-ups at our outpatient clinic for three months using abdominal CT, with no evidence of metastasis or recurrence.
According to the latest WHO classification, IPNB, recognized as a precancerous lesion in cholangiocarcinoma (CCA), is predominantly characterized by papillary or villous growth within the bile duct lumen[1]. More than 30% of patients with IPNB exhibit significant mucus secretion into the lumen[2]. The pathogenesis of IPNB remains unclear; however, investigations have identified major risk factors, including hepatic bile duct stones, hepatic schistosome infection, primary sclerosing cholangitis, congenital biliary anomalies, and exposure to substances, such as chlorinated organic solvents. Other contributing factors include bile duct malformations, familial adenomatous polyposis, and Gardner's syndrome. The progression of IPNB is gradual, and is initiated by inflammation due to biliary stasis, biliary infections, and CCAs, ultimately culminating in a multistage transformation of the bile ducts, marked by proliferation, heterotopic hyperplasia, and carcinoma development[3]. Molecular studies have identified mutations in CTNNB1, STK11, and GNAS in patients with IPNB concurrently with intraductal papillary mucinous neoplasm (IPMN)[4]. Kirsten rat sarcoma viral oncogene mutations are risk factors for IPNB progression and offer potential diagnostic and therapeutic avenues[5]. Tanaka et al[6] identified EVI1 expression in IPNB, highlighting its potential as a prognostic marker. Notably, patients with MUC1-expressing IPNB tumors have a more unfavorable prognosis[7]. Owing to the relatively subtle clinical symptoms of IPNB compared with those of other tumors, a significant number of patients with IPNB are diagnosed after the disease has advanced, leading to bile duct obstruction. Subsequently, these patients gradually develop associated biliary symptoms, including jaundice, abdominal pain, and cholangitis[8]. The current study's findings indicate a higher prevalence of IPNB in East Asia, particularly in regions such as Japan, Korea, and Thailand, where intrahepatic bile duct stones are prevalent[9]. IPNB is also considered the biliary counterparts of IPMNs of the pancreas because of their mucin-hypersecretory properties[10]. To enhance staging and prognostic accuracy, IPNB has been further classified into four distinct subtypes: Pancreaticobiliary, intestinal, eosinophilic, and gastric; the intestinal subtype is the most prevalent and displays the highest incidence of malignancy. Currently, the commonly used modified IPNB staging systems include types 1 (intrahepatic biliary) and 2 (extrahepatic biliary). Approximately 40% of IPNB cases were classified as type 1, and 60% as type 2. Notably, mucin hypersecretion is more prevalent in type 1 IPNB (I-IPNB) compared to type 2[11,12]. Recent studies have demonstrated that the modified two-tier grading system effectively reflects postoperative survival compared to the traditional grading approach, contributing to a comprehensive postoperative assessment of IPNB[13]. Additionally, one research team discovered that the survival rate for type 1 was notably superior to that for type 2 by employing a scoring system encompassing six pathological characteristics (such as location, mucin secretion, and tissue structure)[14]. Compared with CCA, IPNB generally exhibits a more favorable overall prognosis. However, given the potential for malignant transformation, early diagnosis and prompt treatment of IPNB remain central to ongoing research. Our case was incidentally discovered when the patient was admitted to the hospital for surgical treatment of gallbladder stones. The majority of cases were identified as a consequence of disease progression, presenting with biliary symptoms that led them to seek medical attention for laboratory and imaging assessments. Nonetheless, routine laboratory tests, such as ALT/AST/alkaline phosphatase and tumor markers CEA/CA199, lack distinct specificity and often exhibit elevated levels in many patients with typical biliary inflammation[15]. CT and MRI are remain the primary diagnostic tools for imaging evaluation. However, patients with IPNB often present with concurrent biliary inflammation and stones, resulting in a low rate of early clinical diagnosis. Currently, choledochoscopy should be employed in conjunction with CT and MRI for the precise localization of IPNB. Transoral cholangioscopy (POCS) enables the assessment of the scope of bile duct lesions, thereby facilitating the formulation of a tailored surgical strategy[16]. In IPNB cases characterized by substantial mucin secretion, percutaneous transhepatic cholangioscopy (PTCS) offers greater advantages than POCS[17]. The 2012 guidelines identified pertinent predictors, such as obstructive jaundice, duct size, wall nodules, and abrupt size changes, as potential indicators of the extent of malignancy in IPNB[18]. A recent study conducted by a Korean research team revealed that of the 116 cases of I-IPNB, 62 (53.4%) exhibited invasive carcinomas, whereas 61 (76.3%) of the 80 cases of type 2 extrahepatic IPNB displayed invasive carcinomas. Multifactorial analysis indicated that mural nodules of < 12 mm and intensified mural nodules were predictors of malignancy in I-IPNB and type 2 IPNB, respectively. In addition, 43.7% of patients with nonsurgical mucosal alloplastic hyperplasia would develop malignancies within three years[19].
Prompt initiation of surgical treatment is imperative for patients diagnosed with IPNB, likely achieving complete margin-negative (R0), significantly enhancing patient prognosis[20]. Conventional surgical approaches include partial hepatectomy, choledochotomy, pancreaticoduodenectomy, hepatopancreaticoduodenectomy, and liver transplantation. The optimal surgical choice is determined through preoperative multidisciplinary team discussions, PTCS, and POCS integrated with intraoperative cholangioscopy. Recently, a Japanese research team effectively employed red dichroic imaging technology for precise tumor extent assessment during preoperative POCS in a 75-year-old IPNB patient, consequently facilitating a successful pancreaticoduodenectomy[21]. Palliative modalities are frequently employed in patients with severe preoperative bile duct inflammation or in advanced cases that are not amenable to surgical intervention. These include endoscopic nasobiliary drainage and endoscopic retrograde cholangiopancreatography biliary stenting aimed to mitigate the patient's symptoms. Innovative techniques, such as radiofrequency ablation and photodynamic therapy have emerged as viable treatment alternatives[22,23]. A Korean research team successfully alleviated jaundice in a patient with advanced IPNB using argon plasma coagulation, and the current follow-up period extends beyond two years[24]. Advancements in novel technologies have the potential to enhance patient survival significantly. Despite IPNB's notably improved overall prognosis compared with CCA, with a 5-year survival rate exceeding 80%[25], ongoing regular follow-up remains imperative. Notably, approximately 13%-29% of patients with surgically resected IPNB experience recurrence within a short timeframe. This recurrence rate escalates to 47%-62% in cases where the patient is diagnosed with invasive IPNB[26].
In conclusion, IPNB, recognized as a precancerous lesion, has a favorable overall prognosis. However, the precise pathogenesis and mechanisms underlying IPNB progression remain unclear. Furthermore, nonspecific clinical manifestations pose a challenge for early diagnosis. Meticulous preoperative imaging, intraoperative cholangioscopy, and prompt pathological evaluation of resection margins are crucial for the informed selection of an appropriate surgical strategy. A comprehensive exploration of molecular targeted therapy coupled with vigilant postoperative monitoring will aid in formulating a more informed and rational treatment protocol for individuals affected by IPNB.
We extend our gratitude to the patients for their valuable contributions to this case report. Our appreciation also goes to the Department of General Surgery at the Second Affiliated Hospital of Jiaxing University for their support in managing this case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yildiz K, Turkey S-Editor: Qu XL L-Editor: A P-Editor: Qu XL
| 1. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2422] [Article Influence: 484.4] [Reference Citation Analysis (3)] |
| 2. | Wang X, Cai YQ, Chen YH, Liu XB. Biliary tract intraductal papillary mucinous neoplasm: report of 19 cases. World J Gastroenterol. 2015;21:4261-4267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Nakanuma Y, Kakuda Y, Sugino T, Sato Y, Fukumura Y. Pathologies of Precursor Lesions of Biliary Tract Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 4. | Zen Y, Akita M. Neoplastic Progression in Intraductal Papillary Neoplasm of the Bile Duct. Arch Pathol Lab Med. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Sun T, Zuo T, Hui P, Cai G. Significance of KRAS mutation testing in biliary brushing cytology specimens: A 10-year retrospective review. Cancer Cytopathol. 2022;130:558-565. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Tanaka M, Shibahara J, Ishikawa S, Ushiku T, Morikawa T, Shinozaki-Ushiku A, Hayashi A, Misumi K, Tanaka A, Katoh H, Sakuma K, Kokudo T, Inagaki Y, Arita J, Sakamoto Y, Hasegawa K, Fukayama M. EVI1 expression is associated with aggressive behavior in intrahepatic cholangiocarcinoma. Virchows Arch. 2019;474:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Bennett S, Marginean EC, Paquin-Gobeil M, Wasserman J, Weaver J, Mimeault R, Balaa FK, Martel G. Clinical and pathological features of intraductal papillary neoplasm of the biliary tract and gallbladder. HPB (Oxford). 2015;17:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E, Lee SG, Min YI. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, Sakai Y, Yokosuka O, Miyazaki M. Intraductal papillary neoplasms of the bile duct. Int J Hepatol. 2014;2014:459091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Kubota K, Nakanuma Y, Kondo F, Hachiya H, Miyazaki M, Nagino M, Yamamoto M, Isayama H, Tabata M, Kinoshita H, Kamisawa T, Inui K. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association. J Hepatobiliary Pancreat Sci. 2014;21:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Kubota K, Jang JY, Nakanuma Y, Jang KT, Haruyama Y, Fukushima N, Furukawa T, Hong SM, Sakuraoka Y, Kim H, Matsumoto T, Lee KB, Zen Y, Kim J, Miyazaki M, Choi DW, Heo JS, Endo I, Hwang S, Nakamura M, Han HS, Uemoto S, Park SJ, Hong EK, Nanashima A, Kim DS, Kim JY, Ohta T, Kang KJ, Fukumoto T, Nah YW, Seo HI, Inui K, Yoon DS, Unno M. Clinicopathological characteristics of intraductal papillary neoplasm of the bile duct: a Japan-Korea collaborative study. J Hepatobiliary Pancreat Sci. 2020;27:581-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Nakanuma Y, Uesaka K, Okamura Y, Terada T, Fukumura Y, Kakuda Y, Sugino T, Sato Y, Taek JK, Park YN. Reappraisal of pathological features of intraductal papillary neoplasm of bile duct with respect to the type 1 and 2 subclassifications. Hum Pathol. 2021;111:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Nakanuma Y, Jang KT, Fukushima N, Furukawa T, Hong SM, Kim H, Lee KB, Zen Y, Jang JY, Kubota K. A statement by the Japan-Korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage. J Hepatobiliary Pancreat Sci. 2018;25:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Onoe S, Ebata T, Yokoyama Y, Igami T, Mizuno T, Yamaguchi J, Watanabe N, Otsuka S, Nakamura S, Shimoyama Y, Nagino M. A clinicopathological reappraisal of intraductal papillary neoplasm of the bile duct (IPNB): a continuous spectrum with papillary cholangiocarcinoma in 181 curatively resected cases. HPB (Oxford). 2021;23:1525-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Jung G, Park KM, Lee SS, Yu E, Hong SM, Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol. 2012;57:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Tringali A, Milluzzo SM, Ardito F, Laurenzi A, Ettorre GM, Barbaro B, Ricci R, Giuliante F, Boškoski I, Costamagna G. Peroral-cholangioscopy to plan surgery for protruding biliary lesions: report of four cases. Ther Adv Gastrointest Endosc. 2022;15:26317745221139735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Tsuyuguchi T, Sakai Y, Sugiyama H, Miyakawa K, Ishihara T, Ohtsuka M, Miyazaki M, Yokosuka O. Endoscopic diagnosis of intraductal papillary mucinous neoplasm of the bile duct. J Hepatobiliary Pancreat Sci. 2010;17:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 19. | Han SY, Kim DU, Nam HS, Kang DH, Jang SI, Lee DK, Shin DW, Cho KB, Yang MJ, Hwang JC, Kim JH, So H, Bang SJ, Sung MJ, Kwon CI, Lee DW, Cho CM, Cho JH. Comparison of the Malignant Predictors in Intrahepatic and Extrahepatic Intraductal Papillary Neoplasm of the Bile Duct. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Chan WH, Chen CM, Wang SY, Wu RC, Chen TC, Lee HK, Lin CH, Yeh CN. Intraductal papillary neoplasm of the bile duct presenting with hepatogastric fistula: a case report and literature review. Front Oncol. 2023;13:1193918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Koiwai A, Hirota M, Murakami K, Katayama T, Kin R, Endo K, Kogure T, Takasu A, Sakurai H, Kondo N, Takami K, Yamamoto K, Katayose Y, Satoh K. Direct peroral cholangioscopy with red dichromatic imaging 3 detected the perihilar margin of superficial papillary extension in a patient with intraductal papillary neoplasm of the bile duct. DEN Open. 2023;3:e228. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Natov NS, Horton LC, Hegde SR. Successful endoscopic treatment of an intraductal papillary neoplasm of the bile duct. World J Gastrointest Endosc. 2017;9:238-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Bechmann LP, Hilgard P, Frilling A, Schumacher B, Baba HA, Gerken G, Zoepf T. Successful photodynamic therapy for biliary papillomatosis: a case report. World J Gastroenterol. 2008;14:4234-4237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Arai J, Kato J, Toda N, Kurokawa K, Shibata C, Kurosaki S, Funato K, Kondo M, Takagi K, Kojima K, Ohki M, Seki M, Tagawa K. Long-term survival after palliative argon plasma coagulation for intraductal papillary mucinous neoplasm of the bile duct. Clin J Gastroenterol. 2021;14:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Kim JR, Jang KT, Jang JY, Lee K, Kim JH, Kim H, Kim SW, Kwon W, Choi DW, Heo J, Han IW, Hwang S, Kim WJ, Hong SM, Kim DS, Yu YD, Kim JY, Nah YW, Park HW, Choi HJ, Han HS, Yoon YS, Park SJ, Hong EK, Seo HI, Park DY, Kang KJ, Kang YN, Yu HC, Moon WS, Lim CS, Bae JM, Jo S, Lee W, Roh YH, Jeong JS, Jeong CY, Lee JS, Song IS, Kim KH, Kim HG, Cho CH, Joo SH, Won KY, Kim HJ, Choi JH, Chu CW, Lee JH, Park IY, Lee H, Lee SE, Kim HS, Lee HK, Cho MS, Han KM. Clinicopathologic analysis of intraductal papillary neoplasm of bile duct: Korean multicenter cohort study. HPB (Oxford). 2020;22:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |