Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.276
Peer-review started: November 6, 2023
First decision: November 22, 2023
Revised: December 5, 2023
Accepted: December 25, 2023
Article in press: December 25, 2023
Published online: January 16, 2024
Processing time: 66 Days and 0.2 Hours
Venous thromboembolism (VTE) is a potentially fatal complication of hepa
To evaluate the utility of monitoring plasma D-dimer levels in the early diagnosis of VTE after hepatectomy.
The medical records of patients who underwent hepatectomy at our institution between January 2017 and December 2020 were retrospectively analyzed. Patients were divided into two groups according to whether or not they developed VTE after hepatectomy, as diagnosed by contrast-enhanced computed tomography and/or ultrasonography of the lower extremities. Clinicopathological factors, including demographic data and perioperative D-dimer values, were compared between the two groups. Receiver operating characteristic curve analysis was per
In total, 234 patients who underwent hepatectomy were, of whom (5.6%) were diagnosed with VTE following hepatectomy. A comparison between the two groups showed significant differences in operative time (529 vs 403 min, P = 0.0274) and blood loss (530 vs 138 mL, P = 0.0067). The D-dimer levels on postoperative days (POD) 1, 3, 5, 7 were significantly higher in the VTE group than in the non-VTE group. In the multivariate analysis, intraoperative blood loss of > 275 mL [odds ratio (OR) = 5.32, 95% confidence interval (CI): 1.05-27.0, P = 0.044] and plasma D-dimer levels on POD 5 ≥ 21 μg/mL (OR = 10.1, 95%CI: 2.04-50.1, P = 0.0046) were independent risk factors for VTE after hepatectomy.
Monitoring of plasma D-dimer levels after hepatectomy is useful for early diagnosis of VTE and may avoid routine prophylactic anticoagulation in the postoperative period.
Core Tip: This is the first retrospective study to evaluate the utility of monitoring plasma D-dimer levels in the early diagnosis of venous thromboembolism (VTE) after hepatectomy. Thirteen (5.6%) patients were diagnosed with VTE following hepatectomy and none of the patients required intensive care unit management (or oxygen administration) due to worsening respiratory status. The VTE group tended to have significantly elevated postoperative plasma D-dimer. Elevated D-dimer on postoperative day 5 and increased intraoperative blood loss were risk factors in the development of VTE after hepatectomy. Monitoring of plasma D-dimer levels after hepatectomy may allow early detection of asymptomatic VTE and may help avoid routine postoperative anticoagulation.
- Citation: Miyake T, Yanagimoto H, Tsugawa D, Akita M, Asakura R, Arai K, Yoshida T, So S, Ishida J, Urade T, Nanno Y, Fukushima K, Gon H, Komatsu S, Asari S, Toyama H, Kido M, Ajiki T, Fukumoto T. Utility of plasma D-dimer for diagnosis of venous thromboembolism after hepatectomy. World J Clin Cases 2024; 12(2): 276-284
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/276.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.276
Venous thromboembolism (VTE) comprises pulmonary embolism (PE) and deep vein thrombosis (DVT). The association between VTE and cancer is well recognized. Although some reports describing the incidence of VTE in surgery for gastrointestinal cancer have been described, few reports on the incidence of VTE in patients with cancer after hepatobiliary-pancreatic (HBP) surgery currently exist[1-3]. VTE is a potentially fatal complication of surgery. Moreover, the risk of VTE in patients with cancer is estimated to be four to seven-fold higher than that in the general population[4-6]. VTE is associated with a worse prognosis and increased medical costs among patients with cancer[7-9]. Therefore, guidelines recommend that patients with a middle-to-high risk of VTE should be managed using strategies such as compression stockings, intermittent pneumatic compression (IPC) devices, and anticoagulant therapy[10]. According to the relevant guidelines, cancer patients undergoing HBP surgery are typically classified as being at high risk for VTE. HBP surgery is often a highly invasive operation. However, the use of postoperative prophylactic anticoagulant therapy in patients undergoing HBP surgery is controversial.
D-dimer levels are useful clinical biomarkers for detecting VTE[11,12]. We hypothesized that measuring this marker would be beneficial for diagnosing VTE following hepatectomy without postoperative prophylactic anticoagulant therapy. We routinely measured plasma D-dimer levels after hepatectomy from January 2017 onwards, and used this marker as an indicator for detecting VTE. The aim of this study was to provide real-world data on the incidence of VTE after hepatectomy without postoperative prophylactic anticoagulant therapy, and to investigate the significance of plasma D-dimer monitoring for the early detection and treatment of VTE.
The current study was conducted using data extracted from a prospective database. We analyzed all consecutive patients who underwent hepatectomy for primary malignant disease at the Department of Hepato-Biliary-Pancreatic Surgery, Kobe University, between January 2017 and December 2020. Demographic and clinicopathological variables, including age, sex, body mass index, diagnosis, surgical details (type and extent of resection), surgical approach, co-morbidity, smoking history, history of VTE, use of anticoagulant therapy, operation time, estimated blood loss volume, and post
For patients administered anticoagulants, anticoagulant medication was discontinued preoperatively in accordance with our institutional standards. All patients in this study underwent mechanical prophylaxis, including the use of com
Plasma D-dimer levels were measured preoperatively and on postoperative days (PODs) 1, 3, 5, and 7, and contrast-enhanced chest-extremity computed tomography (CE-CT) and/or ultrasound (US) was performed when plasma D-dimer levels were 20 μg/mL or higher, and when they were less than 20 μg/mL but did not below 10 μg/mL throughout the week. The cutoff value of 20 μg/mL plasma D-dimer was set with reference to previous reports[11]. Anticoagulation therapy was considered if VTE was diagnosed.
Major hepatectomy was defined as right/left hemi-hepatectomy, right/left tri-segmentectomy, and central bi-seg
DVT was defined as phlebitis, thrombophlebitis, and venous embolism or thrombosis of deep vessels of the lower extremity[10]. PE was defined as a disease in which the pulmonary vein was occluded by thromboembolism. In the present study, VTE was defined as DVT and PE within 30 d after surgery, in accordance with the guidelines[9]. Cases of portal vein thrombosis were excluded as they are generally not included in the definition of DVT included in this study.
Patients were divided into two groups, namely those who developed VTE within 30 d of surgery [VTE (+)] and those who did not [VTE (-)]. The VTE (-) group was defined as patients with no symptoms of VTE and no VTE detected on radiographic imaging from the perioperative period to discharge and at follow-up every 1-2 mo.
Continuous variables are presented as medians with ranges, and categorical variables are presented as frequencies and percentages. χ2 test was performed to compare the two groups. Receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff value of D-dimer for VTE after hepatectomy. Univariate and multivariate analyses were performed using logistic regression analysis to identify significant predictors for VTE after hepatectomy. Statistical significance was set at P < 0.05. Statistical analyses were performed using JMP Pro 16 software (SAS Institute, Cary, NC, United States).
A total of 234 patients who underwent hepatectomy were enrolled in this study. Major and minor hepatectomies were performed in 59 (25%) and 175 patients (75%), respectively. The patients included in this study were aged 33-93 years (mean age: 72 years), and comprised 177 (76%) men and 57 (24%) women. The baseline characteristics of the patients are shown in Table 1. Thirteen (5.6%, DVT/PE/DVT and PE: 11/1/1, respectively) patients were diagnosed with VTE after hepatectomy.
| Variables | Hepatectomy (n = 234) |
| Age, yr | 72 (33-93) |
| Sex (male/ female) (%) | 177/57 (76/24) |
| Disease (%) | |
| Hepatocellular carcinoma | 198 (84) |
| Intrahepatic cholangiocarcinoma | 11 (5) |
| Perihilar cholangiocarcinoma | 25 (11) |
| Surgical procedures | |
| Major hepatectomy | 59 (25) |
| Minor hepatectomy | 175 (75) |
| Laparotomy | 40 |
| Laparoscopic | 135 |
| Operation time (min) | 410 (92-762) |
| Blood loss (mL) | 150 (0-5330) |
| VTE | 13 (5.6) |
| DVT | 12 |
| PE | 2 |
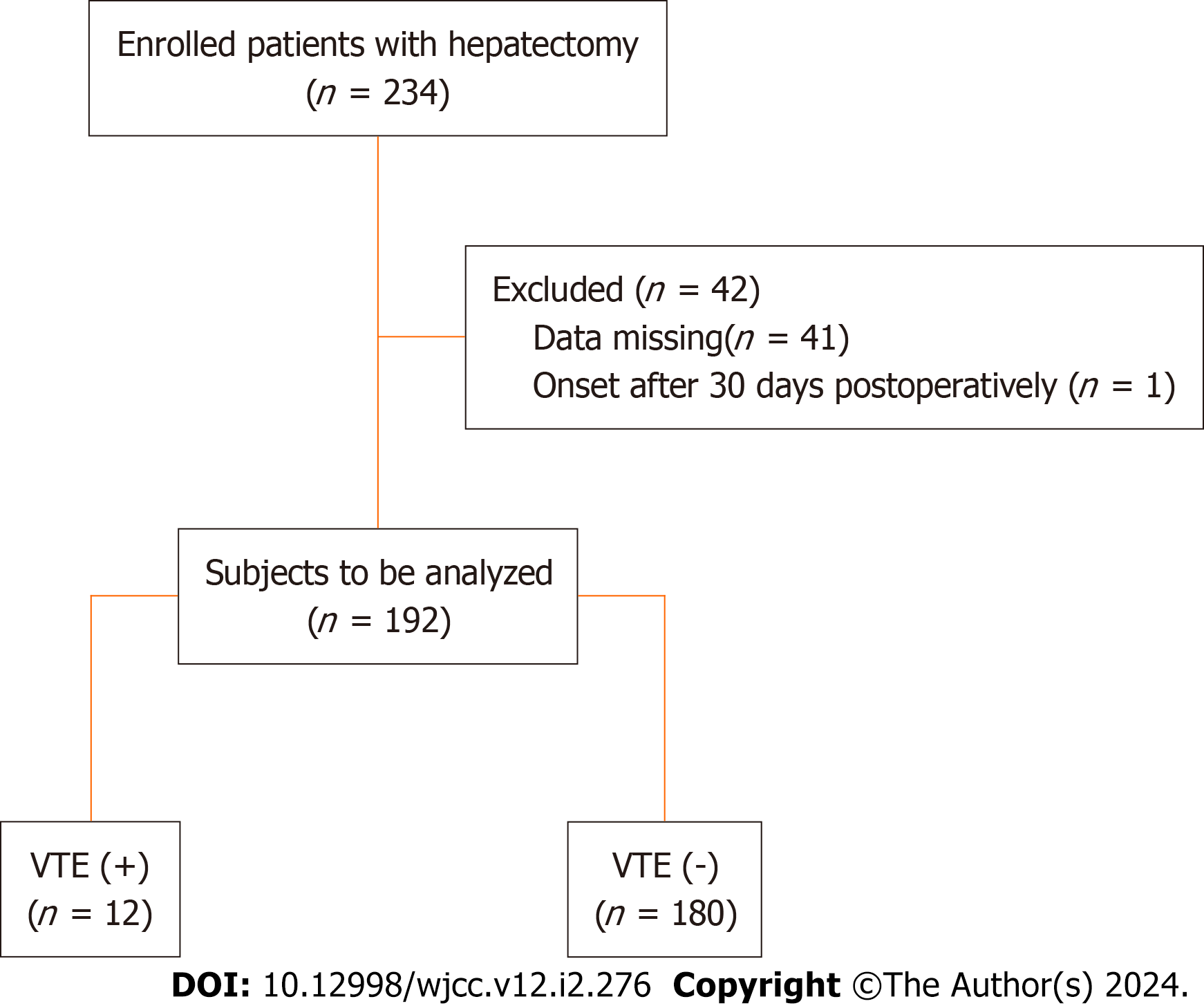
Of the 234 patients who underwent hepatectomy, 192 were studied to determine the correlation between plasma D-dimer levels and VTE, and 42 were excluded (41 for missing data and one which involved onset after 30 d postoperatively) (Figure 1). CE-CT and/or US were performed in 41 patients (22.4%) during the study period. Of these, 34 patients had D-dimer levels greater than 20 μg/mL, of whom 11 developed VTE; 7 patients had D-dimer levels between 10 and 20 μg/mL, of whom only one developed VTE.
The comparison of VTE (+) and VTE (-) showed significant differences in operative time (529 vs 403 min, P = 0.0274) and blood loss (530 vs 138 mL, P = 0.0067), but not in other parameters (Table 2). In VTE cases, all patients were asymp
| Variables | VTE (+) (n = 12) | VTE (-) (n = 180) | P value |
| Age, yr (range) (median) | 73 (59-84) | 72 (33-91) | 0.4635 |
| Sex | |||
| Male/female | 9 (75)/3 (25) | 135 (75)/45 (25) | 0.65 |
| BMI (kg/m2) (median) | 23.4 (17.7-28.3) | 22.7 (13.1-38.9) | 0.7864 |
| Disease | |||
| HCC | 8 | 148 | |
| IHCC | 0 | 11 | |
| PHCC | 4 | 21 | |
| Major/minor hepatectomy (%) | 6 (50)/6(50) | 45 (25)/135(75) | 0.0576 |
| Laparoscopy (%) | 4 (33) | 109 (61) | 0.0635 |
| Smoking (%) | 8 (67) | 109 (61) | 0.6744 |
| Hypertension (%) | 9 (75) | 95 (53) | 0.1347 |
| Diabetes mellitus (%) | 5 (42) | 64 (36) | 0.6692 |
| Cardiovascular disease (%) | 1 (8) | 20 (11) | 0.7653 |
| Anticoagulation (%) | 2 (17) | 19 (11) | 0.5114 |
| History of VTE | 0 | 0 | - |
| DVT/PE | 11/2 | 0/0 | |
| Operation time (min) (median) | 529 (250-611) | 403 (92-762) | 0.0274 |
| Blood loss (mL) (median) | 530 (10-2515) | 138 (10-5330) | 0.0067 |
| Postoperative complication (≥ CDIIIb) | 1 (8) | 4 (2) | 0.1984 |
| Ddimer (μg/mL) (median) | |||
| POD1 (range) | 10.4 (5-21.7) | 6 (1.1-48.5) | 0.005 |
| POD3 (range) | 16.7 (4.7-54.6) | 7.3 (1.1-75) | 0.0029 |
| POD5 (range) | 29.5 (12.9-65) | 13.3 (2.7-167.3) | 0.0163 |
| POD7 (range) | 26.1 (15.1-75.8) | 15.5 (2.2-138.9) | 0.0029 |
| No. | Disease | Extent of resection | Date of diagnosis | D-dimer (time of diagnosis) | Method of diagnosis | Diagnosis | Treatment |
| 1 | HCC + GC | Partial (S7) | POD 7 | 20.6 | US | DVT | Anticoagulant |
| 2 | HCC | Segmental (S5 + 8) | POD 15 | 32.3 | US | DVT | Anticoagulant |
| 3 | HCC | Segmental (S6 + 7) | POD 7 | 55.6 | US | DVT | Anticoagulant |
| 4 | HCC | Segmental (S4) + partial (S8) | POD 3 | 30.2 | US | DVT | Observation |
| 5 | HCC | Right lobectomy + partial (S2) | POD 7 | 23.1 | US | DVT | Observation |
| 6 | PHCC | Left hemihepatectomy | POD 7 | 75.8 | CECT | PE | Anticoagulant |
| 7 | PHCC | Left hemihepatectomy | POD 6 | 57.8 | CECT | PE/DVT | Anticoagulant |
| 8 | HCC | Segmental (S5 + 8) | POD 7 | 25.5 | US | DVT | Observation |
| 9 | PHCC | Left hemihepatectomy | POD 21 | 17.4 | US | DVT | Observation |
| 10 | PHCC | Left hemihepatectomy | POD 7 | 15.1 | CECT | PE | Anticoagulant |
| 11 | HCC | Segmental (S6 + 7) | POD 11 | 37.3 | US | DVT | Anticoagulant |
| 12 | HCC | Right hemihepatectomy | POD 7 | 43.6 | US | DVT | Observation |
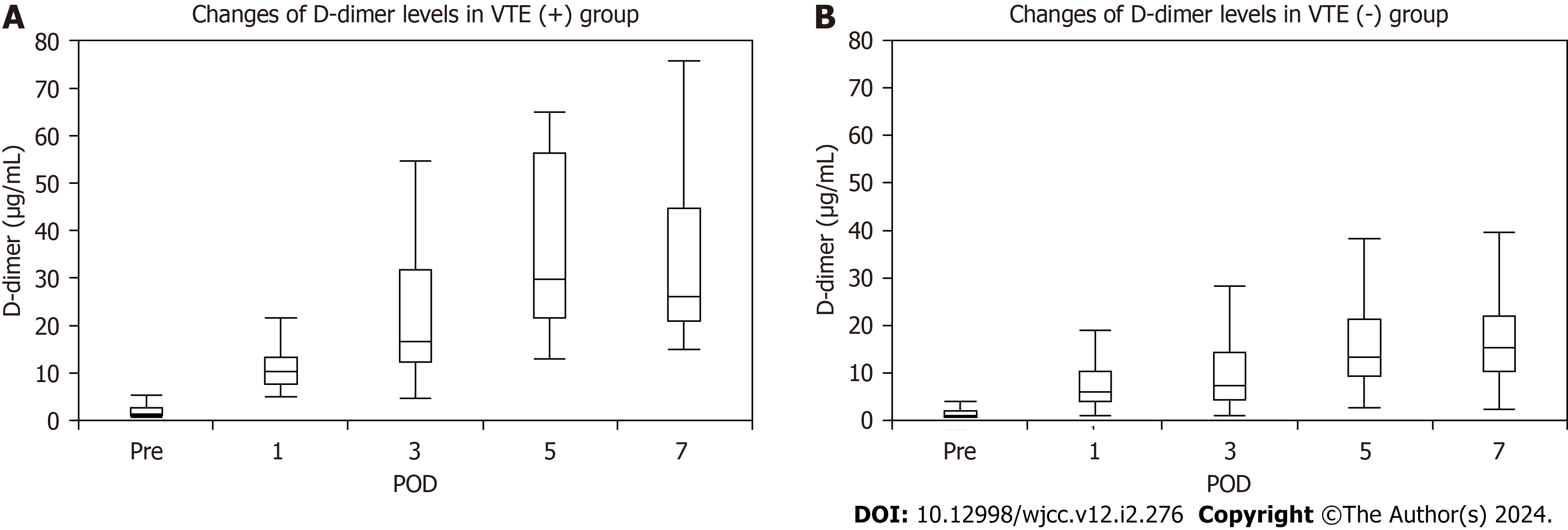
Plasma D-dimer levels increased gradually on POD 1, 3, 5, and 7 in each group. Even though the preoperative plasma D-dimer levels did not differ significantly, levels on POD 1, 3, 5, and 7 were significantly higher in the VTE (+) group than in the VTE (-) group [10.4 (5-21.7) vs 6 (1.1-48.5), P = 0.009; 16.7 (4.7-54.6) vs 7.2 (1.1-75), P = 0.002; 29.5 (12.9-65) vs 13.25 (2.7-167.3), P < 0.0001; 26.1 (15.1-75.8) vs 15.45 (2.2-138.9), P = 0.0005] (Figure 2).
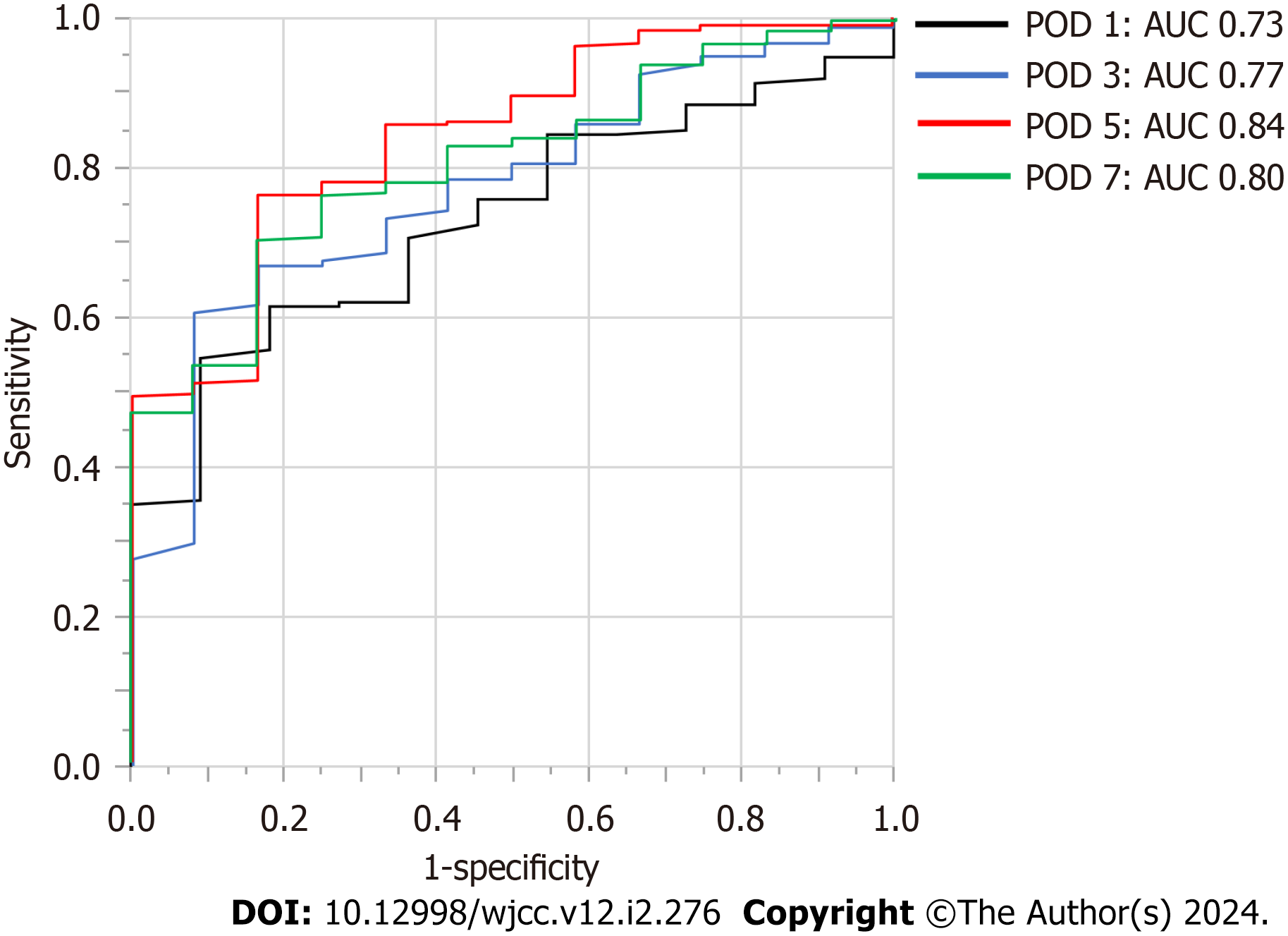
The optimal cutoff values for plasma D-dimer levels on POD 1, 3, 5, and 7 for VTE were determined using the area under the curve (AUC) of the ROC curves in this cohort, and the results were as follows: POD 1: 6 μg/mL, AUC 0.73; POD 3: 10 μg/mL, AUC 0.77; POD 5: 21 μg/mL, AUC 0.84; POD 7: 20 μg/mL, AUC 0.80. The AUC value for POD 5 was the highest and was statistically significant among the AUC values on POD 1, 3, 5, and 7 (Figure 3).
Multivariate analysis revealed intraoperative blood loss of > 275 mL [odds ratio (OR) = 5.32, 95% confidence interval (CI): 1.05-27.0, P = 0.044] and plasma D-dimer levels of ≥ 21 μg/mL on POD 5 (OR = 10.1, 95%CI: 2.04-50.1, P = 0.0046) as independent risk factors for VTE after hepatectomy (Table 4).
| Variables | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Sex (female) | 1.0 | 0.26-3.86 | 1.0 | |||
| Age ≥ 70 yr | 1.2 | 0.35-4.19 | 0.7585 | |||
| Major hepatectomy | 3 | 0.10-1.09 | 0.0682 | |||
| Minimally invasivehepatectomy | 3.07 | 0.89-10.6 | 0.0755 | |||
| BMI ≥ 25 kg/m2 | 1.17 | 0.34-4.04 | 0.8078 | |||
| HT | 2.68 | 0.70-10.2 | 0.1484 | |||
| DM | 1.29 | 0.39-4.25 | 0.6700 | |||
| Cardiovascular disease | 0.72 | 0.09-5.93 | 0.7662 | |||
| Smoking | 1.3 | 0.38-4.49 | 0.6751 | |||
| Anticoagulation | 1.7 | 0.25-8.31 | 0.5157 | |||
| Ope time ≥ 462 (min) | 5.3 | 1.39-20.3 | 0.0147 | 3.37 | 0.80-14.2 | 0.0978 |
| Blood loss ≥ 275 (mL) | 10 | 2.12-47.1 | 0.0036 | 5.32 | 1.05-27.0 | 0.0435 |
| Blood transfusion | 4.48e-7 | 0.9902 | ||||
| DD (POD 5) ≥ 21 (μg/mL) | 14.8 | 3.11-70.1 | < 0.0001 | 10.1 | 2.04-50.1 | 0.0046 |
| Complication (≥ CDIIIb) | 4.0 | 0.41-38.8 | 0.2322 | |||
The overall symptomatic VTE rate after hepatectomy was approximately 3% in previous retrospective analyses of the national database in the United States[13]. The risk of VTE after hepatectomy is proportional to the resected liver volume, and ranged from 2.1% after partial hepatectomy to 5.8% after extended hepatectomy[11]. However, the frequency of VTE after hepatectomy remains unknown in Japan[11,14].
Hayashi et al[14] recently reported that asymptomatic VTE developed in 12.2% of patients after HBP surgery for malignant and benign tumors. Our data showed that asymptomatic VTE occurred in 13 (5.6%) patients after hepatectomy for hepatocellular carcinoma and bile duct cancer.
The incidence of asymptomatic VTE in our study was relatively high compared to that of symptomatic VTE in the United States[13]. This may be because we proactively performed CE-CT and/or US for patients with higher plasma D-dimer levels, and asymptomatic VTE was diagnosed in this study.
The United States guidelines for treating VTE recommend anticoagulation therapy after surgery for malignant tumors, and routine anticoagulation after hepatectomy has been reported to be safe[15,16], but the incidence of postoperative VTE is rare in Japan. In addition, there are some risks associated with the use of anticoagulants, and the use of anticoagulants in cases where there is no need to use them is debatable. Our hospital does not routinely provide prophylactic post
Onda et al[11] previously reported resected liver weight as a risk factor for VTE after hepatectomy, while Hue et al[13] reported that the VTE rate was higher after open hepatectomy than after minimally invasive hepatectomy. The present study revealed that the risk factors for VTE after hepatectomy included intraoperative blood loss > 275 mL, and plasma D-dimer levels ≥ 21 μg/mL on POD 5. In addition, prolonged bed rest, obesity, coexisting cardiopulmonary disease, smoking, and a history of VTE are generally cited as risk factors for VTE; however, the present study found no significant differences in these parameters. This may be because the number of cases was small, the surgery was aimed at malignant tumors, and body mass index or other patient backgrounds did not differ significantly. Although several studies have reported that blood transfusions increased the incidence of VTE in cancer surgery[17-19], few studies have reported the relationship between blood loss and thrombosis to date. Nielsen et al[20] showed that massive blood loss requiring transfusion increased the risk of postoperative VTE in bariatric surgery.
Measurement of plasma D-dimer in this study is useful for the early detection of postoperative VTE, but this protocol requires high medical costs. Among the 41 patients who tested based on screening with plasma D-dimer, 21 had D-dimer levels of ≥ 21 μg/mL on POD 5, and 10 had VTE. Therefore, measuring D-dimer levels on POD 5 alone is likely sufficient to detect VTE, resulting in reduced medical costs. However, two cases could not be detected, meaning that all cases could not be covered using this method. As mentioned above, postoperative D-dimer monitoring appears to be useful for the early diagnosis of VTE. However, each measurement method has its own advantages and disadvantages, and further study is needed.
This study has several limitations. First, this was a single-center, retrospective study involving a small number of patients, which may have introduced selection bias. Second, it is not clear whether the VTE (-) group really did not have VTE, as imaging studies were not performed on all cases in this study. Therefore, well-designed prospective studies would be needed to clarify the efficacy of plasma D-dimer monitoring after hepatectomy.
Monitoring plasma D-dimer levels after hepatectomy can be useful for the early diagnosis of VTE and may avoid routine prophylactic anticoagulation in the postoperative period.
Venous thromboembolism (VTE) after hepatectomy is a potentially fatal complication.
The motivation of this study is that monitoring plasma D-dimer could be useful in the early diagnosis of VTE after hepatectomy.
To uncover the incidence of VTE after hepatectomy without postoperative prophylactic anticoagulant therapy and to investigate the significance of plasma D-dimer monitoring for the early detection and treatment of VTE.
This was a retrospective study on the development of VTE after hepatectomy for malignant disease was performed at a single institution over a 3-year period. We compared patients categorized based on the presence or absence of VTE to examine risk factors for the development of postoperative VTE.
Thirteen (5.6%) patients were diagnosed with VTE following hepatectomy. Elevation of D-dimer on postoperative day 5 and increased intraoperative blood loss were risk factors for the development of VTE after hepatectomy.
We proposed that monitoring of plasma D-dimer levels after hepatectomy is useful for early diagnosis of VTE, and may avoid routine prophylactic anticoagulation in the postoperative period.
We demonstrated monitoring D-dimer as an alternative to routine postoperative anticoagulation for early diagnosis of postoperative VTE.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nah YW, South Korea S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH
| 1. | Ohashi Y, Ikeda M, Kunitoh H, Sasako M, Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, Ibusuki K, Sakon M. Venous thromboembolism in cancer patients: report of baseline data from the multicentre, prospective Cancer-VTE Registry. Jpn J Clin Oncol. 2020;50:1246-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Patel H, Sun H, Hussain AN, Vakde T. Advances in the Diagnosis of Venous Thromboembolism: A Literature Review. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | van Es N, Ventresca M, Di Nisio M, Zhou Q, Noble S, Crowther M, Briel M, Garcia D, Lyman GH, Macbeth F, Griffiths G, Iorio A, Mbuagbaw L, Neumann I, Brozek J, Guyatt G, Streiff MB, Baldeh T, Florez ID, Gurunlu Alma O, Agnelli G, Ageno W, Marcucci M, Bozas G, Zulian G, Maraveyas A, Lebeau B, Lecumberri R, Sideras K, Loprinzi C, McBane R, Pelzer U, Riess H, Solh Z, Perry J, Kahale LA, Bossuyt PM, Klerk C, Büller HR, Akl EA, Schünemann HJ; IPDMA Heparin Use in Cancer Patients Research Group. The Khorana score for prediction of venous thromboembolism in cancer patients: An individual patient data meta-analysis. J Thromb Haemost. 2020;18:1940-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1459] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 5. | Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1601] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 6. | Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 7. | Rochefort CM, Verma AD, Eguale T, Lee TC, Buckeridge DL. A novel method of adverse event detection can accurately identify venous thromboembolisms (VTEs) from narrative electronic health record data. J Am Med Inform Assoc. 2015;22:155-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Liebman HA. Cancer prognosis in patients with venous thromboembolism (VTE) and patients with clinical and laboratory biomarkers predictive of VTE risk. Thromb Res. 2018;164 Suppl 1:S19-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Bradley CT, Brasel KJ, Miller JJ, Pappas SG. Cost-effectiveness of prolonged thromboprophylaxis after cancer surgery. Ann Surg Oncol. 2010;17:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | JCS Joint Working Group. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ J. 2011;75:1258-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Onda S, Furukawa K, Haruki K, Hamura R, Shirai Y, Yasuda J, Shiozaki H, Gocho T, Shiba H, Ikegami T. D-dimer-based screening for early diagnosis of venous thromboembolism after hepatectomy. Langenbecks Arch Surg. 2021;406:883-892. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Kodama J, Seki N, Masahiro S, Kusumoto T, Nakamura K, Hongo A, Hiramatsu Y. D-dimer level as a risk factor for postoperative venous thromboembolism in Japanese women with gynecologic cancer. Ann Oncol. 2010;21:1651-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Hue JJ, Katayama E, Markt SC, Rothermel LD, Hardacre JM, Ammori JB, Winter JM, Ocuin LM. Association Between Operative Approach and Venous Thromboembolism Rate Following Hepatectomy: a Propensity-Matched Analysis. J Gastrointest Surg. 2021;25:2778-2787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Hayashi H, Shimizu A, Kubota K, Notake T, Sugenoya S, Masuo H, Hosoda K, Yasukawa K, Kobayashi R, Soejima Y. Asymptomatic Venous Thromboembolism After Hepatobiliary-Pancreatic Surgery: Early Detection Using D-dimer and Soluble Fibrin Monomer Complex Levels. Ann Gastroenterol Surg. 2022;6:109-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 15. | Tzeng CW, Katz MH, Fleming JB, Pisters PW, Lee JE, Abdalla EK, Curley SA, Vauthey JN, Aloia TA. Risk of venous thromboembolism outweighs post-hepatectomy bleeding complications: analysis of 5651 National Surgical Quality Improvement Program patients. HPB (Oxford). 2012;14:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Baltatzis M, Low R, Stathakis P, Sheen AJ, Siriwardena AK, Jamdar S. Efficacy and safety of pharmacological venous thromboembolism prophylaxis following liver resection: a systematic review and meta-analysis. HPB (Oxford). 2017;19:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res. 2012;129:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Abu-Rustum NR, Richard S, Wilton A, Lev G, Sonoda Y, Hensley ML, Gemignani M, Barakat RR, Chi DS. Transfusion utilization during adnexal or peritoneal cancer surgery: effects on symptomatic venous thromboembolism and survival. Gynecol Oncol. 2005;99:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377-2381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Nielsen AW, Helm MC, Kindel T, Higgins R, Lak K, Helmen ZM, Gould JC. Perioperative bleeding and blood transfusion are major risk factors for venous thromboembolism following bariatric surgery. Surg Endosc. 2018;32:2488-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |