Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.3845
Revised: May 7, 2024
Accepted: May 13, 2024
Published online: July 6, 2024
Processing time: 111 Days and 23.3 Hours
Stromal cell derived factor-1 (SDF-1) plays a pivotal role in the recruitment of stem cells to injured livers. However, the changes of SDF-l in patients with hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACLF) have yet to be elucidated.
To study the SDF-1 changes in patients with HBV-related ACLF.
30 patients with HBV-related ACLF, 27 patients with chronic hepatitis B and 20 healthy individuals are involved in our study. The SDF-l mRNA expression in liver tissue was detected by quantitative real-time polymerase chain reaction. Immunohistochemical staining was performed to illustrate the expression of SDF-l, CXC receptor 4 (CXCR4) and Ki67. The serum SDF-l concentrations were also detected by enzyme-linked immunosorbent assays.
The expression of SDF-1 mRNA from ACLF patients was remarkably higher than that from other patients (both P < 0.05). The expression of SDF-l, CXCR4 and Ki67 from ACLF were the highest among the three groups (all P < 0.01). The serum SDF-l levels in ACLF patients were significantly lower than that in other patients (both P < 0.01). Moreover, in ACLF patients, the serum SDF-1 Levels were positively correlated with serum total bilirubin and international normalized ratio. In addition, the serum SDF-l levels in survival were significantly lower compared with the non-survivals (P < 0.05). The area under the curve for the serum SDF-1 level in predicting 28-d mortality was 0.722 (P < 0.05).
This study provides the SDF-1 changes in patients with HBV-related ACLF. The SDF-1 Level at admission may serve as a promising prognostic marker for predicting short-term prognosis.
Core Tip: In this study, we investigated the changes in stromal cell derived factor-1 (SDF-1) in hepatitis B virus (HBV) associated acute-on-chronic liver failure (ACLF) patients. 30 HBV related ACLF patients, 27 chronic hepatitis B patients, and 20 healthy participants participated in our study. Our research findings provide information on the changes in SDF-1 in HBV associated ACLF patients. The level of SDF-1 at admission can serve as a promising prognostic marker for predicting short-term prognosis.
- Citation: Zhang L, Wang JY, Zhao CY, Shen C, Chen MR, Tian ZY. Prognostic role of the stromal cell derived factor-1 in patients with hepatitis B virus-related acute-on-chronic liver failure. World J Clin Cases 2024; 12(19): 3845-3853
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/3845.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.3845
An estimated 400 million people are infected with the hepatitis B virus (HBV), which is a common and frequent cause of chronic liver disease worldwide[1]. For various reasons, some cases will progress to liver failure, among which acute-on-chronic liver failure (ACLF) is more common. The features of ACLF are rapid progression and high incidence of short- and medium-term mortality, ranging from 50% to 90%, caused by multi-organ failure[2,3]. In this case, orthotopic liver transplantation remains the most effective treatment. Unfortunately, the feasibility is extremely limited by the shortage of available donor organ, high cost and organ rejection. Clearly, the vast majority of patients with ACLF can’t rely on organ transplantation. In order to overcome these problems, alternative approaches have been proposed. Bone marrow stem cells (BMSC) are well known as cells that can differentiate into variable cell types and maintain self-renewal activity[4]. Hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) within the bone marrow (BM) have the capacity to populate liver and differentiate into functional hepatocyte to replace damaged liver tissue[5]. Despite the extensive use of HSCs in clinical transplantation, the molecular mechanisms that govern their mobilization are still poorly understood. Migration of HSCs and MSCs into the liver micro-environment is dependent on dynamic and complex regulatory processes.
To date, the most prominent stem cell homing factor is the chemokine stromal cell derived factor-1 (SDF-1). SDF-1, a member of the CXC chemokine family, was first identified from murine BM stromal cells and found to support the proliferation of B-cell progenitors[6]. SDF-1 is expressed in several tissues and organs, such as skin, lung, thymus, kidney and liver and possesses a variety of biologic activities[7,8]. One of the functions of SDF-1 is to direct cell migration along the SDF-1 gradient and its expression has been reported to increase during liver injury and inflammation[9]. SDF-1 binds to chemokine CXC receptor 4 (CXCR4), a marker of hematopoietic, endothelial, neural, muscle, and liver stem cells, which is a G protein-linked receptor composed of 352 amino acids[10]. The interactions between SDF-1 and CXCR4 play a pivotal role in the recruitment of stem cells to injured livers. However, the expression of SDF-1 and CXCR4 in patients with HBV-related ACLF has yet to be elucidated. The Ki67 protein is widely used as a marker of cell proliferation, which is expressed through the cell cycle, except in the G0 phase[11].
In the present study, we detected the expression of SDF-1 mRNA in liver tissue, the expression and the distribution of intrahepatic SDF-1, CXCR4 and Ki67, as well as serum levels of SDF-1 in patients with HBV-related ACLF, and we further explored the variation of serum SDF-1 Levels in ACLF patients with different outcomes to analyze the role of SDF-1 and CXCR4 in the homing of liver stem cells.
This study was an open control clinical research. All participants consecutively were admitted to our department (the Third Affiliated Hospital of Hebei Medical University), from March 2021 to November 2022, including 30 patients with HBV-related ACLF, 27 patients with chronic hepatitis B (CHB). Meanwhile, 20 healthy individuals were selected as control group matched with experimental groups for sex and age. The diagnostic criteria of ACLF, defined by the Asian Pacific Association for the Study of the Liver (APASL)[12], was adopted. The CHB patients were diagnosed according to the Asia-Pacific consensus statement on the management of CHB (a 2012 update)[13]. Individuals who had coinfection of hepatitis A, C, E viruses or HIV, alcoholic liver disease, autoimmune disease, pregnancy, malignancy, or serious illness in other systems were all excluded. The study was conducted in compliance with the Declaration of Helsinki. The research use of these samples was approved by the Ethics Committee of the Third Affiliated Hospital of Hebei Medical University and the written informed consent was obtained from the patients or their families prior to enrollment.
Blood samples were drawn under fasting conditions from participants within 24 h after admission. Liver specimens were collected from ten explanted livers of HBV-related ACLF, ten biopsied livers of CHB, and eight healthy donors. Some of the tissue samples were fixed by 10% formalin, others were stored in liquid nitrogen.
Serum concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), and direct bilirubin (DBIL) were detected by fully Automatic Biochemical Analyzer (AU2700, Olympus, Tokyo, Japan). Prothrombin activity and international normalized ratio (INR) were measured using fully Automatic Coagulometer (Ac.T 5diff AL, Beckman-Coulter Inc., Brea, CA, United States). HBV serological markers including HBsAg, hepatitis B e antigen (HBeAg), anti-HBe, and anti-hepatitis B core were assessed using commercially enzyme immunoassay kits (Yingkexinchuang Sci-Tech Co., Ltd., Xiamen, China). Quantitative detection of serum HBV DNA was performed by real-time polymerase chain reaction (PCR) with a limited range from 5.0 × 102 to 1.0 × 109 copies/mL (DA Gene, Zhongshan University, Guangzhou, China).
Total RNA was isolated from 50 mg of liver tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) following the manufacturer’s instructions. The extracted RNA was reverse transcribed using the PrimeScriptTM RT reagent kit (Fermentas, Ontario, Canada). SDF-1 mRNA was detected by quantitative real-time PCR (ABI PRISM 7500; Roche Molecular Systems, Inc., Alameda, CA, United States) and GoTaq Green Master Mix (Promega Biotech, Beijing, China). A typical protocol for the PCR was as follows: a 2-min denaturation step at 95 ℃, followed by 35 cycles of amplification consisting of denaturation at 95 ℃ for 10 s, annealing at 56 ℃ for 30 s, and extension at 68 ℃ for 45 s. The expression levels of SDF-1 gene were normalized to the level of GAPDH. The sequences of primers were: SDF-1: forward: 5’- TGAGAGCTCGCTTTGAGTGA -3’and reverse: 5’-CACCAGGACCTTCTGTGGAT-3’ (size: 233 bp). Samples were twice assayed in triplicate and relative expression of SDF-1 was calculated by using the 2-△△Ct method.
Paraffin-embedded livers were cut into 5-μm thick sections, which were deparaffinized by xylene and rehydrated with a graded ethanol series. Antigen retrieval was performed in 10 mmol/L sodium citrate buffer (PH 6.0) via pressure cooking for 15 min. After quenched endogenous peroxidase activity in 3% H2O2 for 15 min, the sections were incubated overnight at 4 ℃ with either monoclonal antibody of mouse-anti human SDF-1 (1:200 dilution; R&D Systems, Minneapolis, MN, United States) or Ki67 (1:200 dilution; Dako, Glostrup, Denmark) or polyclonal antibody of rabbit-anti-human CXCR4 (1:250 dilution, Boster Biotechnology, Wuhan, China). And then the sections were incubated with biotinylated secondary antibodies (Zhongshan Biotech, Beijing, China) at 37 ℃ for 40 min. The bound antibody washed in PBS was detected with 3,3’-diaminobenzidine substrate kit (Zhongshan Biotech, Beijing, China) and then counterstained with hematoxylin. To determine the integral optical density score, the immunoreactivity was quantified by Image Pro Plus software (Media Cybernetics, Silver Spring, MD, United States).
Blood was spun at 2000 g for 10 min at 4 ℃, and the serum was stored at -80℃. According to the manufacturer’s protocol, SDF-1 was measured by using enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, United States).
Statistical analysis was performed by SPSS software version 13.0 (SPSS Inc., Chicago, IL, United States). The results were expressed as mean ± SD. The continuous variables among multi-groups were analyzed by one way ANOVA or the Kruskal-Wallis test depending on data distribution, and pairwise comparisons between groups were performed using the LSD-t or Mann-Whitney test. The independent-samples t-test or Mann-Whitney test was performed between two groups. Categorical variables including sex, viral load was compared by Pearson’s χ2 test. The relationship between the SDF-1 and other parameters was detected by the Spearman’s correlation. The prognostic value of the serum SDF-1 level with significant difference was assessed by the area under the receiver operating characteristic (ROC) curves. P values less than 0.05 were considered statistically significant.
A total of 57 HBV-infected patients, including 30 ACLF patients and 27 CHB patients, as well as 20 healthy individuals, were recruited in the study. The baseline demographic and clinical characteristics are shown in Table 1. There was no significant difference in sex ratio and age among the three groups. As expected, markers of liver injury including ALT, AST, TBIL, DBIL and INR were significantly higher in ACLF patients than that in CHB patients and healthy controls (all P < 0.01). The HBV DNA load was comparable between ACLF patients and CHB patients (P > 0.05).
| HBV-related ACLF (n = 30) | CHB (n = 27) | Healthy controls (n = 20) | P value | |
| Age (yr) | 42 ± 14 | 41 ± 15 | 39 ± 14 | 0.641 |
| Gender (male/female) | 25/5 | 17/10 | 13/7 | 0.179 |
| Albumin (g/L) | 29.7 ± 4.5 | 40.1 ± 6.0 | 44.4 ± 4.3 | < 0.001 |
| ALT (U/L) | 400.9 ± 318.8 | 189.7 ± 121.2 | 21.1 ± 10.2 | < 0.001 |
| AST (U/L) | 362.2 ± 249.1 | 147.3 ± 87.0 | 20.6 ± 6.9 | < 0.001 |
| TBIL (µmol/L) | 381.6 ± 178.1 | 41.5 ± 38.8 | 12.0 ± 3.6 | < 0.001 |
| DBIL (µmol/L) | 216.4 ± 111.3 | 19.7 ± 23.8 | 3.7 ± 1.2 | < 0.001 |
| INR | 2.6 ± 0.9 | 1.1 ± 0.1 | - | < 0.001 |
| PTA (%) | 35.2 ± 12.1 | 98.1 ± 16.1 | - | < 0.001 |
| HBVDNA (log10 copies/mL) | 4.7 ± 1.6 | 5.5 ± 1.6 | - | 0.084 |
| HBeAg (+)/HBeAg (-) | 11/19 | 15/12 | - | 0.153 |
| 30-d mortality | 19 (63.3%) | 0 (0) | 0 (0) | - |
The SDF-l mRNA expression detection was performed on the liver tissues from 8 HBV-related ACLF patients, 10 CHB patients and 8 healthy controls. The expression of SDF-l mRNA (3.4 ± 1.02) in ACLF patients was significantly higher than that in CHB patients (2.16 ± 1.19) and healthy controls (1.00) (P = 0.001). Thus, ACLF patients exhibited higher SDF-1 mRNA levels in liver tissues.
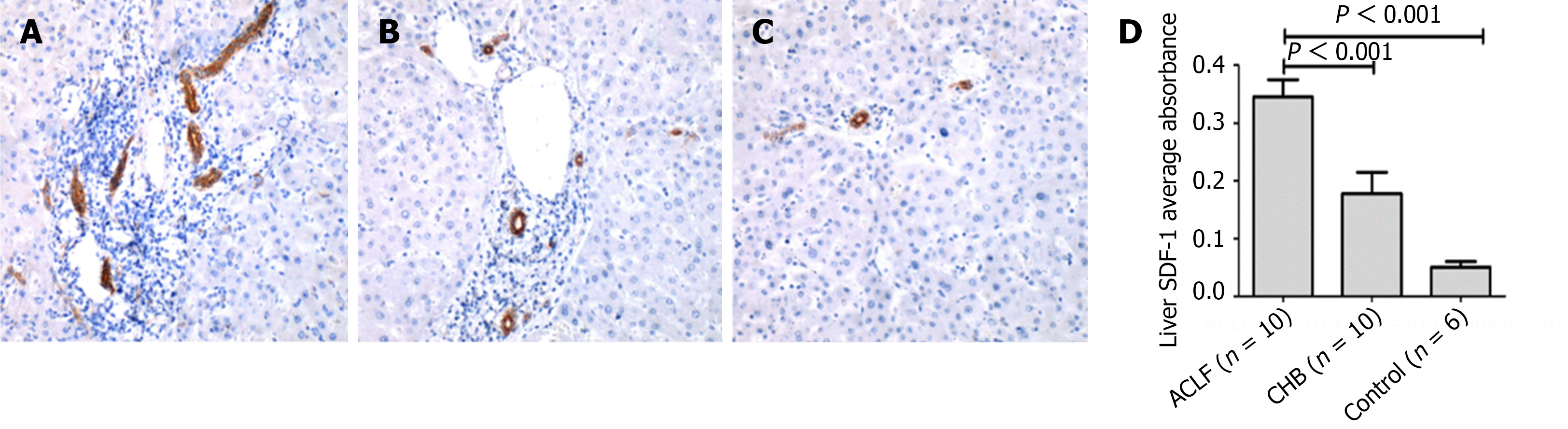
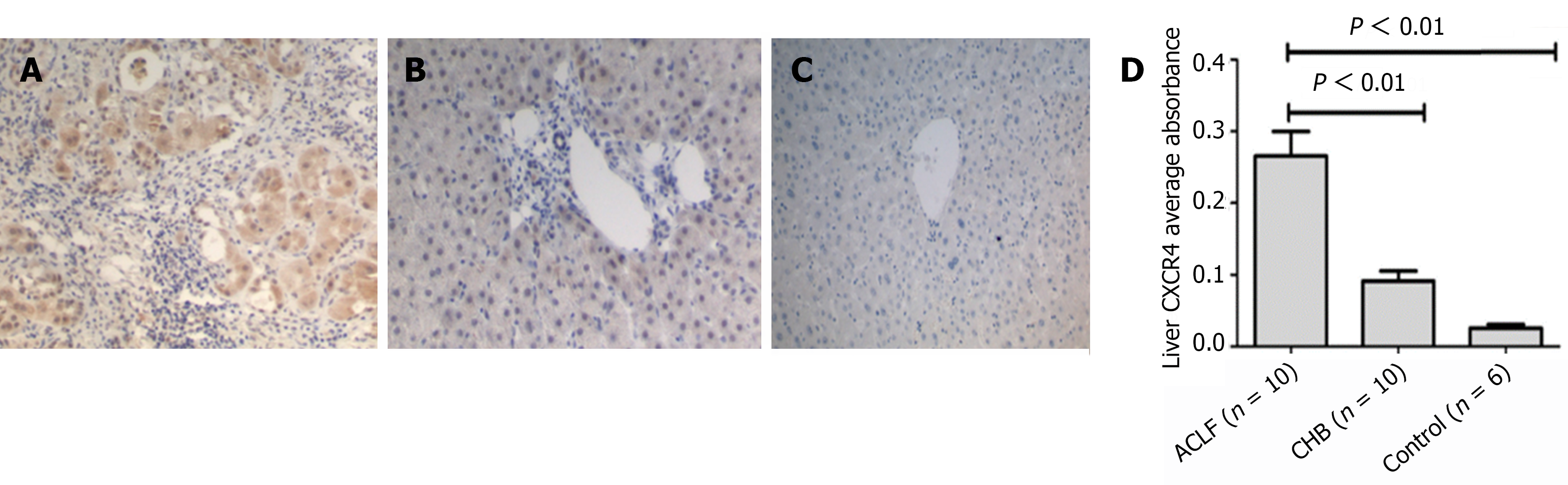
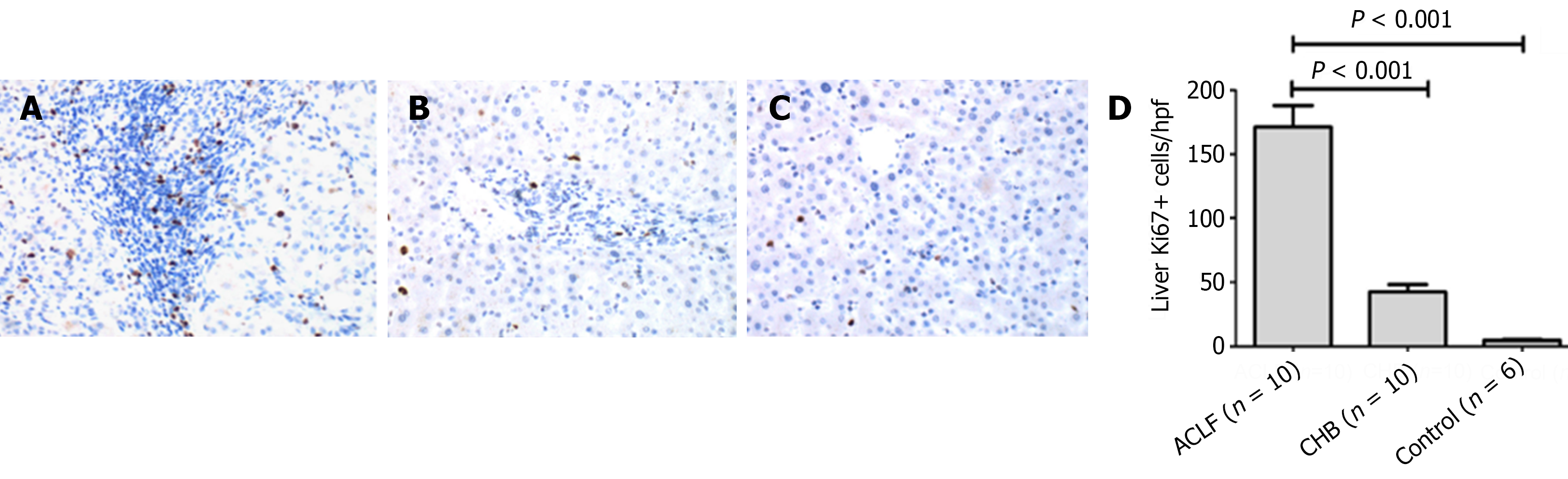
The SDF-l, CXCR4 and Ki67 expression detection was performed on the liver tissues from 10 HBV-related ACLF patients, 10 CHB patients and 6 healthy controls. The average absorbance of SDF-l was respectively 0.345 ± 0.095 in ACLF patients, 0.178 ± 0.116 in CHB patients and 0.051 ± 0.022 in healthy controls. The expression of SDF-l was highest in ACLF group, and lowest in healthy controls. Moreover, in ACLF patients, SDF-l distribution was extended to bile ductule and hepatic progenitor cells. Meanwhile, obvious ductular reaction can be observed. However, SDF-l was only expressed in bile duct in healthy controls (Figure 1). CXCR4 staining was observed predominantly in the membrane and cytoplasm of bile duct epithelium and hepatic progenitor cells. Quantitative analysis showed that the expression of CXCR4 in ACLF group (0.266 ± 0.109) was significantly higher than that either in CHB group (0.092 ± 0.044) or in healthy control (0.026 ± 0.011) (both P < 0.01) (Figure 2). Likewise, the expression of Ki67 detected in liver sections in ACLF patients (171.2 ± 52.8/hpf) was higher than that either in CHB patients (42.4 ± 17.8/hpf) or in healthy controls (4.7 ± 1.9/hpf) (both P < 0.01). The distribution of Ki67 was predominantly in the portal areas, and minorly in the liver parenchyma (Figure 3). Taken together, these findings supported the notion that the SDF-1, CXCR4, Ki67 expressions were more likely to elevate in liver tissues from ACLF patients.
The serum SDF-l level detection was performed in 30 HBV-related ACLF patients, 27 CHB patients and 20 healthy controls. The serum SDF-l levels in ACLF patients (1717.33 ± 458.07 pg/mL) were significantly lower than that in CHB patients (2638.96 ± 574.04 pg/mL) and healthy controls (2378.20 ± 660.09 pg/mL) (both P < 0.01). Thus, the lower serum concentrations of SDF-1 were a feature of ACLF patients.
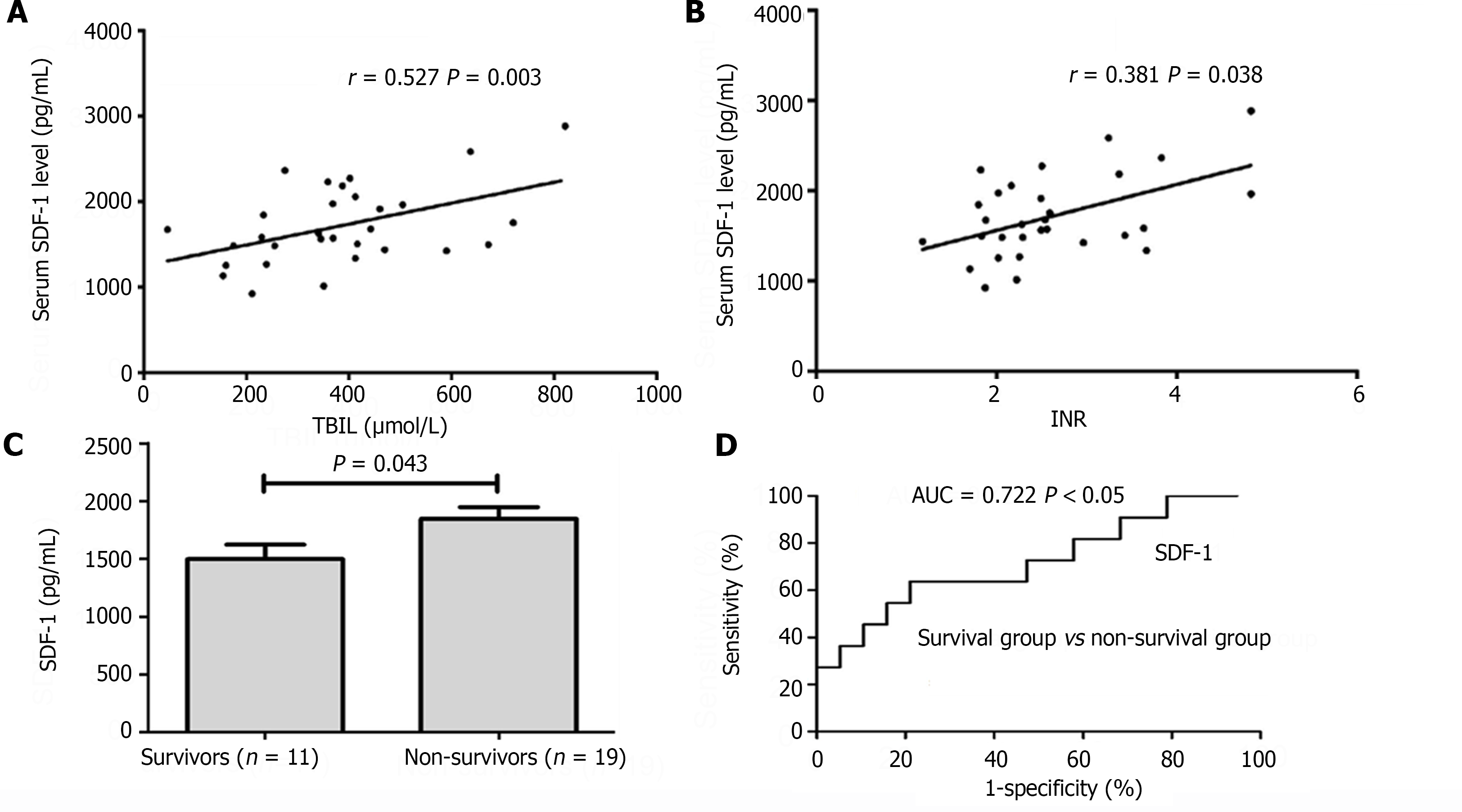
To better understand the clinical significance of the SDF-1 Levels in HBV-related ACLF patients, we further analyzed the relationship between serum SDF-1 Levels and ALT, TBIL, INR, which reflect different aspect of liver function, and the relationship between serum SDF-1 levels and the MELD score, which is recommended as a prognostic score for ACLF. The serum SDF-1 levels were positively correlated with TBIL (r = 0.53, P = 0.003) and INR (r = 0.381, P = 0.038) (Figure 4A and B). But no relationship was found between the serum SDF-l levels and ALT levels, neither was between the serum SDF-1 levels and the MELD score (both P > 0.05).
The HBV-related ACLF patients (n = 30) were divided into survival group (n = 11) and non-survival group (n = 19) on the 28th day of admission. Table 2 describes the baseline characteristics of the above two groups. There was no significant difference in sex ratio, age, HBV DNA level, HBeAg status between the two groups. The serum SDF-l levels in survival (1497.69 ± 408.55 pg/mL) were significantly lower compared with the non-survival (1844.50 ± 445.84 pg/mL) (P < 0.05) (Figure 4C). The ROC analysis showed that the area under the curve of serum SDF-1 levels to predict 28-d mortality was 0.722 (P < 0.05, 95% confidence interval 0.526 to 0.919) when the cut-off value was 1500.71 pg/mL. Its sensitivity and specificity were 78.9% and 63.6%, respectively (Figure 4D).
| Survivors (n = 11) | Non-survivors (n = 19) | P value | |
| Age (yr) | 40 ± 13 | 44 ± 14 | 0.467 |
| Gender (male/female) | 8/3 | 17/2 | 0.327 |
| Albumin (g/L) | 30.9 ± 5.2 | 29.1 ± 4.1 | 0.305 |
| ALT (U/L) | 471.8 ± 319.3 | 359.8 ± 319.9 | 0.189 |
| AST (U/L) | 415.2 ± 279.7 | 331.6 ± 232.1 | 0.451 |
| TBIL (µmol/L) | 315.9 ± 182.8 | 419.6 ± 168.4 | 0.126 |
| DBIL (µmol/L) | 173.5 ± 89.6 | 241.3 ± 117.1 | 0.109 |
| INR | 2.0 ± 0.4 | 2.9 ± 0.9 | 0.001 |
| CREA (µmol/L) | 84.4 ± 40.4 | 94.3 ± 62.9 | 0.747 |
| HBVDNA (log10 copies/mL) | 4.8 ± 1.3 | 4.7 ± 1.8 | 0.808 |
| HBeAg (+)/HBeAg (-) | 5/6 | 6/13 | 0.696 |
| MELD score | 23.2 ± 6.8 | 28.8 ± 7.0 | 0.041 |
Previous studies have confirmed that if chronic/extensive damage is inflicted on the liver or hepatocyte proliferation is blocked, BMSCs have the capacity to differentiate into functional hepatocyte[14]. Thus, migration of stem cells from bone marrow to injured liver is a prerequisite. In recent decades, chemokines have been discovered to act as a strong chemoattractant for stem cells, among which SDF-1 and its receptor CXCR4 play a unique biological role[15]. SDF-1 has been demonstrated to participate in the concert of these cells homing from extrahepatic sources to the liver in rodents. However, there is currently not much knowledge about the expression of SDF-1/CXCR4 in patients with HBV-related ACLF.
In the present study, we found that SDF-1 expression was significantly increased in the liver tissues of HBV-related ACLF patients. Moreover, SDF-1 enhances survival of hepatic progenitor cells through autocrine or paracrine pathways. We have also observed that the peripheral SDF-1 levels were markedly lower in ACLF patients. These results indicate that SDF-1 levels increase remarkably in liver tissue and decline dramatically in peripheral blood of ACLF patients. We speculate this reduction is due to degradation of the chemokine by proteolytic enzymes. In vitro, SDF-1 is cleaved by various metalloproteinase enzymes. CXCR4 is widely expressed on bone marrow progenitor cells and early tissue-committed stem cells, including liver progenitor cells[16]. Neutralization of the CXCR4 receptor with an anti-CXCR4 antibody significantly inhibit the homing of human cord blood or mobilized peripheral blood CD34+ stem cells to the liver of irradiated NOD/SCID mice. Moreover, CXCR4+ stem cells can migrate and home along the concentration gradient of SDF-1 in vivo and in vitro[17]. We found the massive accumulation of CXCR4+ cells in livers of HBV-related ACLF patients, and the location of cell population in the membrane and cytoplasm of bile duct epithelium and hepatic progenitor cells. Based on the results mentioned above, SDF-l increases in the injured liver and decreases in peripheral blood. Thus, we proposed that the SDF-l gradient between the injured liver and peripheral blood in patients with HBV-related ACLF may promote the homing of CXCR4+ BMSCs to the liver. Multiple factors influence the homing of BMSCs. Some studies reported that the efficiency of human MSCs homing was influenced by the recipient animals’ age. In our study, age was comparable in the three groups in this study.
The Ki67 protein is associated with cell proliferation. Furthermore, we found that Ki67+ cells localized mainly in the portal areas and necro-inflammatory regions. We also found that intrahepatic Ki67 expression was higher in patients with HBV-related ACLF than that in CHB patients and healthy controls. Our findings show that when massive hepatocyte death, liver progenitor cells participate in liver regeneration, which are in accordance with previous reports[18]. Moreover, some studies have shown that bone marrow is a source of hepatic progenitor cells in injured livers[19]. We speculate that due to the SDF-1gradient between the injured liver and peripheral blood in patients with HBV-related ACLF, the intrahepatic Ki67 and CXCR4 expression was higher in ACLF patients than that in other groups.
Interestingly, SDF-1 levels were observed to be positively correlated with TBIL and INR in ACLF patients. However, we didn’t find any correlation between SDF-1 levels and ALT levels or MELD score. The MELD score consists of three objective, i.e. serum INR, TBIL and creatinine levels and serum creatinine which is influenced by muscle mass, protein dietary intake is strongly powered in the score[20,21]. This may be the reason why we didn’t conclude a well correlation between SDF-1 and MELD score in this research. Serum concentrations of alpha-fetoprotein (AFP), variably elevated during liver injury, have been suggested to be of prognostic importance in ACLF, higher values being associated with a better survival rate. However, we found admission AFP were not actually higher but lower in survivors compared with non-survivors. The above results suggest that SDF-1 Levels may be a predictive value for the severity of ACLF.
After 28-d follow up, we further explored the changes of serum SDF-1 levels in different prognostic HBV-related ACLF patients and performed a preliminary analysis to determine the differences of SDF-1 levels in survivors and non-survivors. The results demonstrated that the serum SDF-1 levels were lower in survival compared with the non-survivors on admission. The lower levels seemed to predict the greater concentration gradient of SDF-1 between injured liver and peripheral blood and more BMSCs’ migration to injured liver. These BMSCs contribute to hepatocyte repopulation through cell fusion or trans-differentiation[22]. Furthermore, MSCs secrete several cytokines, such as transforming growth factor beta, hepatocyte growth factor, epidermal growth factor, IL-6, which stimulate liver regeneration, inhibit hepatocellular apoptosis and attenuate tissue damage caused by excessive inflammation[23,24]. Therefore, the lower levels of peripheral blood SDF-l may indicate better short-term prognosis in ACLF patients.
In conclusion, the SDF-l gradient, which increases in the injured liver, and decreases in peripheral blood in patients with HBV-related ACLF, may facilitate the homing and engraftment of BMSCs to the liver. Furthermore, our data indicated the lower levels of peripheral blood SDF-l may induce the migration of more stem cells, and therefore may indicate better short-term prognosis in ACLF patients. The SDF-1/CXCR4 may provide potential strategies for the treatment of HBV-related ACLF in the future.
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J Hepatol. 2023;79:461-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 2. | Ngu NLY, Flanagan E, Bell S, Le ST. Acute-on-chronic liver failure: Controversies and consensus. World J Gastroenterol. 2023;29:232-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Martin P, Nguyen MH, Dieterich DT, Lau DT, Janssen HLA, Peters MG, Jacobson IM. Treatment Algorithm for Managing Chronic Hepatitis B Virus Infection in the United States: 2021 Update. Clin Gastroenterol Hepatol. 2022;20:1766-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Lagarkova MA. Such Various Stem Cells. Biochemistry (Mosc). 2019;84:187-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Yang H, Chen J, Li J. Isolation, culture, and delivery considerations for the use of mesenchymal stem cells in potential therapies for acute liver failure. Front Immunol. 2023;14:1243220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Ichiseki T, Shimasaki M, Ueda S, Hirata H, Souma D, Kawahara N, Ueda Y. Efficacy of Rectal Systemic Administration of Mesenchymal Stem Cells to Injury Sites via the CXCL12/CXCR4 Axis to Promote Regeneration in a Rabbit Skeletal Muscle Injury Model. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Yahya I, Morosan-Puopolo G, Brand-Saberi B. The CXCR4/SDF-1 Axis in the Development of Facial Expression and Non-somitic Neck Muscles. Front Cell Dev Biol. 2020;8:615264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Meng Z, Xin L, Fan B. SDF-1α promotes subchondral bone sclerosis and aggravates osteoarthritis by regulating the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. BMC Musculoskelet Disord. 2023;24:275. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Abubakr S, Hazem NM, Sherif RN, Elhawary AA, Botros KG. Correlation between SDF-1α, CD34 positive hematopoietic stem cells and CXCR4 expression with liver fibrosis in CCl4 rat model. BMC Gastroenterol. 2023;23:323. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Xue Y, Li Z, Wang Y, Zhu X, Hu R, Xu W. Role of the HIF1α/SDF1/CXCR4 signaling axis in accelerated fracture healing after craniocerebral injury. Mol Med Rep. 2020;22:2767-2774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Ma X, Wu Y, Zhang T, Song H, Jv H, Guo W, Ren G. Ki67 Proliferation Index as a Histopathological Predictive and Prognostic Parameter of Oral Mucosal Melanoma in Patients without Distant Metastases. J Cancer. 2017;8:3828-3837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 592] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 13. | Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, Amarapurkar D, Cooksley G, Jafri W, Mohamed R, Hou JL, Chuang WL, Lesmana LA, Sollano JD, Suh DJ, Omata M. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 792] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 14. | Danilova IG, Yushkov BG, Kazakova IA, Belousova AV, Minin AS, Abidov MT. Recruitment of macrophages and bone marrow stem cells to regenerating liver promoted by sodium phthalhydrazide in mice. Biomed Pharmacother. 2019;110:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Wang S, Gao S, Li Y, Qian X, Luan J, Lv X. Emerging Importance of Chemokine Receptor CXCR4 and Its Ligand in Liver Disease. Front Cell Dev Biol. 2021;9:716842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Wu SZ, Li YL, Huang W, Cai WF, Liang J, Paul C, Jiang L, Wu ZC, Xu M, Zhu P, Wang Y. Paracrine effect of CXCR4-overexpressing mesenchymal stem cells on ischemic heart injury. Cell Biochem Funct. 2017;35:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Xiu G, Li X, Yin Y, Li J, Li B, Chen X, Liu P, Sun J, Ling B. SDF-1/CXCR4 Augments the Therapeutic Effect of Bone Marrow Mesenchymal Stem Cells in the Treatment of Lipopolysaccharide-Induced Liver Injury by Promoting Their Migration Through PI3K/Akt Signaling Pathway. Cell Transplant. 2020;29:963689720929992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Abou Monsef Y, Kutsal O. Immunohistochemical evaluation of hepatic progenitor cells in different types of feline liver diseases. J Vet Med Sci. 2021;83:613-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Haga H, Yan IK, Takahashi K, Matsuda A, Patel T. Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stem Cells Improve Survival from Lethal Hepatic Failure in Mice. Stem Cells Transl Med. 2017;6:1262-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 20. | Peng Y, Qi X, Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine (Baltimore). 2016;95:e2877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 334] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 21. | Morales-Arráez D, Ventura-Cots M, Altamirano J, Abraldes JG, Cruz-Lemini M, Thursz MR, Atkinson SR, Sarin SK, Kim W, Chavez-Araujo R, Higuera-de la Tijera MF, Singal AK, Shah VH, Kamath PS, Duarte-Rojo A, Charles EA, Vargas V, Jager M, Rautou PE, Rincon D, Zamarripa F, Restrepo-Gutiérrez JC, Torre A, Lucey MR, Arab JP, Mathurin P, Louvet A, García-Tsao G, González JA, Verna EC, Brown RS Jr, Argemi J, Fernández-Carrillo C, Clemente A, Alvarado-Tapias E, Forrest E, Allison M, Bataller R. The MELD Score Is Superior to the Maddrey Discriminant Function Score to Predict Short-Term Mortality in Alcohol-Associated Hepatitis: A Global Study. Am J Gastroenterol. 2022;117:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | Azevedo CM, Solano de Freitas Souza B, Andrade de Oliveira S, Paredes BD, Barreto ES, Neto HA, Ribeiro dos Santos R, Pereira Soares MB. Bone marrow-derived cells migrate to the liver and contribute to the generation of different cell types in chronic Schistosoma mansoni infection. Exp Parasitol. 2015;159:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Kholodenko IV, Kholodenko RV, Yarygin KN. The Crosstalk between Mesenchymal Stromal/Stem Cells and Hepatocytes in Homeostasis and under Stress. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 449] [Article Influence: 56.1] [Reference Citation Analysis (0)] |