Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.3791
Revised: May 14, 2024
Accepted: May 24, 2024
Published online: July 6, 2024
Processing time: 106 Days and 19.7 Hours
The incidence and mortality of lung cancer have increased annually. Accurate diagnosis can help improve therapeutic efficacy of interventions and prognosis. Percutaneous lung biopsy is a reliable method for the clinical diagnosis of lung cancer. Ultrasound-guided percutaneous lung biopsy technology has been widely promoted and applied in recent years.
To investigate the diagnostic value of contrast-enhanced ultrasound (CEUS)-guided percutaneous biopsy in peripheral pulmonary lesions.
We retrospectively collected data on 237 patients with peripheral thoracic focal lesions who underwent puncture biopsy at Wuxi People’s Hospital. The patients were randomly divided into two groups: The CEUS-guided before lesion punc
Accurate pathological results were obtained for 92.83% (220/237) of peripheral lung lesions during the first biopsy, with an accuracy rate of 95.8% (113/118) in the contrast group and 89.9% (107/119) in the control group. The difference in the area under the curve (AUC) between the contrast and the control groups was not statistically significant (0.952 vs 0.902, respectively; P > 0.05). However, when the lesion diameter ≥ 5 cm, the diagnostic AUC of the contrast group was higher than that of the control group (0.952 vs 0.902, respectively; P < 0.05). In addition, the average number of puncture needles in the contrast group was lower than that in the control group (2.58 ± 0.53 vs 2.90 ± 0.56, respectively; P < 0.05).
CEUS guidance can enhance the efficiency of puncture biopsy of peripheral pulmonary lesions, especially for lesions with a diameter ≥ 5 cm. Therefore, CEUS guidance has high clinical diagnostic value in puncture biopsy of peripheral focal lung lesions.
Core Tip: Percutaneous lung biopsy is the most reliable method for clinical diagnosis of lung cancer. In this study, the data of patients with peripheral thoracic focal lesions were collected retrospectively, and comparisons were made between conventional ultrasound-guided examination (control) and contrast-enhanced ultrasound (CEUS)-guided percutaneous biopsy in the diagnosis of peripheral pulmonary lesions. The results showed that CEUS guidance can improve the efficiency and provide significant diagnostic value for puncture biopsy of peripheral pulmonary lesions.
- Citation: Jiang X, Chen J, Gu FF, Li ZR, Song YS, Long JJ, Zhang SZ, Xu TT, Tang YJ, Gu JY, Fang XM. Evaluating the efficacy of percutaneous puncture biopsy guided by contrast-enhanced ultrasound for peripheral pulmonary lesions. World J Clin Cases 2024; 12(19): 3791-3799
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/3791.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.3791
Lung cancer currently ranks first in worldwide incidence and mortality rate among all cancer types. The imaging diagnosis of lung cancer primarily involves chest X-ray, computed tomography (CT), and positron emission tomography[1]. Percutaneous lung biopsy is the most reliable method for the clinical diagnosis of lung cancer. The histological and molecular diagnosis of lung cancer specimens obtained by puncture biopsy can provide a basis for selecting reasonable and effective treatment plans, especially for patients who have lost the opportunity for surgery and are left with various medication intervention options[2]. Ultrasound-guided percutaneous biopsy has been extensively performed on various visceral lesions, and the technology has been widely promoted and applied in recent years[3]. Compared with CT and magnetic resonance imaging, ultrasound-guided percutaneous biopsy of peripheral lung malignancies has many advan
However, when necrosis or atelectasis occurs in the lesion area, the possibility of missed diagnosis by grayscale and color ultrasound-guided puncture biopsy increases. To improve the detection rate of lung cancer in lung puncture biopsy, clinicians often increased the number of biopsy needles. However, blind puncture biopsy would increase the incidence of complications such as bleeding, emphysema, and pleural reactions and may potentially increase the possibility of tumor metastasis[5].
Contrast-enhanced ultrasound (CEUS) is widely used for ultrasound diagnosis of various tissues and organs[6,7]. Research has shown that CEUS can accurately visualize the microvascular perfusion of pulmonary lesions[8]. This study compared between the diagnostic efficacy puncture biopsy guided by CEUS and that by conventional ultrasound in peripheral lung lesions. The diagnostic value and advantages of CEUS in puncture biopsy of peripheral focal lung lesions were also explored.
Clinical data: This study included 237 patients (175 male and 62 female), who underwent ultrasound-guided percu
Inclusion criteria: (1) Adults aged > 18 years; and (2) Preoperative CT confirming the presence of subpleural lung lesions or suspected malignant tumors.
Exclusion criteria: (1) Prolonged prothrombin time > 1.5 or platelet count < 30000; (2) Presence of a right-to-left intracardiac shunt; (3) Severe pulmonary hypertension (pulmonary artery pressure exceeding 90 mmHg); (4) Uncontrolled systemic hypertension (i.e., systolic blood pressure exceeding 140 mmHg); (5) Massive pleural effusion; (6) Adult respiratory distress syndrome; and (7) Pregnancy or breastfeeding.
A Phillips iU22 color Doppler ultrasonic diagnostic instrument equipped with a convex array probe was used in the study at a probe frequency of 2-5 MHz. A semiautomatic biopsy gun (Weihai Jierui, 18G × 200 mm, China). The contrast agent used was SonoVue (Bracco, Milan, Italy).
All patients underwent conventional ultrasound to determine the size, location, echo, and adjacency of lesions in comparison with chest CT scans. Color Doppler flow imaging was used to identify the presence of intercostal blood along the puncture path and internal and peripheral blood flow signals of the lesion. Following routine examinations, patients in the control group underwent percutaneous lung puncture biopsy guided by real-time B-ultrasound. This process involved avoiding intercostal and large blood vessels within the lesion and taking 2-3 random biopsy specimens from within the lesion. If the obtained puncture tissues were unsatisfactory, 1-2 additional needles could be used under the condition that the patient had no complications.
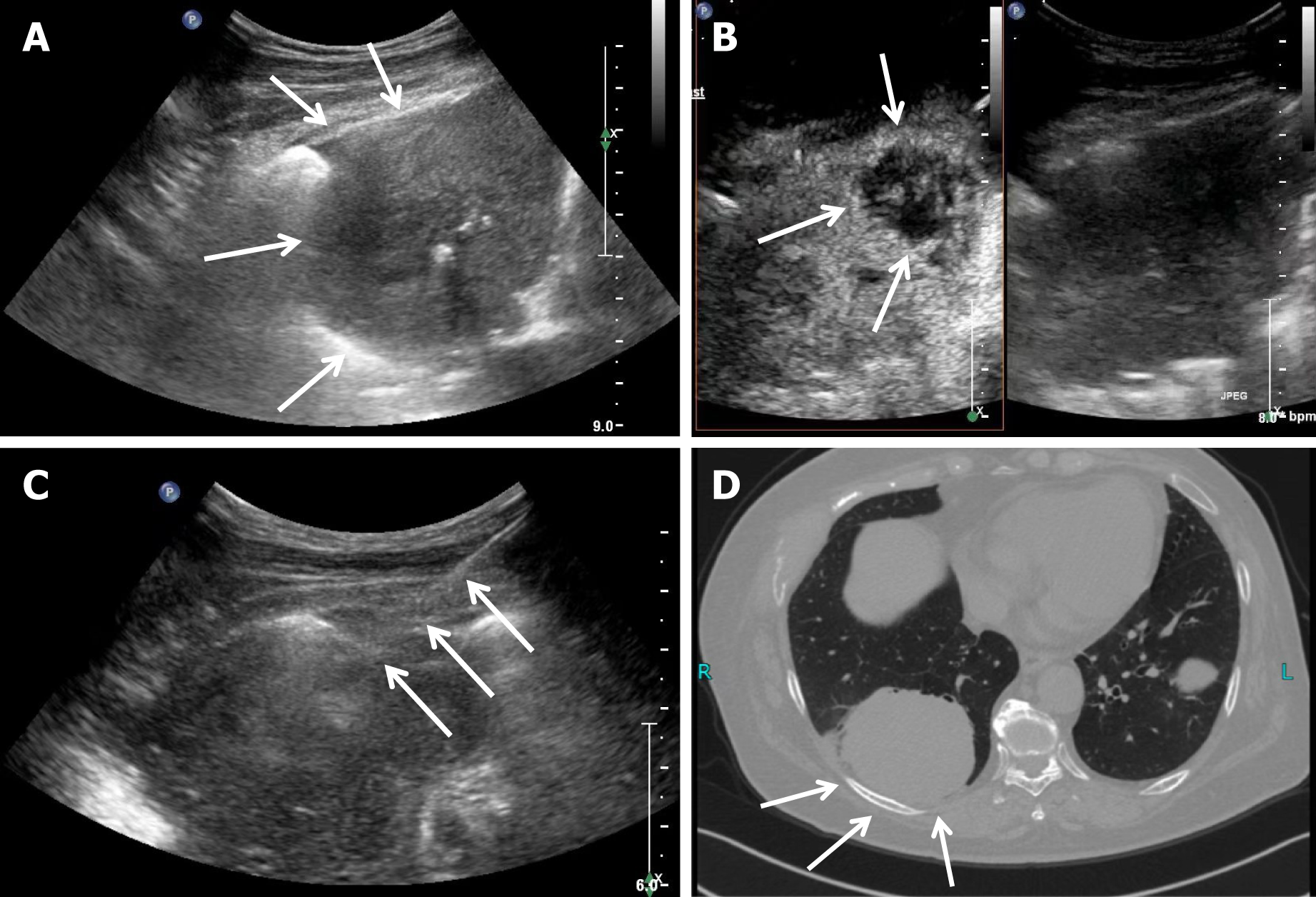
Patients in the contrast group underwent CEUS examination after conventional ultrasound examination. The SonoVue contrast agent was mixed with 5 mL saline and fully oscillated. Subsequently, 2.4 mL of this suspension was extracted and injected through a superficial vein in the elbow, followed by 5 mL normal saline. The contrast enhancement and distribution within the lesion were observed. After CEUS, 1-2 needles were used to puncture the enhanced area of the bronchial arterial phase in the lesion (Figure 1), and 1-2 punctures were randomly performed on other areas. The number of puncture needles and complications that occurred during or after the procedure were recorded. All lung puncture tissues were fixed in formalin solution and numbered individually before being sent for pathological examination.
All lesions underwent puncture biopsy guided by conventional or CEUS. The diagnosis was mainly based on the pathological and histological findings of the first biopsy. If the first biopsy could not establish a diagnosis, a second puncture biopsy or surgery would be performed to establish the pathology.
Statistical analysis was performed using SPSS 25 software. Count data were expressed as mean ± SD and analyzed using the independent sample t test. The χ2 test was performed to compare constituent ratios. The efficacy of puncture biopsy between the contrast and control groups was analyzed using the receiver operating characteristic (ROC) curve, and the DeLong test was used to compare the areas under the curve (AUCs) between the two groups. A P value < 0.05 was con
The CEUS and control groups showed no significant differences in terms of age (68.0 ± 11.0 years vs 68.1 ± 9.6 years, respectively; P > 0.05), sex distribution (87 male/31 female vs 88 male/31 female, respectively; P > 0.05), and smoker status percentage (68% vs 69%, respectively; P > 0.05).
Based on measurements performed on chest CT, the average diameter of the biopsy lesions was 43.1 ± 15.8 mm in the CEUS group and 42.0 ± 14.0 mm in the control group, with no significant difference (P > 0.05). The number and ratio of cases with lesions 1-5 cm in diameter were 83 (50.6%) in the contrast group and 81 (49.4%) in the control group. The number of lesions with diameter > 5 cm were 35 (47.9%) and 38 (52.1%), respectively. No significant difference was observed between the two groups (χ2 = 0.143; P = 0.71).
The locations of the punctured lesions in the contrast and control groups were distributed as follows: Upper lobe [44 (37%) vs 42 (35.6%), respectively], middle lobe [30 (25.2%) vs 39 (33.1%)], respectively, and lower lobe [45 (37.8%) vs 37 (31.4%), respectively]. No significant difference was found in the needle biopsy sites between the two groups (P > 0.05) (Table 1).
| CEUS group | Control group | χ2/t value | P value | |
| Age | 68.0±11.0 | 68.1±9.6 | 0.095 | 0.93 |
| Gender | 0.001 | 0.96 | ||
| Male | 87 (73.7) | 88 (73.9) | ||
| Female | 31 (26.3) | 31 (26.1) | ||
| Smoking history | 68 (57.6) | 69 (58) | 0.001 | 0.96 |
| Location | 1.997 | 0.37 | ||
| Upper leaf | 44 (37) | 42 (35.6) | ||
| Middle leaf | 30 (25.2) | 39 (33.1) | ||
| Lower leaf | 45 (37.8) | 37 (31.4) | ||
| Diameter (mm) | 43.1 ± 15.8 | 42.0 ± 14.0 | 0.282 | 0.58 |
| 1-5 cm | 83 (50.6) | 81 (49.4) | 0.143 | 0.71 |
| ≥ 5 cm | 35 (47.9) | 38 (52.1) |
Accurate pathological results were obtained for 92.83% (220/237) of peripheral lung lesions for the first ultrasound-guided biopsy. The success rate in the CEUS group was 95.76% (113/118), with 60 cases of lung adenocarcinoma, 26 cases of lung squamous cell carcinoma, 4 cases of small-cell lung cancer, and 5 cases of metastatic lung cancer. Eighteen benign lesions were found, comprising 10 cases of pneumonia, 1 case of tuberculosis, 2 cases of pulmonary fibrosis, 2 cases of normal lung tissue, and 3 cases of other diagnoses. In addition, the first puncture did not obtain clear pathological results for 5 cases, of which 4 were confirmed by the second puncture or surgery as malignant lesions and 1 local chronic inflammation.
The success rate in the control group for the first biopsy was 89.92% (107/119), which included 52 cases of lung adenocarcinoma, 19 cases of lung squamous cell carcinoma, 4 cases of small-cell lung cancer, and 7 cases of metastatic lung cancer. Of the 25 benign lesions identified, 13 were cases of pneumonia, 4 of pulmonary fibrosis, 2 of normal lung tissue, and 6 of other diagnoses. Among 12 cases with unclear pathologies, 11 were later confirmed as malignant lesions and 1 as tuberculosis.
No significant difference was observed in the pathological diagnosis between the two groups (P > 0.05) (Table 2).
| CEUS group | Control group | χ2 value | P value | |
| Confirmed cases | 113 (95.8) | 107 (89.9) | 3.042 | 0.081 |
| Undiagnostic biopsy | 5 (4.2) | 12 (10.1) | ||
| Pathology results | 7.93 | 0.541 | ||
| Adenocarcinoma | 60 (50.8) | 52 (43.6) | ||
| Squamous cell carcinoma | 26 (22.0) | 19 (16.1) | ||
| Small cell carcinoma | 4 (3.4) | 4 (3.4) | ||
| Metastatic cancer | 5 (4.2) | 7 (5.9) | ||
| Pneumonia | 10 (8.5) | 13 (10.9) | ||
| Tuberculosis | 1 (0.8) | 0 (0) | ||
| Pulmonary fibrosis | 2 (1.7) | 4 (3.4) | ||
| Normal | 2 (1.7) | 2 (1.7) | ||
| Others | 3 (2.5) | 6 (5.0) |
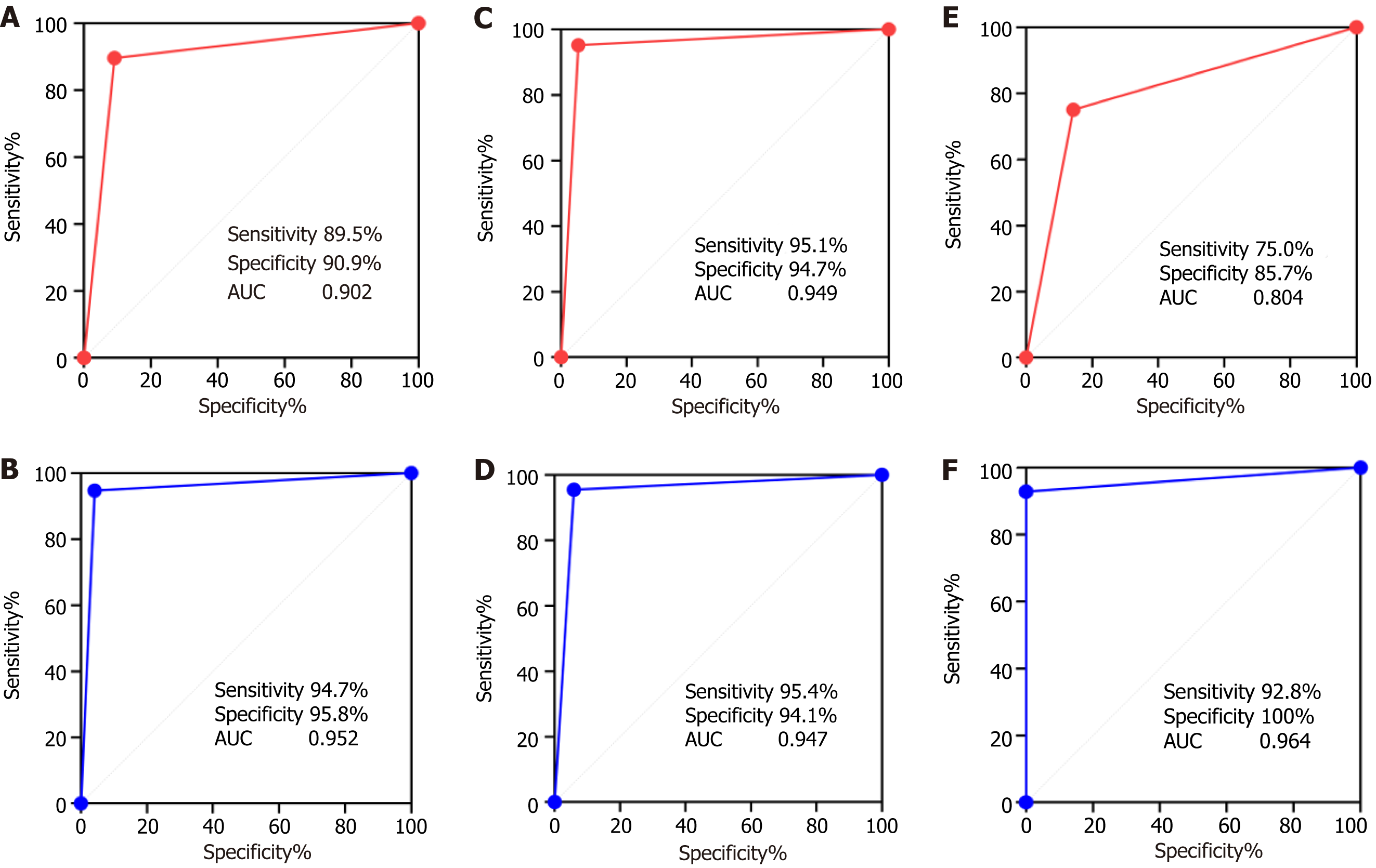
The diagnostic efficiency of the first lung puncture biopsy in both groups was evaluated using the AUCs of the ROC curves. The sensitivity, specificity, and AUC of lung puncture biopsy in the CEUS group were 94.7%, 95.8%, and 0.952, respectively. In the control group, these were 89.5%, 90.9%, and 0.902, respectively. No significant differences were found between the two groups (P > 0.05).
A further subgroup analysis was performed based on lesion diameter. For lesions 1-5 cm in diameter, the sensitivity, specificity, and AUC in the CEUS group were 95.4%, 94.1%, and 0.947, respectively. In the control group, these were 95.1%, 94.7%, and 0.949, respectively. When the lesion diameter ≥ 5 cm, the sensitivity, specificity, and AUC in the CEUS group were 92.8%, 100%, and 0.964, respectively, and 75.0%, 85.7%, and 0.804, respectively, in the control group (Figure 2). The AUC of the contrast group was significantly higher than that of the control group (0.949 vs 0.804, respectively; P < 0.05) (Table 3).
| CEUS group | Control group | z value | P value | |
| Total number (n = 237) | 118 | 119 | ||
| Sensitivity (95%CI) | 94.7% (88.15-97.71) | 89.5% (81.29-94.40) | 1.093 | 0.274 |
| Specificity (95%CI) | 95.8% (79.76-99.79) | 90.9% (76.43-96.86) | 0.796 | 0.426 |
| AUC (95%CI) | 0.952 (0.896-0.983) | 0.902 (0.835-0.949) | 1.282 | 0.200 |
| 1-5 cm (n = 164) | 83 | 81 | ||
| Sensitivity (95%CI) | 95.4% (87.47-98.76) | 95.1% (86.71-98.68) | 0.134 | 0.893 |
| Specificity (95%CI) | 94.1% (73.02-99.70) | 94.7% (75.36-99.73) | 0.081 | 0.936 |
| AUC (95%CI) | 0.949 (0.877-0.986) | 0.947 (0.876-0.985) | 0.037 | 0.970 |
| ≥ 5 cm (n = 73) | 35 | 38 | ||
| Sensitivity (95%CI) | 92.8% (77.35-98.73) | 75.0% (55.10-88.00) | 1.178 | 0.075 |
| Specificity (95%CI) | 100% (64.57-100.0) | 85.7% (60.06-97.46) | 1.051 | 0.293 |
| AUC (95%CI) | 0.964 (0.840-0.998) | 0.804 (0.643-0.914) | 2.271 | 0.023 |
The average number of puncture needles used in the CEUS group was significantly lesser than that in the control group (2.58 ± 0.53 vs 2.90 ± 0.56, respectively; P < 0.05) (Table 4).
| Groups | Cases | Total number of puncture cases | Average number of puncture cases |
| CEUS group | 118 | 298 | 2.58 ± 0.53 |
| Control group | 119 | 345 | 2.90 ± 0.56 |
| χ2/t value | - | - | 4.567 |
| P value | - | - | 0.000 |
No major complications were noted during or after the puncture biopsy in this study; however, 12 cases (5.1%) of mild complications were observed. The complication rate in the CEUS group as 4.2% (5/118), whereas that in the control group was 5.9% (7/119). Minor bleeding from the puncture needle tract occurred in 5 cases (2 in the CEUS group, 3 in the control group). All bleeding ceased after a brief period of compression. Chest pain accompanied by a slight cough was reported in 4 cases (2 in the CEUS group, 2 in the control group), all of which improved after rest. Furthermore, 3 cases of mild pneumothorax (1 in the CEUS group and 2 in the control group) were detected by postoperative ultrasound and confirmed by follow-up CT. No symptoms such as hemoptysis or dyspnea were recorded. These cases underwent clinical follow-up observation.
Ultrasound-guided puncture biopsy has certain inherent limitations, considering lung tissue is a gas-containing organ. Therefore, the puncture biopsy of lung tumors mainly relies on CT guidance. However, peripheral lung lesions can be clearly visualized using real-time ultrasound. Ultrasound-guided puncture biopsy for peripheral pulmonary lesions offers several advantages, such as no radiation exposure, precision, convenience and flexibility in adjusting the needle insertion angle, which make ultrasound-guided biopsy more effective than a CT-guided one[9]. However, with necrosis in lung tumors, distinguishing between necrotic and active areas in peripheral lung masses becomes difficult with conventional ultrasound, causing a 20% failure rate for puncture biopsy. CEUS can enhance microvascular perfusion in peripheral lung tumors, and the enhanced areas often indicate active areas[10]. Bai et al[11] showed that CEUS of lung lesion shows a significant difference in the lesion arrival time for benign and malignant lesions, with a later arrival time indicating a higher likelihood of malignancy.
Operators can distinguish the active areas within the entire tumor through enhancement intensity and phase changes. Under CEUS guidance, the puncture of necrotic and inflammatory areas in large tumors can be avoided, thereby im
In the present study, 92.83% (220/237) of cases were successfully given a pathological diagnosis. Although no significant difference was observed between the CEUS group and control groups, the sensitivity, specificity and AUC of the CEUS group were higher than those of the control group. Zhang et al[12] explored ultrasound-guided percutaneous needle biopsy for peripheral lung lesions and possible complications. They analyzed 92 patients and found that the diagnostic success rate of one puncture did not differ significantly between the CEUS and noncontrast groups (98.1% vs 90.0%, respectively; P > 0.05), which is similar to our finding. Zhou et al[13] also investigated 315 cases of CEUS-guided percutaneous lung biopsies. The study achieved a diagnostic accuracy rate of 92.6%, which is comparable with that in our study.
Further subgroup analysis revealed that when the tumor diameter was 1-5 cm, no significant difference was found in the success rate of lung malignant tumor puncture between the two groups. However, when the tumor diameter ≥ 5 cm, the AUC efficiency of lung puncture in the CEUS group was higher than that in the control group (0.964 vs 0.804, respectively; P < 0.05). This may be due to the lower necrosis rate when the lesion is small since, an increase in the lung tumor size induces a corresponding increase in the possibility of tumor necrosis and bleeding. Necrotic and active areas of lung tumors are difficult to distinguish on conventional ultrasound; CEUS addresses this by visualizing necrotic or liquefied areas within the tumor. Under CEUS, necrotic areas can be effectively avoided during puncture, thereby improving the puncture success rate. Quarato et al[14] showed that the detection rate of necrosis was 8% for lung lesions 1-2 cm in diameter, 31% when the diameter was 2-5 cm, and 100% when the diameter was > 5 cm. In addition, as the tumor diameter increases, the mixed echoes generated by secondary inflammation and atelectasis after tumor necrosis increases the difficulty of diagnosis by ordinary ultrasound, increasing the rate of false negative results in ordinary ultrasound-guided biopsy puncture.
In this study, 12 patients developed complications, with an overall incidence rate of 5.1%. The incidence rate of complications in the CEUS group was 4.2%, which is slightly lower than that in the control group (5.9%). All patients improved after conservative treatment. Previous reports have shown that the complications rate in ultrasound-guided lung biopsy is 2%-15%[15-18], which is consistent with the rates reported in the present study.
In ultrasound-guided puncture biopsy, the accuracy of lung puncture increases with the number of puncture needles used; however, each additional needle potentially raises the risk of complications. Patients often refuse multiple puncture biopsies due to associated pain and fear[19]. In the present study, the average number of puncture needles in the CEUS group was lesser than that in the control group; however, the puncture efficiency remained unaffected. This indicates that CEUS-guided puncture biopsy is more precise and can reduce the pain and risk of complications for patients.
This study has some limitations. First, it was a retrospective study conducted at a single center with a relatively small sample size, which may have unintentionally introduced selection bias. Second, the outcomes of ultrasound-guided puncture are intrinsically linked to the surgeon’s experience, limiting the generalizability of the results. Lastly, not all cases were reexamined by CT postoperatively, which indicates a possibility that complications may have been underestimated on postoperative ultrasound examination and clinical symptoms assessment. Further studies with a larger sample size and multicenter clinical studies need to be conducted to verify our findings.
In conclusion, CEUS can enhance the visualization of active areas within peripheral pulmonary focal lesions by improving the visualization of microvessels. Especially for lesions with a diameter ≥ 5 cm, CEUS can improve puncture area selection and accuracy, especially by visualizing inflamed and necrotic tissue. Our findings demonstrate that CEUS adds clinical diagnostic value to puncture biopsy of peripheral lung lesions.
We would like to thank all the workers who have contributed to this research.
| 1. | Harrison S, Judd J, Chin S, Ragin C. Disparities in Lung Cancer Treatment. Curr Oncol Rep. 2022;24:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Abrishami Kashani M, Campbell-Washburn AE, Murphy MC, Catalano OA, McDermott S, Fintelmann FJ. Magnetic Resonance Imaging for Guidance and Follow-up of Thoracic Needle Biopsies and Thermal Ablations. J Thorac Imaging. 2022;37:201-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 3. | Granata A, Distefano G, Pesce F, Battaglia Y, Suavo Bulzis P, Venturini M, Palmucci S, Cantisani V, Basile A, Gesualdo L. Performing an Ultrasound-Guided Percutaneous Needle Kidney Biopsy: An Up-To-Date Procedural Review. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Stigt JA, Groen HJ. Percutaneous ultrasonography as imaging modality and sampling guide for pulmonologists. Respiration. 2014;87:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Liao MY, Zhou YF, Tian ZX, Luo R, Qu YJ, Xu LY. [The factor analysis of the incidence of complication in CT-guided lung automated cutting needle biopsy with extrapleural locating method]. Zhonghua Yi Xue Za Zhi. 2010;90:1747-1751. [PubMed] |
| 6. | Erlichman DB, Weiss A, Koenigsberg M, Stein MW. Contrast enhanced ultrasound: A review of radiology applications. Clin Imaging. 2020;60:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Yusuf GT, Wong A, Rao D, Tee A, Fang C, Sidhu PS. The use of contrast-enhanced ultrasound in COVID-19 Lung imaging. J Ultrasound. 2022;25:319-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Chen W, Zhang Y, Tang J, Wei D, Liao H, Zhang S, He L, Tang Q. Correlations between contrast-enhanced ultrasound and microvessel density in non-small cell lung cancer: A prospective study. Front Oncol. 2023;13:1086251. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Safai Zadeh E, Görg C, Prosch H, Jenssen C, Blaivas M, Laursen CB, Jacobsen N, Dietrich CF. WFUMB Technological Review: How to Perform Contrast-Enhanced Ultrasound of the Lung. Ultrasound Med Biol. 2022;48:598-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Hong-Xia Z, Wen H, Ling-Gang C, Wen-Jia C, Shuo L, Li-Juan D, Hai-Man S, Yang Z. A New Method for Discriminating between Bronchial and Pulmonary Arterial Phases using Contrast-Enhanced Ultrasound. Ultrasound Med Biol. 2016;42:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Bai J, Yang W, Wang S, Guan RH, Zhang H, Fu JJ, Wu W, Yan K. Role of Arrival Time Difference Between Lesions and Lung Tissue on Contrast-Enhanced Sonography in the Differential Diagnosis of Subpleural Pulmonary Lesions. J Ultrasound Med. 2016;35:1523-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Zhang H, Guang Y, He W, Cheng L, Yu T, Tang Y, Song H, Liu X, Zhang Y. Ultrasound-guided percutaneous needle biopsy skill for peripheral lung lesions and complications prevention. J Thorac Dis. 2020;12:3697-3705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Zhou D, Zhang Y, Chen W, Jiang J, Chen Y, Zhou X, Tang Q. Enhanced ultrasound-guided vs non-enhanced ultrasound-guided percutaneous needle biopsy in tissue cellularity of lung malignancies: a propensity score matched study. Quant Imaging Med Surg. 2022;12:5056-5067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Quarato CMI, Cipriani C, Dimitri L, Lacedonia D, Graziano P, Copetti M, De Cosmo S, Simeone A, Scioscia G, Foschino Barbaro M, Sperandeo M. Assessing value of contrast-enhanced ultrasound vs. conventional transthoracic ultrasound in improving diagnostic yield of percutaneous needle biopsy of peripheral lung lesions. Eur Rev Med Pharmacol Sci. 2021;25:5781-5789. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Lee MH, Lubner MG, Hinshaw JL, Pickhardt PJ. Ultrasound Guidance Versus CT Guidance for Peripheral Lung Biopsy: Performance According to Lesion Size and Pleural Contact. AJR Am J Roentgenol. 2018;210:W110-W117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Mychajlowycz M, Alabousi A, Mironov O. Ultrasound- Versus CT-Guided Subpleural Lung and Pleural Biopsy: An Analysis of Wait Times, Procedure Time, Safety, and Diagnostic Adequacy. Can Assoc Radiol J. 2021;72:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Guo YQ, Liao XH, Li ZX, Chen YY, Wang SD, Wang JH, Liao XS, Luo Y. Ultrasound-Guided Percutaneous Needle Biopsy for Peripheral Pulmonary Lesions: Diagnostic Accuracy and Influencing Factors. Ultrasound Med Biol. 2018;44:1003-1011. [PubMed] [DOI] [Full Text] |
| 18. | Khan RA, Kumar V, Taimur M, Khan MA, Arshad MM, Amjad MA. Diagnostic Yield of Ultrasound-guided Trucut Biopsy in Diagnosis of Peripheral Lung Malignancies. Cureus. 2019;11:e4802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Gaffney CD, Vertosick EA, Carlsson SV, Lin X, Wolchasty N, Hardbattle R, Vickers AJ, Ehdaie B. A brief mind-body intervention to reduce pain and anxiety during prostate needle biopsy: a clinically integrated randomized controlled trial with 2-staged consent. Urol Oncol. 2023;41:484.e1-484.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |