Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3539
Revised: April 23, 2024
Accepted: May 7, 2024
Published online: June 26, 2024
Processing time: 172 Days and 0.8 Hours
Few studies have reported an association between an increased risk of acquiring cancers and survival in patients with 4q deletion syndrome. This study presents a rare association between chromosome 4q abnormalities and fallopian tube high-grade serous carcinoma (HGSC) in a young woman.
A 35-year-old woman presented with acute dull abdominal pain and a known chromosomal abnormality involving 4q13.3 duplication and 4q23q24 deletion. Upon arrival at the emergency room, her abdomen appeared ovoid and distended with palpable shifting dullness. Ascites were identified through abdominal ultrasound, and computed tomography revealed an omentum cake and an enlarged bilateral adnexa. Blood tests showed elevated CA-125 levels. Para
This study demonstrates a rare correlation between a chromosome 4q abnormality and HGSC. UBE2D3 may affect crucial cancer-related pathways, including P53, BRCA, cyclin D, and tyrosine kinase receptors, thereby possibly contributing to cancer development. In addition, ADH1 and DDIT4 may be potential influencers of both carcinogenic and therapeutic responses.
Core Tip: In this report, we present a case of high-grade serous carcinoma (HGSC) in the fallopian tube of a young woman, associated with a chromosomal 4q abnormality. We provide updated insights on the symptoms, signs, diagnosis, and treatment specific to HGSC linked with chromosomal abnormalities. Given the rare nature of chromosomal abnormality-associated HGSC, we have formulated a strategic approach for its diagnosis and management.
- Citation: Zhang KC, Chu SY, Ding DC. High-grade serous carcinoma of the fallopian tube in a young woman with chromosomal 4q abnormality: A case report. World J Clin Cases 2024; 12(18): 3539-3547
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3539.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3539
Ovarian cancer had been the fifth most common cancer in women and the fourth most common cause of cancer-related deaths in women from 1999 to 2009 in the United States[1,2]. Epithelial ovarian tumors comprise 90% of ovarian cancers, of which approximately 80% are serous carcinomas that arise from the fallopian tube epithelium[1-3]. Epidemiological data have shown that the oldest age during which ovarian cancer is detected is 60-65 years in the United Kingdom and Taiwan, with 90% of cases occurring after 45 years of age[4,5]. Numerous factors contribute to an increased risk of ovarian cancer. These include early onset of menstruation (early menarche), delayed cessation of menstrual cycles (late menopause), a family history of breast or ovarian cancer, and mutations in the BRCA1 or BRCA2 genes[6-8]. Early-stage ovarian cancers are often asymptomatic, whereas mild or frequent symptoms such as gastrointestinal discomfort, abdominal pain or masses, loss of appetite and weight, abnormal vaginal bleeding, and dyspnea due to pleural effusion occur in more advanced cases[9]. Although the exact causes of ovarian cancers remain unclear, different gene mutations allowing us to distinguish between high- and low-grade serous ovarian carcinomas have been identified through pathology[2,10]. Thus, genetic mutations may be critical in serous ovarian carcinomas.
Chromosome 4 is a large chromosome that contains multiple critical protein-coding genes[11,12]. Interstitial or terminal deletion of 4q results in 4q deletion syndrome, also called 4q syndrome, which is a rare chromosomal disorder with an estimated incidence of 1 per 100000 people[13]. The most common phenotypes of 4q deletion syndrome typically involve anomalies in craniofacial, digital, skeletal, and cardiac features, and this condition is associated with a mortality rate of approximately 23%[14-17]. Most surviving probands show delayed neurodevelopment and antenatal or postnatal growth deficiencies[15]. However, in some patients, slow growth and intellectual disability may occur without any apparent physical abnormalities, making it difficult to recognize this disorder[18].
Owing to its rarity and frequent underdiagnosis, few studies have identified an association between an increased risk of developing cancers and 4q deletion syndrome. Herein, we report a rare association between chromosome 4q abnormality and high-grade serous carcinoma (HGSC) of the fallopian tube in a 35-year-old woman.
Acute abdominal dull pain was noted.
A 35-year-old female with a chromosomal abnormality (4q13.3 duplication and 4q23q24 deletion) had been in her usual state of health until a month ago. Her activities of daily living were partially dependent, and she was able to attend the daycare center for education. One month before admission, she experienced abdominal distension without diarrhea, vomiting, and changes in eating habits. Thus, her mother visited a pharmacy and bought symptom-relieving medications.
She had a sudden onset of acute dull abdominal pain at the right upper quadrant, without specific precipitating or relieving factors. She rated her pain as an 8/10 and mentioned that it kept her up at night. Her mother immediately visited the emergency department of our hospital for evaluation.
Five years ago, the patient first visited our hospital because of cataract due to phacoemulsification in both eyes at 30 years of age. Four years prior, she visited our pediatric outpatient department as she did not experience her menstrual cycle for a year. Our pediatrician found that her hand joints were contracted and deformed. Unintentional body weight loss of 30 kg/year, moderate mental retardation, and delayed development without a rehabilitation program were also observed. Her karyotype was 46, XX with normal metabolic and hormone disease screening test results. Complete blood count test found that she had microcytic anemia with a family history of alpha thalassemia from her father's side. Blood electrophoresis revealed normal hemoglobin (Hb) A1, HbA2, and HbH levels.
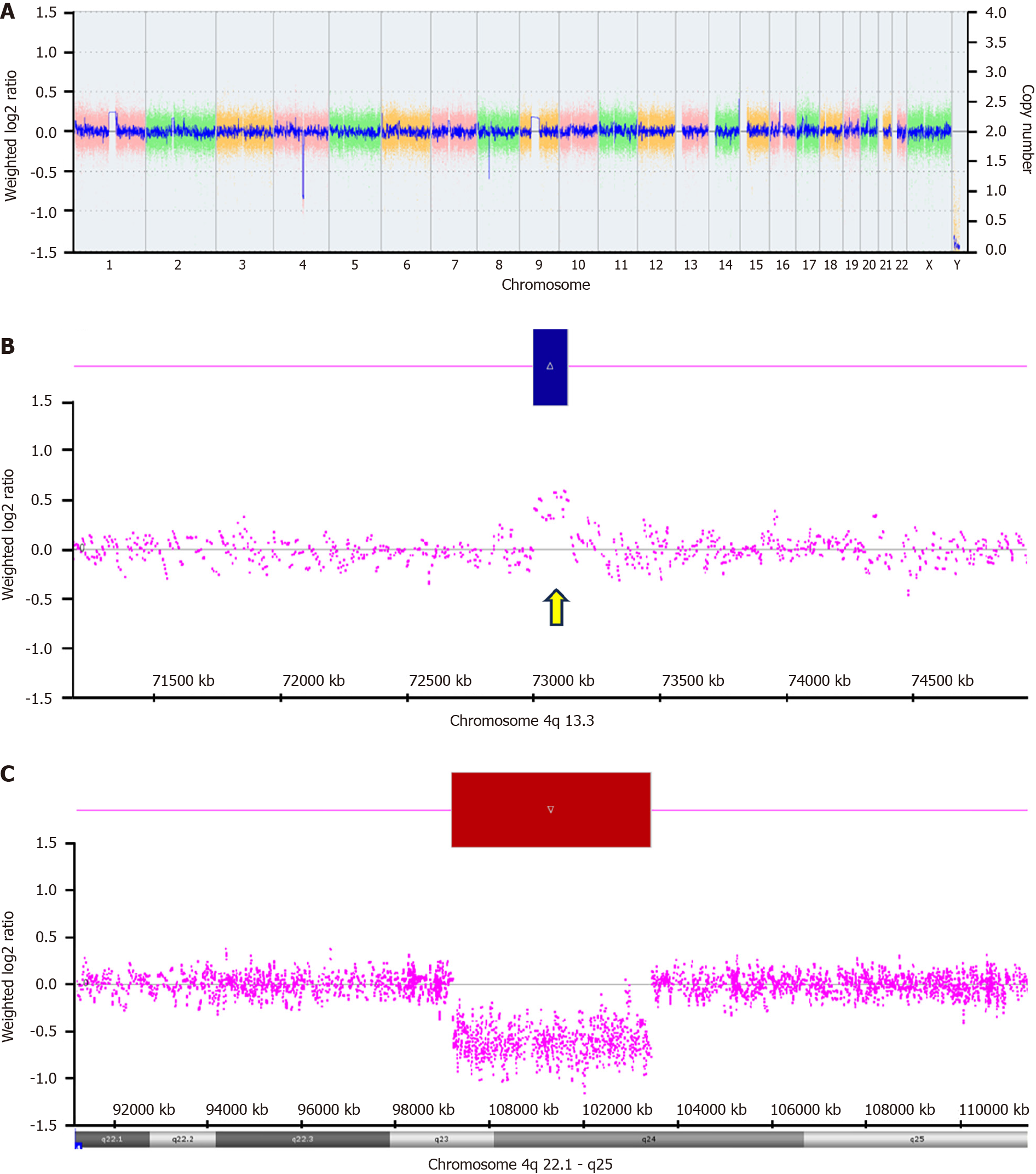
After receiving education on nutritional support, her menstrual cycles returned to normal. Two years ago, her menstrual cycle became absent again, and her pelvic echogram showed a right ovarian cyst approximately 1.3 cm2 × 1.4 cm2 in size without other positive findings. Whole-exome sequencing revealed 46 XX, 4q13.3 duplication, and 4q23q24 deletions affecting multiple genes (Figure 1).
Radiography of her joints showed osteoarthritis of the bilateral hip joints with large subchondral cysts at the right acetabular roof and femoral head; osteoarthritis of the bilateral knee joints; multiple joint destruction, deformity, and subluxation at most of the metacarpophalangeal, proximal interphalangeal, and distal interphalangeal joints in both hands; and diffuse joint space narrowing with subchondral and marginal erosions at the radiocarpal joint and intercarpal joints of bilateral wrists. She had come for regular follow-up 1 year prior.
The patient complained of frequent abdominal bloating and constipation. Amenorrhea was also observed for half a year.
The patient was a freelance worker, nonsmoker, and nonalcoholic consumer and had regular menstrual cycles since she was 12 years of age. Her parents had no history of cancer. Her uncle and grandmother had skin cancer and leukemia, respectively. She had a brother 5 years older, who was mentally and physically normal.
On examination, the patient was alert and fully oriented. Her temperature was 36.2 °C, blood pressure was 100/60 mmHg, pulse was 82 beats per minute, respiratory rate was 20 breaths/min, and oxygen saturation was 97% while breathing room air. The patient was malnourished and pale. Her height, weight, and body mass index were 145 cm, 37 kg, and 17.6 kg/m2, respectively. The abdomen was ovoid and distended. Bowel sounds are typical in auscultation. A tympanic sound was heard at the top of the abdomen but it did not become dull when shifting downwards. A shifting dullness test was performed thereafter, showing positive findings. Abdominal tenderness was observed only in the right upper quadrant without rebounding pain, and Murphy’s sign was negative. Her heart sounds were regular and without murmurs. Breathing sounds with pleural friction rubs were auscultated in the bilateral lung fields. Her extremities were warm, with pitting edema. Her right hip showed a decreased range of motion and muscle power, which were otherwise normal in the knees, shoulders, elbows, and left hip. Her hands contracted with stiffness at the metacarpal phalangeal, proximal interphalangeal, and distal interphalangeal joints and bilateral radiocarpal and intercarpal joints. The capillary refilling time of the toe was > 2 s. Results of the remaining examinations were normal.
Laboratory analyses revealed microcytic anemia, hypoalbuminemia, hypoproteinemia, and elevated C-reactive protein levels. Cancer markers showed increased cancer antigen 125 (241.7 U/mL), while the other markers were within the normal reference range. Paracentesis showed cloudy ascites with a white blood cell count of over 1000 cells/μL. Moreover, the amylase level in ascites was below 1000 IU/L, suggesting the absence of pancreatic ascites. The serum-ascites albumin gradient was 0.5 g/dL, suggesting that ascites was not caused by portal hypertension. Culture and gram staining results were negative for bacterial infections (Table 1).
| Variable | On admission | Reference range |
| Hematocrit (%) | 31.5 | 36-46 |
| Hemoglobin (g/dL) | 8.7 | 12-16 |
| White cell count (μL) | 10480 | 3500-11000 |
| Prothrombin time (s) | 9.9 | 8-12 |
| Total protein (g/dL) | 5.1 | 6.4-8.9 |
| Albumin (g/dL) | 3.3 | 3.5-5.7 |
| CRP (mg/dL) | 9.39 | < 1 |
| AFP (ng/mL) | 1.9 | < 9 |
| CEA (ng/mL) | 0.3 | < 3 |
| CA125 (U/mL) | 241.7 | < 35 |
| CA19-9 (U/mL) | 2.7 | < 35 |
| Ascites | ||
| Appearance | Cloudy | - |
| WBC count (μL) | 1087 | - |
| Amylase (IU/L) | 134 | - |
| Albumin (g/dL) | 2.6 | - |
| Gram stain | Negative | Negative |
| Bacteria culture | No growth | No growth |
Kidney, ureter, and bladder radiography showed hydroperitoneum. Computed tomography from the lower chest to the pelvis revealed carcinomatous peritonitis and an omental cake.
Stage IIIC HGSC of the fallopian tube.
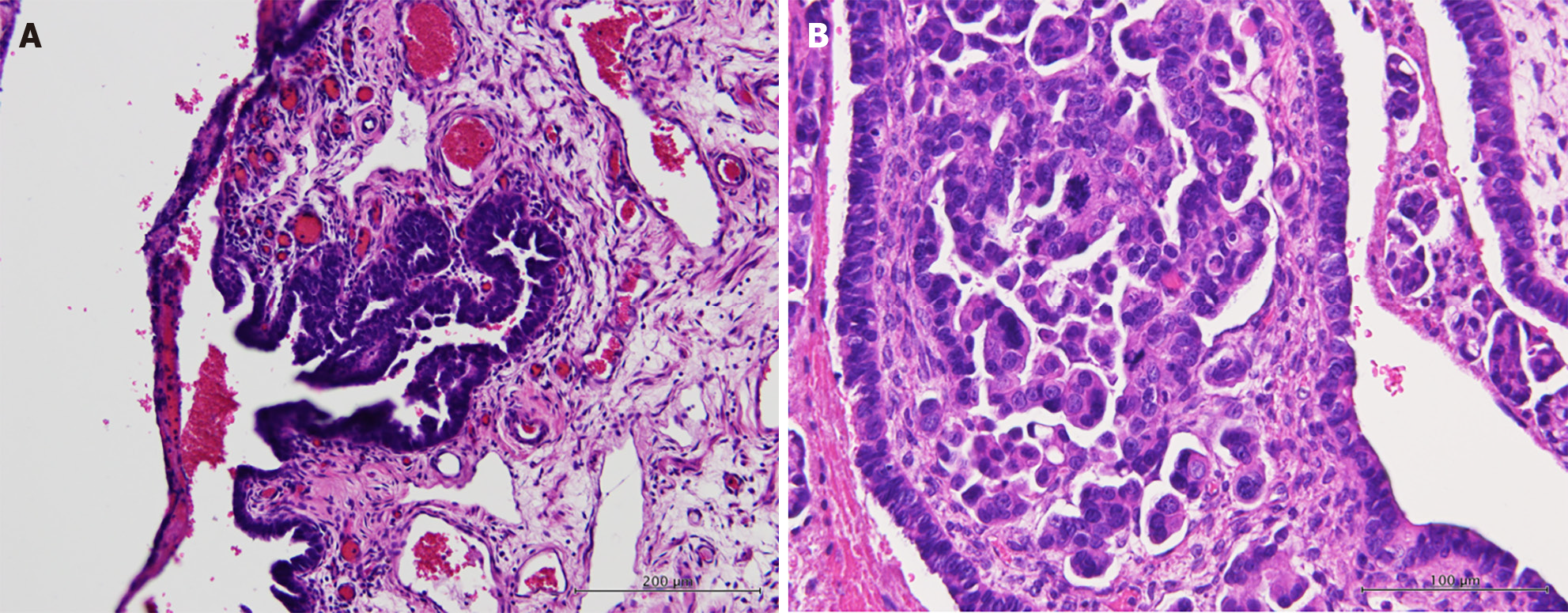
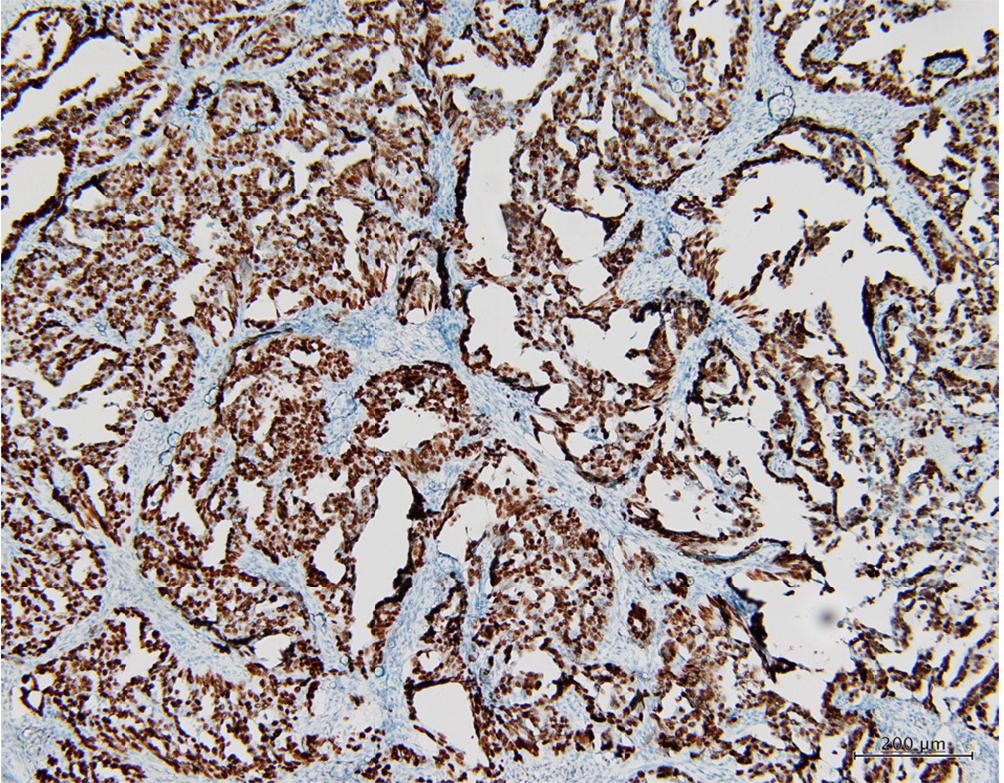
With the impression of secondary bacterial peritonitis of unknown origin, pelvic ultrasonography was performed, demonstrating increased bilateral adnexal mass with carcinomatosis in the cul-de-sac. Additional immunohistochemical staining of the ascites showed CK7(+), CK20(-), CDX2(-), PAX8(-), and TTF-1(-) expression, indicating that the cancer may have originated from the ovaries. Staging surgery for ovarian cancer was performed using frozen-section pathology, demonstrating HGSC. Histopathological examination confirmed that the primary site was the right fallopian tube, showing serous tubal intraepithelial carcinoma and HGSC (Figure 2). Moreover, HGSC was found in the bilateral ovaries, uterine corpus, greater omentum, peritoneum, bilateral pelvic lymph nodes, para-aortic lymph nodes, and appendix. The tumor size in the bilateral ovaries was > 2 cm with lymph node metastasis to the paraaortic lymph nodes (pT3cN1a, FIGO stage IIIC). Additional immunohistochemical staining of the tumor revealed mutational overexpression of p53 (Figure 3) and strong positive estrogen receptor expression (60%). MSH6 and PMS2 proteins retained expression in the nucleus.
Postoperatively, she received two courses of chemotherapy with carboplatin (dosage: area under the curve = 5) and paclitaxel (dosage: 135 mg/m2). However, pancytopenia was observed after the first course of chemotherapy. She recovered after subcutaneous injection of granulocyte colony-stimulating factor, packed erythrocytes, and platelet transfusion. The chemotherapy dose was reduced to 80% of the standard dose. The patient was closely monitored thereafter. The patient's symptom onset and diagnostic timeline are presented in Table 2.
| Year-old | Incident |
| 12 | Menarche |
| 26 | No mense for one year, hand joints were contracted and deformed, Unintentional body weight loss of 30 kg/year, moderate mental retardation, and delayed development |
| 26 | Karyotype was 46, XX |
| 30 | Cataract due to phacoemulsification in both eyes |
| 33 | The menstrual cycle became absent again |
| 33 | NGS showed 4q13.3 duplication, and 4q23q24 deletions |
| 35 | High-grade serous carcinoma of the ovary, stage IIIC |
Chromosome 4q defects, including deletions, loss of heterogeneity, and translocations, have been observed in colon, lung, and multiple myeloma cancer tissues, including deletion, loss of heterogeneity, and translocation[19-21]. Frequent loss of chromosome 4q has been observed in the analysis of ovarian cancer cell lines and tissues using comparative genetic hybridization[22,23]. However, the critical mutated genes in aggressive HGSCs are TP53 and BRCA on chromosome 17[2,10]. Few studies have shown that the loss of critical genes in 4q deletions results in an increased risk of developing HGSC. To our knowledge, this study is the first to report the potential association between specific genes within the critical region of the 4q23q24 deletion and HGSC, as well as multiple aging-related diseases, such as osteoarthritis and cataracts.
In our case, the patient lost one copy of the 4q23q24 region, which affected multiple genes, as listed in Table 1. Several of the genes involved may be associated with HGSC. The ubiquitin-conjugating enzyme E2 D3 (UBE2D3) gene encodes the UBE2D3 protein (Ubc5Hc), which regulates ubiquitination[24]. Ubiquitination targets proteins for proteasomal degradation and requires the thioester cascade of the E1-E2-E3 complex to transfer ubiquitin to target proteins[25]. Recent studies have shown that the ubiquitination and deubiquitination of multiple pathways are associated with tumorigenesis and cancer metabolism[26,27]. BRCA proteins are essential for repairing DNA mutations that have been found to increase the risk of ovarian cancer[28]. The RING domain in the BRCA1-BARD1 protein orients the UBE2D3 protein towards the ubiquitination of H2A for homologous recombination to repair DNA double-strand breaks[29]. Moreover, mutations in the BRCA1-BARD1 RING domain have been linked to familial breast and ovarian cancer, showing that structural compactness and reduced significant interactions may be a possible reason for cancer by preventing ubiquitination and hindering DNA repair[30].
P53 mutations are commonly observed in advanced ovarian cancers, as in our case[31]. Mutations in the TP53 gene encodes the mutant P53 protein, which mainly induces mutations affecting cell proliferation or mutagen exposure[32]. The mutant P53 protein loses its wild-type tumor-suppressive function and acquires oncogenic properties, enabling tumor metastasis, proliferation, and cell survival[33]. Moreover, mutant p53 exerts dominant-negative activity by abrogating DNA binding and growth suppression of wild-type p53[34]. Mutant p53 is regulated by many of the same signals that regulate wild-type p53[35]. The loss of degrading enzymes of wild-type p53, including murine double minute 2 (Mdm2), an E3 ubiquitin ligase that targets p53 for proteolytic degradation, stabilizes mutant p53, resulting in an earlier age of tumor onset in mice[36]. UBE2D3 protein knockdown causes the accumulation of Mdm2 and p53 in unstressed cells and inhibits the MDM2-mediated ubiquitin-proteasome degradation of p53[37]. In human breast cancer cells, UBE2D3 protein knockdown reduces ubiquitination of cyclin D1, resulting in increased proliferation and cell invasiveness[38]. UBE2D3 also has ubiquitinated tyrosine kinase receptors that are commonly dysregulated in HGSC[39,40]. In summary, the downregulation of UBE2D3 impairs the ubiquitination of multiple proteins, including P53, BRCA, Cyclin D, and tyrosine kinase receptors, with some losing their function, which may lead to cancer.
The class 1 alcohol dehydrogenase (ADH1) family is associated with ovarian carcinogenesis and peritoneal metastasis. ADH1B enhances various factors that additionally facilitate the progression of ovarian cancer[41]. The drug metabolism-associated gene ADH1B and its interactive network are crucial for immune regulation and therapeutic responses[42]. ADH1B downregulation has been observed in ovarian cancer. Therefore, it may be a potential therapeutic target in patients with ovarian cancer. ADH1B downregulation could be linked to drug resistance[43]. Our patient had an ADH1B deletion.
DDIT4 overexpression increases recurrence risk by 18%[44]. DDIT4 may be involved in antitumor therapy resistance, proliferation, and invasion in numerous cancers and participates in DNA-damage repair in specific conditions[45]. Therefore, DDIT4L was deleted in this patient, which may have led to carcinogenesis.
Besides HGSC, the early onset of senile diseases such as cataracts in both eyes and osteoarthritis in the hip in her 30s may have been caused by the deletion of the CISD2 gene. The expression levels of CISD2 decrease in an age-dependent manner during natural aging. CSID2 knockout drives premature aging by causing mitochondria-mediated defects in mice[46]. The lifespan of mice was extended after achieving a persistent level of CISD2 via transgenic expression[47]. Impaired osteogenic differentiation has been observed in CISD2-deficient murine-induced pluripotent stem cells, contributing to the pathogenesis of osteopenia and the lordokyphosis phenotype in CISD2 knockout mice[48]. Mitochondrial dysfunction in chondrocytes derived from CISD2 knockout murine-induced pluripotent stem cells leads to decreased extracellular matrix and increased reactive oxygen species formation, which is commonly observed in the aged chondrocytes of patients with osteoarthritis[49]. Nevertheless, no study has reported that CISD2 deficiency induces cataracts. CISD2 deficiency affects human corneal epithelial cells and impairs epithelial regeneration[50]. These results indicate that CISD2 gene deletion may be associated with premature aging diseases, which may require further attention in our patients for the onset of certain conditions.
This study revealed a rare correlation between chromosome 4q abnormality and fallopian tube HGSC in a 35-year-old woman. UBE2D3 is implicated in cancer-related pathways, affecting P53, BRCA, cyclin D, and tyrosine kinase receptors, and potentially contributing to cancer development. ADH1 and DDIT4 are factors that influence carcinogenesis and therapeutic responses. The clinical significance lies in uncovering this rare association in young individuals and providing insights into key cancer pathways. These findings may affect the diagnosis, prognosis, and treatment strategies for HGSC with similar genetic abnormalities, emphasizing the need for personalized approaches in ovarian cancer management. Further research is required to identify novel therapeutic targets for tailored treatment.
We thank Dr. Yu-Tai Shih for his assistance with X-ray reading.
| 1. | Ledermann JA, Sessa C, Colombo N; ESMO Guidelines Committee. appendix 7: Ovarian cancer: eUpdate published online September 2016 (http://www.esmo.org/Guidelines/Gynaecological-Malignancies). Ann Oncol. 2016;27:v145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C; ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi24-vi32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 531] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 3. | Hong MK, Chu TY, Ding DC. The fallopian tube is the culprit and an accomplice in type II ovarian cancer: A review. Tzu Chi Med J. 2013;25:203-205. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Teng YH, Liu FC, Huang SY, Kuo CF, Yu HP. Epidemiology and Mortality of Ovarian Cancer in Taiwan: A Population-Based Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Redman C, Duffy S, Bromham N, Francis K; Guideline Development Group. Recognition and initial management of ovarian cancer: summary of NICE guidance. BMJ. 2011;342:d2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Gong TT, Wu QJ, Vogtmann E, Lin B, Wang YL. Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies. Int J Cancer. 2013;132:2894-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Tehranifar P, Wu HC, Shriver T, Cloud AJ, Terry MB. Validation of family cancer history data in high-risk families: the influence of cancer site, ethnicity, kinship degree, and multiple family reporters. Am J Epidemiol. 2015;181:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Schrader KA, Hurlburt J, Kalloger SE, Hansford S, Young S, Huntsman DG, Gilks CB, McAlpine JN. Germline BRCA1 and BRCA2 mutations in ovarian cancer: utility of a histology-based referral strategy. Obstet Gynecol. 2012;120:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Armstrong DK, Alvarez RD, Backes FJ, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Chen LM, Chitiyo VC, Cristea M, DeRosa M, Eisenhauer EL, Gershenson DM, Gray HJ, Grisham R, Hakam A, Jain A, Karam A, Konecny GE, Leath CA III, Leiserowitz G, Liu J, Martin L, Matei D, McHale M, McLean K, Miller DS, Percac-Lima S, Remmenga SW, Schorge J, Stewart D, Thaker PH, Vargas R, Hendrickson AW, Werner TL, Zsiros E, Dwyer MA, Hang L. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J Natl Compr Canc Netw. 2022;20:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 10. | Barnes BM, Nelson L, Tighe A, Burghel GJ, Lin IH, Desai S, McGrail JC, Morgan RD, Taylor SS. Distinct transcriptional programs stratify ovarian cancer cell lines into the five major histological subtypes. Genome Med. 2021;13:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 11. | Hillier LW, Graves TA, Fulton RS, Fulton LA, Pepin KH, Minx P, Wagner-McPherson C, Layman D, Wylie K, Sekhon M, Becker MC, Fewell GA, Delehaunty KD, Miner TL, Nash WE, Kremitzki C, Oddy L, Du H, Sun H, Bradshaw-Cordum H, Ali J, Carter J, Cordes M, Harris A, Isak A, van Brunt A, Nguyen C, Du F, Courtney L, Kalicki J, Ozersky P, Abbott S, Armstrong J, Belter EA, Caruso L, Cedroni M, Cotton M, Davidson T, Desai A, Elliott G, Erb T, Fronick C, Gaige T, Haakenson W, Haglund K, Holmes A, Harkins R, Kim K, Kruchowski SS, Strong CM, Grewal N, Goyea E, Hou S, Levy A, Martinka S, Mead K, McLellan MD, Meyer R, Randall-Maher J, Tomlinson C, Dauphin-Kohlberg S, Kozlowicz-Reilly A, Shah N, Swearengen-Shahid S, Snider J, Strong JT, Thompson J, Yoakum M, Leonard S, Pearman C, Trani L, Radionenko M, Waligorski JE, Wang C, Rock SM, Tin-Wollam AM, Maupin R, Latreille P, Wendl MC, Yang SP, Pohl C, Wallis JW, Spieth J, Bieri TA, Berkowicz N, Nelson JO, Osborne J, Ding L, Sabo A, Shotland Y, Sinha P, Wohldmann PE, Cook LL, Hickenbotham MT, Eldred J, Williams D, Jones TA, She X, Ciccarelli FD, Izaurralde E, Taylor J, Schmutz J, Myers RM, Cox DR, Huang X, McPherson JD, Mardis ER, Clifton SW, Warren WC, Chinwalla AT, Eddy SR, Marra MA, Ovcharenko I, Furey TS, Miller W, Eichler EE, Bork P, Suyama M, Torrents D, Waterston RH, Wilson RK. Generation and annotation of the DNA sequences of human chromosomes 2 and 4. Nature. 2005;434:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Goldfrank D, Schoenberger E, Gilbert F. Disease genes and chromosomes: disease maps of the human genome. Chromosome 4. Genet Test. 2003;7:351-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 13. | Strehle EM, Yu L, Rosenfeld JA, Donkervoort S, Zhou Y, Chen TJ, Martinez JE, Fan YS, Barbouth D, Zhu H, Vaglio A, Smith R, Stevens CA, Curry CJ, Ladda RL, Fan ZJ, Fox JE, Martin JA, Abdel-Hamid HZ, McCracken EA, McGillivray BC, Masser-Frye D, Huang T. Genotype-phenotype analysis of 4q deletion syndrome: proposal of a critical region. Am J Med Genet A. 2012;158A:2139-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camaño P, Dauwerse JG, Snider L, Straasheijm KR, van Ommen GJ, Padberg GW, Miller DG, Tapscott SJ, Tawil R, Frants RR, van der Maarel SM. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. 2010;329:1650-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 579] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 15. | Strehle EM, Bantock HM. The phenotype of patients with 4q-syndrome. Genet Couns. 2003;14:195-205. [PubMed] |
| 16. | Xu W, Ahmad A, Dagenais S, Iyer RK, Innis JW. Chromosome 4q deletion syndrome: narrowing the cardiovascular critical region to 4q32.2-q34.3. Am J Med Genet A. 2012;158A:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Markiewicz MR, Verschueren D, Assael LA. Chromosome 4q deletion syndrome: craniofacial characteristics associated with monosomy of the long arm of chromosome 4q. Cleft Palate Craniofac J. 2010;47:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Caliebe A, Waltz S, Jenderny J. Mild phenotypic manifestations of terminal deletion of the long arm of chromosome 4: clinical description of a new patient. Clin Genet. 1997;52:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Brosens RP, Belt EJ, Haan JC, Buffart TE, Carvalho B, Grabsch H, Quirke P, Cuesta MA, Engel AF, Ylstra B, Meijer GA. Deletion of chromosome 4q predicts outcome in stage II colon cancer patients. Cell Oncol (Dordr). 2011;34:215-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Girard L, Zöchbauer-Müller S, Virmani AK, Gazdar AF, Minna JD. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res. 2000;60:4894-4906. [PubMed] |
| 21. | Keats JJ, Maxwell CA, Taylor BJ, Hendzel MJ, Chesi M, Bergsagel PL, Larratt LM, Mant MJ, Reiman T, Belch AR, Pilarski LM. Overexpression of transcripts originating from the MMSET locus characterizes all t(4;14)(p16;q32)-positive multiple myeloma patients. Blood. 2005;105:4060-4069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Kiechle M, Jacobsen A, Schwarz-Boeger U, Hedderich J, Pfisterer J, Arnold N. Comparative genomic hybridization detects genetic imbalances in primary ovarian carcinomas as correlated with grade of differentiation. Cancer. 2001;91:534-540. [PubMed] |
| 23. | Lambros MB, Fiegler H, Jones A, Gorman P, Roylance RR, Carter NP, Tomlinson IP. Analysis of ovarian cancer cell lines using array-based comparative genomic hybridization. J Pathol. 2005;205:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Roman-Trufero M, Dillon N. The UBE2D ubiquitin conjugating enzymes: Potential regulatory hubs in development, disease and evolution. Front Cell Dev Biol. 2022;10:1058751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 25. | Scheffner M, Nuber U, Huibregtse JM. Protein ubiquitination involving an E1-E2-E3 enzyme ubiquitin thioester cascade. Nature. 1995;373:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 760] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 26. | Deng L, Meng T, Chen L, Wei W, Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther. 2020;5:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 509] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 27. | Sun T, Liu Z, Yang Q. The role of ubiquitination and deubiquitination in cancer metabolism. Mol Cancer. 2020;19:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 28. | Guo T, Dong X, Xie S, Zhang L, Zeng P. Cellular Mechanism of Gene Mutations and Potential Therapeutic Targets in Ovarian Cancer. Cancer Manag Res. 2021;13:3081-3100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Hu Q, Botuyan MV, Zhao D, Cui G, Mer E, Mer G. Mechanisms of BRCA1-BARD1 nucleosome recognition and ubiquitylation. Nature. 2021;596:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 30. | Sarma H, Kiewhuo K, Jamir E, Sastry GN. In silico investigation on the mutational analysis of BRCA1-BARD1 RING domains and its effect on nucleosome recognition and ubiquitination. Biophys Chem. 2023;300:107070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Cole AJ, Dwight T, Gill AJ, Dickson KA, Zhu Y, Clarkson A, Gard GB, Maidens J, Valmadre S, Clifton-Bligh R, Marsh DJ. Assessing mutant p53 in primary high-grade serous ovarian cancer using immunohistochemistry and massively parallel sequencing. Sci Rep. 2016;6:26191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 32. | Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1479] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 33. | Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1319] [Cited by in RCA: 1280] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 34. | Willis A, Jung EJ, Wakefield T, Chen X. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene. 2004;23:2330-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 232] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Suh YA, Post SM, Elizondo-Fraire AC, Maccio DR, Jackson JG, El-Naggar AK, Van Pelt C, Terzian T, Lozano G. Multiple stress signals activate mutant p53 in vivo. Cancer Res. 2011;71:7168-7175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, Van Pelt CS, Lozano G. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev. 2008;22:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 37. | Saville MK, Sparks A, Xirodimas DP, Wardrop J, Stevenson LF, Bourdon JC, Woods YL, Lane DP. Regulation of p53 by the ubiquitin-conjugating enzymes UbcH5B/C in vivo. J Biol Chem. 2004;279:42169-42181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Mittal MK, Singh K, Misra S, Chaudhuri G. SLUG-induced elevation of D1 cyclin in breast cancer cells through the inhibition of its ubiquitination. J Biol Chem. 2011;286:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Liyasova MS, Ma K, Voeller D, Ryan PE, Chen J, Klevit RE, Lipkowitz S. Cbl interacts with multiple E2s in vitro and in cells. PLoS One. 2019;14:e0216967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Wei Y, Erfani S, Schweer D, de Gouvea R, Qadir J, Shi J, Cheng K, Wu D, Craven R, Wu Y, Olivier T, Baldwin LA, Zhou B, Zhou Y, Zhao W, Yang BB, Ueland FR, Yang XH. Targeting receptor tyrosine kinases in ovarian cancer: Genomic dysregulation, clinical evaluation of inhibitors, and potential for combinatorial therapies. Mol Ther Oncolytics. 2023;28:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Gharpure KM, Lara OD, Wen Y, Pradeep S, LaFargue C, Ivan C, Rupaimoole R, Hu W, Mangala LS, Wu SY, Nagaraja AS, Baggerly K, Sood AK. ADH1B promotes mesothelial clearance and ovarian cancer infiltration. Oncotarget. 2018;9:25115-25126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Xu Z, Peng B, Kang F, Zhang W, Xiao M, Li J, Hong Q, Cai Y, Liu W, Yan Y, Peng J. The Roles of Drug Metabolism-Related ADH1B in Immune Regulation and Therapeutic Response of Ovarian Cancer. Front Cell Dev Biol. 2022;10:877254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Liu X, Gao Y, Zhao B, Li X, Lu Y, Zhang J, Li D, Li L, Yin F. Discovery of microarray-identified genes associated with ovarian cancer progression. Int J Oncol. 2015;46:2467-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Pinto JA, Rolfo C, Raez LE, Prado A, Araujo JM, Bravo L, Fajardo W, Morante ZD, Aguilar A, Neciosup SP, Mas LA, Bretel D, Balko JM, Gomez HL. In silico evaluation of DNA Damage Inducible Transcript 4 gene (DDIT4) as prognostic biomarker in several malignancies. Sci Rep. 2017;7:1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 45. | Ding F, Gao F, Zhang S, Lv X, Chen Y, Liu Q. A review of the mechanism of DDIT4 serve as a mitochondrial related protein in tumor regulation. Sci Prog. 2021;104:36850421997273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Chen YF, Kao CH, Chen YT, Wang CH, Wu CY, Tsai CY, Liu FC, Yang CW, Wei YH, Hsu MT, Tsai SF, Tsai TF. CISD2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009;23:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 47. | Wu CY, Chen YF, Wang CH, Kao CH, Zhuang HW, Chen CC, Chen LK, Kirby R, Wei YH, Tsai SF, Tsai TF. A persistent level of CISD2 extends healthy lifespan and delays aging in mice. Hum Mol Genet. 2012;21:3956-3968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Tsai PH, Chien Y, Chuang JH, Chou SJ, Chien CH, Lai YH, Li HY, Ko YL, Chang YL, Wang CY, Liu YY, Lee HC, Yang CH, Tsai TF, Lee YY, Chiou SH. Dysregulation of Mitochondrial Functions and Osteogenic Differentiation in CISD2-Deficient Murine Induced Pluripotent Stem Cells. Stem Cells Dev. 2015;24:2561-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Cheng YH, Chavez E, Tsai KL, Yang KC, Kuo WT, Yang YP, Chiou SH, Lin FH. Effects of thermosensitive chitosan-gelatin based hydrogel containing glutathione on CISD2-deficient chondrocytes under oxidative stress. Carbohydr Polym. 2017;173:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Sun CC, Lee SY, Kao CH, Chen LH, Shen ZQ, Lai CH, Tzeng TY, Pang JS, Chiu WT, Tsai TF. CISD2 plays an essential role in corneal epithelial regeneration. EBioMedicine. 2021;73:103654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |