Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3321
Revised: April 24, 2024
Accepted: May 8, 2024
Published online: June 26, 2024
Processing time: 106 Days and 18.4 Hours
Sudden sensorineural hearing loss (SSNHL), characterized by a rapid and unexplained loss of hearing, particularly at moderate to high frequencies, presents a significant clinical challenge. The therapeutic use of methylprednisolone sodium succinate (MPSS) via different administration routes, in combination with conventional medications, remains a topic of interest.
To compare the therapeutic efficacy of MPSS administered via different routes in combination with conventional drugs for the treatment of mid- to high-frequency SSNHL.
The medical records of 109 patients with mid- to high-frequency SSNHL were analyzed. The patients were divided into three groups based on the route of administration: Group A [intratympanic (IT) injection of MPSS combined with mecobalamin and Ginkgo biloba leaf extract injection], Group B (intravenous injection of MPSS combined with mecobalamin and Ginkgo biloba leaf extract injection), and Group C (single IT injection of MPSS). The intervention effects were compared and analyzed.
The posttreatment auditory thresholds in Group A (21.23 ± 3 .34) were significantly lower than those in Groups B (28.52 ± 3.36) and C (30.23 ± 4.21; P < 0.05). Group A also exhibited a significantly greater speech recognition rate (92.23 ± 5.34) than Groups B and C. The disappearance time of tinnitus, time to hearing recovery, and disappearance time of vertigo in Group A were significantly shorter than those in Groups B and C (P < 0.05). The total effective rate in Group A (97.56%) was significantly greater than that in Groups B and C (77.14% and 78.79%,
IT injection of MPSS combined with conventional treatment demonstrates superior efficacy and safety compared to systemic administration via intravenous infusion and a single IT injection of MPSS. This approach effectively improves patients' hearing and reduces the risk of disease recurrence.
Core Tip: This retrospective analysis compares the therapeutic effectiveness of methylprednisolone sodium succinate (MPSS) administered via different routes in combination with conventional drugs for mid to high-frequency sudden sensorineural hearing loss (SSNHL). Intratympanic (IT) injection of MPSS combined with conventional treatment demonstrates superior efficacy and safety compared to systemic administration via intravenous infusion and solely IT injection of MPSS, leading to significant improvements in patients' hearing thresholds, speech recognition rates, and symptom disappearance times, as well as lower adverse reaction and recurrence rates. This approach provides valuable insights for optimizing treatment strategies for mid to high-frequency SSNHL.
- Citation: Meng XD, Li TT, Deng LM. Therapeutic efficacy of methylprednisolone sodium succinate via diverse administration routes for mid- to high-frequency sudden sensorineural hearing loss. World J Clin Cases 2024; 12(18): 3321-3331
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3321.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3321
High-frequency sudden sensorineural hearing loss (SSNHL) is characterized by a rapid and unexplained loss of hearing within 72 h, with a specific loss in frequencies above 2 kHz and a minimum loss of ≥ 20 dB at frequencies of 2 kHz, 4 kHz, and 8 kHz[1]. Patients typically present with impaired sound perception and sensation, despite no visible damage to the auditory conduction structures of the ear.
The etiology of SSNHL remains a topic of debate, with viral infections, immune disorders, and vascular events being implicated as contributing factors. A study by Azis et al[2] reported that high-frequency SSNHL typically occurs around the age of 41 years, with no significant sex difference in incidence. Bilateral involvement is less common, and triggering factors include mental stress, psychological tension, irregular lifestyle, emotional fluctuations, and sleep disorders[2,3].
Currently, the main treatment options for high-frequency SSNHL involve glucocorticoids and drugs that regulate inner ear circulation. Early and effective treatment is crucial for hearing recovery and prognosis improvement[4]. Methylprednisolone sodium succinate (MPSS), a newly developed glucocorticoid medication, has demonstrated significant efficacy due to its anti-inflammatory, antiallergic, and immunosuppressive effects. MPSS has a shorter half-life than conventional glucocorticoids, making it an attractive option[5,6]. Methylprednisolone has a plasma half-life of approximately 2.5 h, which varies depending on the route of administration and patient-specific factors[5,6]. In contrast, other conventional glucocorticoids, such as dexamethasone and prednisolone, have different pharmacokinetics. Dexamethasone, known for its high potency and long duration of action, has a plasma half-life ranging from 36 to 72 h, which is significantly longer than that of methylprednisolone[5,6]. Prednisolone has a plasma and biological half-life of 12 to 36 h, which is also notably longer than the half-life of methylprednisolone. However, studies by Yao et al[7] revealed that the efficacy of MPSS treatment for high-frequency SSNHL varies depending on the administration method. The traditional systemic use of glucocorticoids has limited effectiveness due to difficulties in crossing the blood-labyrinth barrier. On the other hand, intratympanic (IT) injection of MPSS in the middle ear improves the local drug concentration, leading to advantages such as an early peak drug concentration and a prolonged duration of action[8,9].
High-frequency SSNHL presents a significant clinical challenge due to its sudden onset and potential substantial impact on patients' quality of life[1]. The uncertainty regarding the optimal route of administration of MPSS adds a layer of complexity to the management of this condition[5]. This uncertainty is compounded by conflicting evidence of the efficacy of MPSS for different modes of administration[7,8]. Therefore, understanding the comparative effectiveness of MPSS under different routes of administration is essential not only for optimizing treatment strategies but also for improving patient outcomes[1]. Furthermore, given the varied etiologies and potential contributing factors to mid- to high-frequency SSNHL[2,3], studying the therapeutic efficacy of MPSS administered via different routes, in combination with conventional drugs, is paramount for tailoring individualized treatment regimens to address the specific needs of patients experiencing this audiological challenge.
The sudden and often unexplained nature of SSNHL highlights the need for effective and well-tolerated treatment modalities. Several studies have compared the systemic and IT routes of corticosteroid administration in the management of SSNHL[2,3]. However, what sets this study apart was the comprehensive approach used to compare not only the systemic and IT routes of MPSS administration but also the combined IT administration of neurotrophic factors and vasodilators. In addition to this novel aspect, our study aimed to evaluate the potential synergistic effects of the combination of MPSS with adjunctive therapies, which have shown promise in preclinical and early clinical studies, to provide a more holistic perspective on the treatment outcomes of SSNHL patients[4].
A retrospective analysis of the medical records of 109 patients who were diagnosed with SSNHL and admitted to our hospital between November 2022 and October 2023 was conducted. Specialized otolaryngological examinations, including physical examinations, pure-tone audiometry, and impedance tests, were performed for each patient. Furthermore, magnetic resonance imaging (MRI) of the head and internal auditory canal was carried out to exclude any intracranial abnormalities. The patients were categorized into three groups according to treatment regimen: A, B, and C. Sex, age, disease duration, body mass index and affected ear were not significantly different among the groups (Table 1).
| Group | n | Males/females | Age (yr) | Duration (d) | Body mass index (kg/m²) | Diseased ears (left/right) |
| A | 41 | 23/18 | 46.67 ± 4.28 | 4.17 ± 0.42 | 23.11 ± 1.38 | 25/16 |
| B | 35 | 19/16 | 46.54 ± 4.05 | 4.24 ± 0.46 | 23.14 ± 1.45 | 22/13 |
| C | 33 | 17/16 | 46.75 ± 4.51 | 4.29 ± 0.49 | 23.19 ± 1.33 | 19/14 |
| χ2/t value | - | 0.155 | 0.020 | 0.650 | 0.030 | 0.203 |
| P value | - | 0.925 | 0.979 | 0.523 | 0.970 | 0.903 |
The inclusion criteria for this study were as follows: Aged between 18 and 70 years, a minimum of three consecutive frequencies exhibiting unilateral hearing loss greater than 20 dB without any apparent cause, no prior treatment for SSNHL with other medications before hospitalization, unilateral hearing loss status, normal or nearly normal hearing in the unaffected ear (pure tone average < 30 dB), symptom onset within two weeks, and no history of middle ear inflammation or other relevant diseases in the affected ear prior to the onset of symptoms.
Conversely, the following criteria were used for exclusion: Recent use of ototoxic drugs, pregnancy, severe psychological or psychiatric disorders, inability to complete a four-week follow-up, presence of acoustic neuroma or intracranial tumor-like lesions, family history of sensorineural hearing loss, congenital malformation of the cochlea, and sudden hearing loss caused by trauma.
All patients enrolled in the study underwent comprehensive examinations following admission, which included cranial and internal auditory canal MRI, complete blood count analysis, urinalysis, routine biochemical tests, eleven immunological assays, pure tone audiometry, impedance audiometry, and otoscopy to assess the condition of the tympanic membrane. Notably, none of the patients underwent hyperbaric oxygen therapy.
Based on the different treatment protocols, the patients were divided into three groups: A, B, and C. Patients in Group A received neurotrophic treatment involving the use of mecobalamin (manufactured by Harbin Sanlian Pharmaceutical Co., Ltd., National Drug Approval Number H20044627, specification: 1 mL: 0.5 mg; at a dosage of 1 mg, mixed with 0.9% sodium chloride solution into 100 mL and administered via intravenous drip once a day) and a vasodilator (an injection of Ginkgo biloba leaf extract (manufactured by Chi Sheng Pharma & Biotech Co., Ltd) at a dose of 52.5 mg was administered in 250 mL of 0.9% sodium chloride injection via intravenous drip, twice daily). Patients received adjunctive therapy involving IT injections of MPSS (trade name: Methylprednisolone, Pfizer, concentration: 40 mg/mL). An injection was administered once every other day, totaling five applications in one course. Patients were in the supine position with the affected ear facing upward. After routine disinfection of the external auditory canal, otoscopy was performed to assess the condition of the tympanic membrane. The appropriate size of the otoscope lens was selected based on the patient's external auditory canal size. The observation involved evaluating factors such as color, morphology, congestion at the umbo, retraction, perforation, and plaques on the tympanic membrane. Local anesthesia was achieved by placing a cotton ball soaked with a local anesthetic against the surface of the membrane. After 20 min, the cotton ball was removed. To minimize dizziness during the injection caused by the cold liquid, the small glass bottle containing the MPSS injection could be warmed gently by hand. Slow injection of MPSS was performed in the anterior-inferior quadrant of the tympanic membrane, allowing observation of the injection flow into the middle ear cavity under otoscopy. After reaching the oval window, the injection process was carefully monitored to ensure accurate administration. Using otoscopy, the gradual withdrawal of the needle allowed for visual confirmation of the placement and delivery of the medication into the middle ear through the oval window. Visual inspection through the oval window was crucial to verify the successful administration of the medication and to prevent any undue complications. Each injection dose, ranging between approximately 0.5 and 0.7 mL, was meticulously delivered to achieve the optimal drug concentration and therapeutic efficacy. Fifteen minutes after the injection, the patient's ear was examined for any fluid drainage. If a substantial amount of fluid drained from the middle ear, an additional injection was administered to ensure coverage of the oval window area. For 20 min following the completion of the injection, the patient maintained an upward-facing position of the affected ear, minimizing swallowing and speaking to enable a longer duration of medication retention in the middle ear cavity. During the treatment period, strict measures were implemented to prevent upper respiratory tract infections in the study participants. Patients were instructed to adhere to rigorous hygiene practices, including regular handwashing and the use of face masks in crowded or high-risk environments. Additionally, the study protocol involved monitoring participants for any signs of upper respiratory tract infections, and prompt medical attention was provided at the earliest indication of such symptoms. Special precautions were taken to maintain dryness in the external auditory canal throughout the treatment and postdischarge periods. Patients were provided with detailed instructions on how to keep the external auditory canal dry, emphasizing the importance of avoiding water entry during bathing and swimming activities. Additionally, custom-fit waterproof earplugs were provided to the participants to further safeguard against moisture exposure. The health care team reinforced these instructions during follow-up appointments, and compliance was verified through visual examination of the external auditory canal to ensure the preservation of dry conditions. These measures were instrumental in minimizing the risk of complications associated with moisture exposure during the critical posttreatment phase.
In Group B, which received mecobalamin combined with Ginkgo biloba leaf extract injection, patients received an intravenous infusion of MPSS at a dosage of 0.8 mg/kg/d, with a gradual reduction of 8 mg per day starting on the 6th day and medication discontinuation on the 10th day.
Patients in Group C underwent only IT MPSS injection and did not receive routine treatment.
Considering that noise could exacerbate hearing damage in the affected ear and reduce treatment efficacy, the patients stayed in quiet rooms during their hospitalization. They were advised against increasing the volume of their cell phones due to hearing loss and were instructed to avoid extended periods in public places. Furthermore, the patients were instructed to refrain from active and passive smoking throughout the treatment period, as harmful components in cigarettes can damage blood vessel walls.
A comparison of pure-tone audiometry thresholds among the three groups was performed: The pure-tone audiometry thresholds of the patients in all three groups were measured before and at the time of discharge from the hospital using the Audiometer Astera (Model: Audiometer Astera, Medical Device Registration Number: 20172076107). Prior to the test, earwax was cleared bilaterally, and the device was turned on and calibrated. The patients were instructed to remove glasses, earrings, and other accessories. The testing method was explained to the patients, who were instructed to press a button or raise their hand upon hearing a sound, even if it was very faint. No response was expected if the sound was not audible. The test was performed on one ear at a time, and the minimum sound level that the patient could hear at each frequency was recorded. If the interaural air conduction difference for a certain frequency was ≥40 dB or the air-bone gap at a certain frequency in one ear was > 10 dB, masking was applied. A lower pure tone audiometry threshold indicated better hearing.
A comparison of speech recognition rates among the three groups was performed: Speech recognition rates were evaluated using the Danish Madsen Conera pure-tone audiometer. The speech test material consisted of monosyllabic words from Zheng's monosyllabic word list, which was integrated into the audiometer. The word list was divided into four groups, each containing 25 Chinese characters. The audio files for the word list were delivered through TDH-50P headphones. Monaural listening tests were performed for all patients, and the speech intensity output was consistent for each test, based on the average threshold above 10 dB for frequencies of 0.5, 1, 2, 4, and 8 kHz in the treated ear. After each word was read, the patient was asked to repeat it. Once all 25 Chinese characters were repeated, the audiometer automatically calculated the percentage of correctly repeated characters out of the total characteristics, displaying the speech recognition rate.
A comparison of the time to improvement in relevant symptoms among the three groups was performed: This comparison included the time to tinnitus disappearance, hearing recovery, and dizziness disappearance. The tinnitus disappearance time referred to the time from the start of treatment until tinnitus disappeared. The hearing recovery time was the duration until hearing fully recovered or reached the level before the onset of the illness. The dizziness disappearance time referred to the time from the start of treatment until dizziness symptoms disappeared.
A comparison of the clinical efficacy among the three groups was performed: Clinical efficacy was evaluated according to the criteria outlined in the Diagnosis and Treatment Guidelines for Sudden Deafness (2015)[10]. The evaluation criteria were as follows: Significant improvement: Restoration of the hearing threshold in the affected frequencies to normal or an improvement of ≥ 30 dBHL compared to that before treatment; Effective: An improvement of 15-30 dBHL compared to that before treatment in the hearing threshold of the affected frequencies; and Ineffective: An improvement of < 15 dBHL compared to that before treatment in the hearing threshold of the affected frequencies. The overall effective rate was calculated as follows: (Number of significantly improved cases + number of effective cases)/total number of cases × 100%.
A comparison of the incidence of adverse reactions among the three groups was performed: This comparison included the incidence of various adverse reactions, such as dizziness, nausea/vomiting, rash, abdominal pain/diarrhea, hypertension, tinnitus, fever, and others.
A comparison of recurrence rates among the three groups was performed: The occurrence of recurrence within 6 months of discharge from the hospital was recorded and compared among the three groups.
The collected research data were analyzed utilizing SPSS 25.0 statistical software. Specifically, t tests and chi-square tests were employed for analysis. Descriptive statistics were utilized for continuous variables, which are expressed in the form of means ± SD. A t test was used to compare continuous variables between groups. Count data are represented as percentages and were subjected to group comparisons using the chi-square test. In all tests, a significance level of α = 0.05 was used, and a P value lower than 0.05 was considered to indicate statistical significance.
As shown in Table 2, no significant differences in the pretreatment pure-tone hearing thresholds existed amongst the three groups (52.89 ± 6.27 vs 52.75 ± 6.10 vs 53.29 ± 6.03, F = 0.070, P = 0.932). However, the post-treatment hearing thresholds in Group A (21.23 ± 3.34 vs 28.52 ± 3.36 vs 31.52 ± 4.58, F = 74.460, P < 0.001) were significantly lower than those in Groups B and C, with Group B also showing lower thresholds than Group C (28.52 ± 3.36 vs 31.52 ± 4.58, t = 3.092, P = 0.003). These results indicate that the injection of MPSS into the tympanic cavity, in combination with conventional medication, can directly penetrate the round window membrane and achieve a higher drug concentration in the inner ear. Additionally, local drug application behind the ear can establish an osmotic gradient between the inner ear fluid and the outer lymphatic fluid, facilitating the rapid delivery of the drug to the scala tympani and the outer lymphoid tissue. This condition, in turn, alleviates labyrinthine oedema, regulates cochlear circulation, corrects the ischemic–hypoxic state of the inner ear, effectively improves the hearing thresholds of patients and ensures treatment efficacy and prognosis.
Table 3 shows no significant differences in the pretreatment speech recognition rates amongst the three groups (37.89 ± 4.27 vs 36.75 ± 4.10 vs 37.29 ± 4.03, F = 0.720, P = 0.490). However, the post-treatment speech recognition rates in Group A (92.23 ± 5.34 vs 81.52 ± 8.36 vs 75.96 ± 8.74, F = 45.550, P < 0.001) were significantly higher than those in Groups B and C, with Group B also showing higher rates than Group C (81.52 ± 8.36 vs 75.96 ± 8.74, t = 2.141, P = 0.036). These results indicate that the injection of MPSS into the tympanic cavity, in combination with conventional medication, can effectively improve patients’ speech recognition ability.
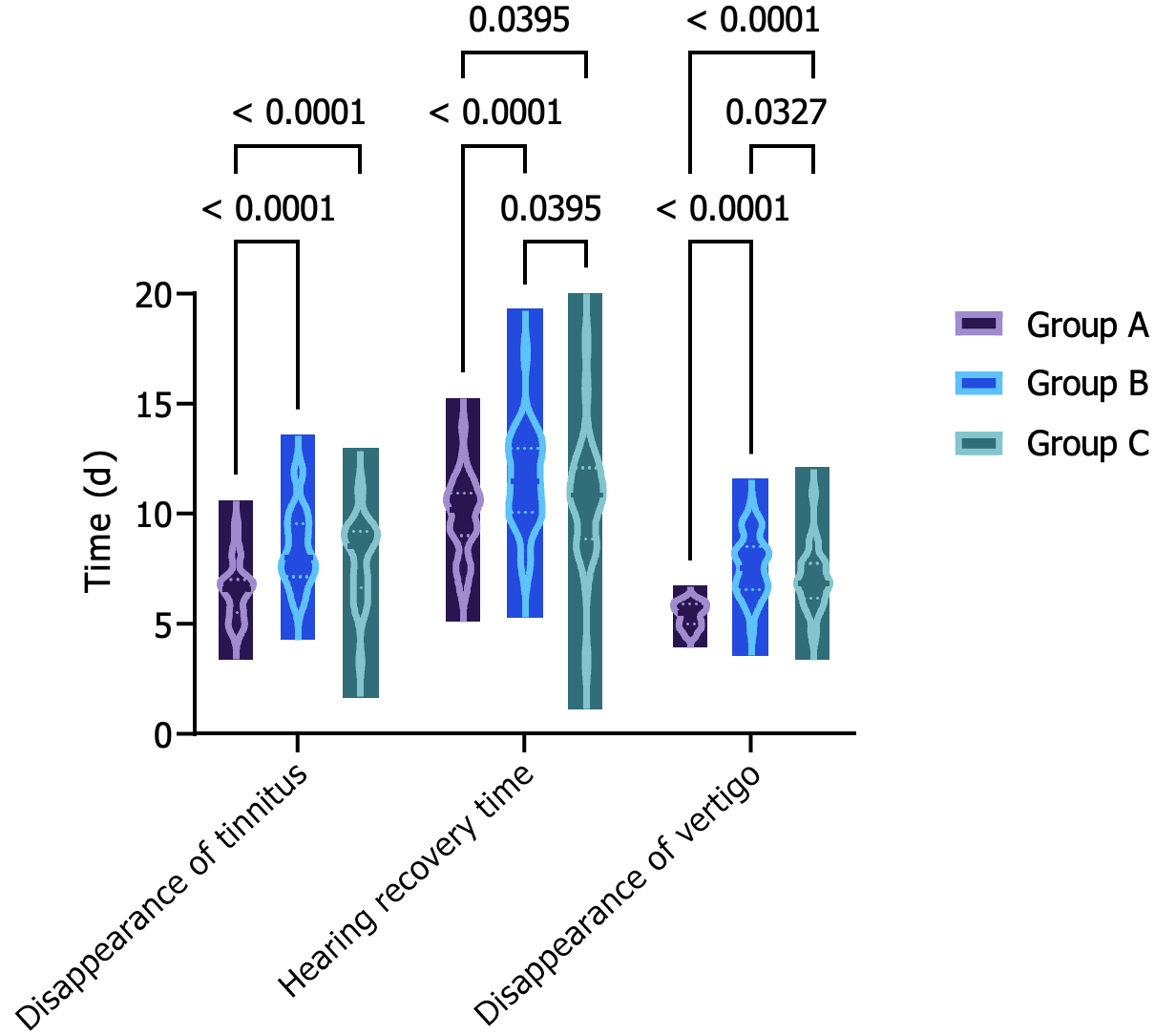
Figure 1 illustrates that the time for tinnitus disappearance (6.53 ± 1.22 vs 8.42 ± 1.65 vs 9.33 ± 1.82 d, F = 31.440, P < 0.001), the time for hearing recovery (9.87 ± 1.67 vs 11.51 ± 2.09 vs 12.69 ± 2.71 d, F = 15.940, P < 0.001) and the time for dizziness disappearance (5.43 ± 0.52 vs 7.65 ± 1.34 vs 8.98 ± 1.45 d, F = 91.440, P < 0.001) in Group A were all shorter than those in Groups B and C, with Group B showing shorter durations than Group C (8.42 ± 1.65 vs 9.33 ± 1.82 d, 11.51 ± 2.09 vs 12.69 ± 2.71 d, 7.65 ± 1.34 vs 8.98 ± 1.45 d, t = 2.162, P = 0.034, t = 2.017, P = 0.048, t = 3.931, P < 0.001). These findings suggest that the IT injection of MPSS in combination with conventional medication treatment can effectively improve the clinical symptoms of patients.
As depicted in Table 4, Group A had 28 significantly effective cases, 12 effective cases and 1 ineffective case; Group B had 11 significantly effective cases, 16 effective cases and 8 ineffective cases; Group C had 8 significantly effective cases, 10 effective cases and 15 ineffective cases. The total effective rate of treatment in Group A (97.56% vs 77.14% vs 54.55%, χ2 = 19.724, P < 0.001) was significantly higher than those in Groups B and C, with Group B being higher than Group C (77.14% vs 54.55%, χ2 = 3.875, P = 0.049). These findings indicate that the IT injection of MPSS in combination with conventional medication treatment provides better therapeutic effects.
Table 5 compares the adverse reactions in Groups A, B and C. The occurrence of adverse reactions in Groups A and C (4.88% and 3.03%, respectively, vs 2.57%, χ2 = 11.443, P = 0.003) was significantly lower than that in Group B. No significant difference was observed between Groups A and C. These findings indicate that the combination of IT MPSS and conventional medication and the sole IT administration of MPSS lead to fewer adverse reactions, reflecting a higher level of safety.
As illustrated in Table 6, the recurrence rate in Group A (2.44% vs 20.00% vs 42.42%, χ2 = 18.147, P < 0.001) was significantly lower than those in Groups B and C, with Group B being lower than Group C (χ2 = 4.001, P = 0.045). This result indicates that the IT injection of MPSS in combination with conventional medication treatment leads to a lower recurrence rate.
For patients with SSNHL with a decline in middle to high frequencies, the pathogenesis is typically associated with damage to outer or inner hair cells. The distribution of ion channels on the basilar membrane of the cochlea differs in these patients, with more basal turn ion channels than apical turn ones, and may even be accompanied with localised vascular supply disturbances in the spiral ligament and hypoxia-induced damage to the spiral organ[11]. From this perspective, clinical recommendations suggest the use of glucocorticoids for treatment because they possess multiple effects, such as anti-inflammatory, immunosuppressive and antiallergic actions. These properties can effectively reduce the harm caused by the immune response whilst reducing the degree of cellular oedema, preventing vascular spasm and alleviating the extent of capillary damage due to inflammatory injury[12]. However, a study by Li et al[13] and Plontke et al[14] revealed that different glucocorticoid administration methods yield varying effects and safety profiles in the treatment of SSNHL with a decline in middle to high frequencies. Therefore, a highly effective and safe method of administering MPSS should be explored to ensure the safety of the prognosis for patients with SSNHL with a decline in middle to high frequencies.
Zhong et al[15] found that the treatment recovery effects for patients with SSNHL were enhanced when MPSS was administered via retroauricular injection, significantly improving the pure-tone audiometry thresholds for these patients, indicating a positive impact of MPSS on improving pure-tone audiometry thresholds. The ‘threshold’ in pure-tone audiometry refers to the minimum sound stimulation that elicits a response in the human ear, and the audiogram is constructed by connecting the thresholds for each frequency. Typically, patients with SSNHL experience a rapid decline in middle- to high-frequency hearing, accompanied with symptoms such as tinnitus, aural fullness, dizziness or vertigo. MPSS has various administration methods, and each method may directly affect the distribution and pharmacological effects of the drug on the body, thereby influencing changes in pure-tone audiometry thresholds. The results of this study showed that the post-treatment pure-tone audiometry thresholds in Group A were significantly lower than those in Groups B and C, and Group B was lower than Group C, confirming that administering MPSS via tympanic injection on top of conventional drug therapy significantly improved the pure-tone audiometry thresholds for patients. As a novel glucocorticoid drug, MPSS can effectively alleviate local cochlear neuroinflammation and oedema, correct inner ear microvascular spasms, promote microvascular circulation and thus improve patients’ hearing thresholds[16,17]. When the drug is administered via the tympanic injection method, MPSS can directly act on the middle ear, reducing local inflammatory exudation, resolving swelling, improving the inflammatory condition in the middle ear and effectively alleviating otitis media in patients with SSNHL, promoting the recovery of hearing. Moreover, MPSS can inhibit platelets, prevent thrombosis, dilate blood vessels and improve microcirculation and organ blood supply and oxygenation, thereby improving the blood and oxygen supply to the cochlea, facilitating the repair of damaged cells, reducing the necrosis of cochlear hair cells and ultimately achieving the goal of restoring middle- to high-frequency hearing and improving pure-tone audiometry thresholds.
The reception and comprehension of language represent the most crucial aspect of the human auditory function. Speech recognition rate refers to the percentage of subjects who can understand and correctly respond to the measured vocabulary or sentences, which not only reflects the functional state of the central auditory system but also evaluates the functional status of the peripheral auditory pathway. Based on the observations, the speech recognition rate in Group A was significantly higher than that in Groups B and C after treatment, and that in Group B was higher than that in Group C, suggesting a positive effect of tympanic injection of MPSS combined with conventional drug therapy on improving speech recognition rates. Li et al[18] selected 50 inpatients with sudden hearing loss as observation subjects, administered IT injection of methylprednisolone and found that the patients’ speech recognition rate significantly improved after treatment, indicating a significant enhancement in their speech discrimination ability. Patients with sudden deafness often experience hearing loss, especially a decline in middle to high frequencies, making high-frequency components in speech difficult to recognise. High-frequency components play a crucial role in the clarity, intonation and consonant recognition of speech. Consequently, when middle- to high-frequency components are impaired, the speech recognition rate of patients will significantly decrease. Tympanic injection involves directly injecting the drug into the middle ear cavity, allowing the drug to act directly on the inner ear structures. By inhibiting the inflammatory response, reducing inner ear oedema and alleviating inner ear pressure, it aims to improve the microenvironment of the inner ear, aiding in the recovery of damaged hair cells or nerve fibres. Furthermore, this method of drug administration ensures high local drug concentrations, reducing systemic side effects, effectively improving middle- to high-frequency hearing loss and enhancing the speech recognition rate.
Patients with high-frequency SSNHL, steeply sloping type, often present with various symptoms such as tinnitus, decreased hearing and dizziness. Failure to promptly intervene with effective measures can lead to the exacerbation of symptoms, potentially resulting in communication barriers, hindering the development of the language system and intellectual growth and further affecting the patient’s quality of life and psychological well-being. Through comparison, this study found that the disappearance time of tinnitus, the recovery time of hearing and the disappearance time of dizziness in Group A were shorter than those in Groups B and C, with those in Group B being shorter than those in Group C. This finding suggests that the combination of IT injection of MPSS and conventional medication therapy in Group A could alleviate patient symptoms more rapidly and shorten the improvement time of relevant symptoms compared with the approaches in Groups B and C[19]. The advantage of IT injection of MPSS under the otoscope is its excellent brightness and the ability to use otoscopes of different angles and lengths to accommodate patients with different external auditory canal conditions, making it particularly suitable for patients with special external auditory canal conditions such as a small external auditory canal or congenital stenosis of the external auditory canal. Furthermore, the distance between the otoscope lens and the eardrum can be adjusted at will, allowing for close observation of the eardrum with clear imaging, and the process of operation can be recorded for comparison during follow-up examinations. Huang et al[20] and Liang et al[21] used electron microscopy for IT injection of glucocorticoids to treat sudden deafness, with the advantage of operability with both hands. On the contrary, for the IT administration in this study, the procedure can be completed with a single-handed puncture. Although electron microscopy can provide stereoscopic imaging, it requires the patient to continuously change head positions to find the appropriate puncture position. Additionally, for patients with a narrow external auditory canal or excessive ear canal hair, the eardrum may appear blurry and difficult to observe, which can be avoided entirely by performing the puncture under direct visualisation with an otoscope. Following the injection, an otoscope will be used to observe the amount of drug that enters the tympanic cavity from the puncture site. If a significant amount of the drug flows out of the puncture site or if the patient exhibits excessive swallowing reflexes at the end of the injection, leading to prolonged opening of the Eustachian tube and subsequent loss of the drug from the middle ear, the drug can be reinjected from the same puncture site under otoscopic visualisation to ensure drug concentration. This approach not only enhances the effect but also expedites symptom relief for the patient, providing assurance for subsequent recovery[19].
Xie et al[22] and Yamamoto et al[23] indicated that IT steroid injection does not damage the inner ear, with insignificant histological or cochlear functional changes. IT injection was shown to be a safe treatment method in the study, with no major complications. This conclusion aligns closely with the results of this study, in which the total effective rate of treatment in Group A was significantly higher than those in Groups B and C, with that in Group B being higher than that in Group C. The incidence of adverse reactions in Groups A and C was significantly lower than that in Group B, and no significant difference existed between those in Groups A and C. The recurrence rate in Group A was significantly lower than those in Groups B and C, with that in Group B being lower than that in Group C. This result indicates that the combination of IT MPSS with conventional medication therapy is indeed effective, significantly improving patients’ clinical symptoms and leading to lower recurrence rates. The reason for this effectiveness lies in the fact that the eardrum injection-to-tympanic administration has the characteristic of drug absorption through the round window, bypassing the blood–labyrinth barrier and leading to elevated and sustained inner ear drug concentrations[24]. The traditional systemic use of MPSS as a treatment for high-frequency, steeply sloping SSNHL has its limitations. Although it is simple to administer, the drug’s interference with the blood–labyrinth barrier makes it challenging to achieve the ideal concentration and duration of action in the inner ear. Increasing the drug dosage or prolonging the medication period may increase the incidence of glucocorticoid-related adverse reactions, thus indicating the treatment’s lack of effectiveness and safety[25,26]. Local administration is a new method proposed to compensate for the deficiencies of systemic administration. IT administration allows the liquid to be absorbed directly through the round window membrane, resulting in increased drug concentrations in the perilymph, with small drug doses to avoid systemic steroid absorption, thus reducing the incidence of adverse reactions[27]. Detailed communication with the patient before IT injection, advising them to keep the ear dry, reduce upper respiratory infections and undergo anti-infection treatment, would result in the healing of all tympanic membrane perforations during follow-up. Effectively explaining the precautions of IT injection to the patient and selecting appropriate puncture points and needles could effectively prevent adverse reactions and significantly reduce recurrence rates.
Compared with intravenous systemic administration and the sole use of IT injection of MPSS, the combined treatment with conventional medication via IT injection demonstrates more ideal effectiveness and safety, remarkably improving patients’ hearing and suppressing disease recurrence.
| 1. | Ghanie A, Paramita L, Widyasari F, Hifni A, Bahar E. Factors Affecting Intratympanic Corticosteroids Injection Therapy Results in Sudden Deafness Patients at Dr Mohammad Hoesin Hospital Palembang. Bioscmed. 2021;5:988-996. [DOI] [Full Text] |
| 2. | Azis FA, Kamilah AS, Restyani AL, Miraghassani A, Widyastuti H. Sudden Sensorineural Hearing Loss (SSNHL): Case Report. MCU. 2022;1:17-20. [DOI] [Full Text] |
| 3. | Essawy WM. Effectiveness of oral corticosteroids (prednisolone) in sensorineural hearing loss post COVID-19. Egypt J Otolaryngol. 2022;38:162. [RCA] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 4. | Liang B, Feng X, Deng R, Liu Y, He Y, Zhang X. Efficacy and safety of post-auricular injection of methylprednisolone sodium succinate and lidocaine in the treatment of acute tinnitus, and its effect on sleep quality. Trop J Pharm Res. 2022;21:1079-1085. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Sanz-sánchez CI, Pérez-arcos JA, Verge-gonzález JC, Cazorla-ramos ÓE. Hipoacusia súbita y COVID-19. Revisión sistemática. ORL. 2022;. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Lyu Y, Zeng F, Yan M, Zhou Z, Zhang W, Liu M, Ke C. Comparison of 2 Different Intratympanic Methylprednisolone Injection Schedules in Combination With Intravenous Dexamethasone for Unilateral Sudden Sensorineural Hearing Loss. Ear Nose Throat J. 2021;100:309S-316S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Yao Q, Jiang Z, Zhang J, Huang S, He S, Feng Y, Wang H, Yin S. Steroid Administration Approach for Idiopathic Sudden Sensorineural Hearing Loss: A National Survey in China. Ann Otol Rhinol Laryngol. 2021;130:752-759. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Prince ADP, Stucken EZ. Sudden Sensorineural Hearing Loss: A Diagnostic and Therapeutic Emergency. J Am Board Fam Med. 2021;34:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 9. | Kousha A, Reed M, Else S. Isolated deafness as a presenting symptom in granulomatosis with polyangiitis. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Shao M, Xiong G, Xiang G, Xu S, Zheng Y, Zhang L. Sudden deafness as an initial presentation of varicella: case report and literature review. Ann Palliat Med. 2021;10:5891-5896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Sun XM, Zhuang SM, Xiao ZW, Luo JQ, Long Z, Lan LC, Zhang HQ, Zhang GP. Autoimmune thyroiditis in patients with sudden sensorineural hearing loss. Laryngoscope Investig Otolaryngol. 2022;7:571-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Cao X, Yi HJ. Audiological characteristics and exploratory treatment of a rare condition of acute-otitis-media-associated sudden sensorineural hearing loss. World J Clin Cases. 2021;9:11311-11319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Li BL, Xu JY, Lin S. Sudden deafness as a prodrome of cerebellar artery infarction: Three case reports. World J Clin Cases. 2022;10:4895-4903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Plontke SK, Meisner C, Agrawal S, Cayé-Thomasen P, Galbraith K, Mikulec AA, Parnes L, Premakumar Y, Reiber J, Schilder AG, Liebau A. Intratympanic corticosteroids for sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2022;7:CD008080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Zhong Z, Wang X, Xu K, Tao J. Clinical Efficacy of Retroauricular Injection of Methylprednisolone Sodium Succinate in the Treatment of Sudden Deafness with Type 2 Diabetes. Comput Math Methods Med. 2022;2022:3097436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 16. | Devantier L, Callesen HE, Jensen LR, Mirian C, Ovesen T. Intratympanic corticosteroid as salvage therapy in treatment of idiopathic sudden sensorineural hearing loss: A systematic review and meta-analysis. Heliyon. 2022;8:e08955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Sialakis C, Iliadis C, Frantzana A, Ouzounakis P, Kourkouta L. Intratympanic Versus Systemic Steroid Therapy for Idiopathic Sudden Hearing Loss: A Systematic Review and Meta-Analysis. Cureus. 2022;14:e22887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Li X, Zhang XY, Wang QJ, Wang DY. Efficacy of methylprednisolone sodium succinate for injection (postotic injection) on the auditory threshold and speech recognition rate of sudden deafness patients. Int J Clin Exp Med. 2015;8:14110-14114. [PubMed] |
| 19. | Yuan Z, Xiang L, Liu R, Yue W. Case report: Bilateral sudden deafness in acute middle cerebellar peduncle infarction: central or peripheral? Front Med (Lausanne). 2023;10:1174512. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Huang KH, Lin HC, Lin CD, Wu PC. Relapsing autoimmune inner ear disease with significant response to methotrexate and azathioprine combination therapy: A case report and mini literature review. Medicine (Baltimore). 2023;102:e33889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Liang Z, Gao M, Jia H, Han W, Zheng Y, Zhao Y, Yang H. Analysis of Clinical Efficacy and Influencing Factors of Nerve Growth Factor (NGF) Treatment for Sudden Sensorineural Hearing Loss. Ear Nose Throat J. 2023;1455613231181711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Xie W, Karpeta N, Liu J, Peng H, Li C, Zhang Z, Liu Y, Duan M. Efficacy of intratympanic or postauricular subperiosteal corticosteroid injection combined with systemic corticosteroid in the treatment of sudden sensorineural hearing loss: A prospective randomized study. Front Neurol. 2023;14:1138354. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 23. | Yamamoto K, Kurioka T, Ohki M, Sano H, Yamashita T. Is repetitive systemic corticosteroid therapy effective for idiopathic sudden sensorineural hearing loss? a retrospective study. Front Neurol. 2023;14:1167128. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Wang H, Zhao Z, Chen S. Local vs Systemic Use of Steroids for Sudden Deafness with Diabetes: A Systematic Review and Meta-Analysis. Ear Nose Throat J. 2023;1455613231170090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Zhu Z, Wu Q, Hu G, Wang X, Chang W, Bin J, Yang W. Efficacy of Ginkgo biloba Extract Combined with Hormones in the Treatment of Sudden Deafness and Its Effect on the Reactivity of Peripheral Blood T Cell Subsets. Comput Math Methods Med. 2022;2022:2903808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Tripathi P, Deshmukh P. Sudden Sensorineural Hearing Loss: A Review. Cureus. 2022;14:e29458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 27. | Rauch SD. Intratympanic steroids for sensorineural hearing loss. Otolaryngol Clin North Am. 2004;37:1061-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |