Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.3200
Revised: March 15, 2024
Accepted: April 23, 2024
Published online: June 16, 2024
Processing time: 118 Days and 16.2 Hours
Glomerulopathy with fibrillary deposits is not uncommon in routine nephropathology practice, with amyloidosis and fibrillary glomerulonephritis being the two most frequently encountered entities. Renal amyloid heavy and light chain (AHL) is relatively uncommon and its biopsy diagnosis is usually limited to cases that show strong equivalent staining for a single immunoglobulin (Ig) heavy chain and a single light chain, further supported by mass spectrometry (MS) and serum studies for monoclonal protein. But polyclonal light chain staining can pose a challenge.
Herein we present a challenging case of renal AHL with polyclonal and polytypic Ig gamma (IgG) staining pattern by immunofluorescence. The patient is a 62-year-old Caucasian male who presented to an outside institution with a serum creatinine of up to 8.1 mg/dL and nephrotic range proteinuria. Despite the finding of a polyclonal and polytypic staining pattern on immunofluorescence, ultrastructural study of the renal biopsy demonstrated the presence of fibrils with a mean diameter of 10 nm. Congo red was positive while DNAJB9 was negative. MS suggested a diagnosis of amyloid AHL type with IgG and lambda, but kappa light chains were also present supporting the immunofluorescence staining results. Serum immunofixation studies demonstrated IgG lambda monoclonal spike. The patient was started on chemotherapy. The chronic renal injury however was quite advanced and he ended up needing dialysis shortly after.
Tissue diagnosis of AHL amyloid can be tricky. Thorough confirmation using other available diagnostic techniques is recommended in such cases.
Core Tip: Amyloidosis and fibrillary glomerulonephritis are the two most commonly encountered glomerulopathies with fibrillary deposits. Accurate diagnosis and differentiation between these two entities are important for patient management. Furthermore, accurate subtyping is also required for amyloidosis cases to further guide treatment. This case report highlights an uncommon diagnostic pitfall that a nephropathologist may encounter while distinguishing light and heavy chain amyloid from fibrillary glomerulopathy. The possible underlying mechanisms are discussed, and we reiterate the importance of a clinical-pathological correlation and the use of multiple available diagnostic modalities if needed, particularly for these overlapping diagnostic entities.
- Citation: Chow MBCY, Bushrow L, Siddiqui I, Chiu A, Hamirani M, Satoskar AA. Congophilic fibrils in the glomeruli with polyclonal immunoglobulin gamma staining - another cause for diagnostic overlap: A case report. World J Clin Cases 2024; 12(17): 3200-3205
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/3200.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.3200
Deposition of electron dense randomly arranged fibrils (without periodicity) in the kidney is seen in two major disease processes-amyloidosis and non-amyloidotic fibrillary glomerulonephritis (FGN). Both consist of abnormally folded proteins.
Proteins forming amyloid are heterogeneous. Up to 36 different protein types and many more variants have been identified in human amyloidosis. A diagnosis of renal amyloidosis mainly relies on the demonstration of congophilic amorphous eosinophilic deposition within the glomeruli (and/or in other compartments in the kidney) with apple green birefringence under polarized light. This is further substantiated by the presence of fibrils, with an average 8 to 10 nm in diameter, on ultrastructural examination. Subtyping of amyloid is critical to inform disease management. The methods widely used are: (1) Immunostaining for the major constituent protein (amyloid-forming protein); and (2) Laser capture with mass spectrometry (MS). Immunostaining is available for a limited number of proteins only, including immunoglobulin (Ig) light chains, Ig heavy chains, serum amyloid A, fibrinogen and transthyretin A (mainly cardiac amyloid, uncommon in the kidney). For the rest, MS is required.
FGN is more uniform in its composition, staining for Ig gamma (IgG) heavy chain along with polyclonal light chains but with slight kappa predominance and typically Congo red negative. The fibril diameter is in the range of 10 to 20 nm, thus differs from that of amyloid fibrils. The Congo red negativity is the most important distinguishing feature from amyloidosis. Recently, MS has demonstrated the heat shock protein DNAJB9 to be consistently present in the deposits of FGN. Immunostaining for DNAJB9 has therefore become a highly sensitive and specific marker for FGN and is particularly useful in the absence of EM[1]. Although rare, cases of “congophilic FGN” have been reported necessitating the use of DNAJB9 immunostaining and EM for correct diagnosis in such cases. Another reported pitfall in the differentiation between FGN and amyloidosis is presented by amyloid heavy and light chain (AHL) due to the presence of concomitant Ig heavy and light chain as seen in FGN (albeit monoclonal).
Light chain amyloidosis is the most common form of renal amyloidosis in North America, associated with underlying monoclonal gammopathy. AHL which is also associated with monoclonal gammopathy occurs much rarely. For majority of the reported cases of AHL, the amyloid fibrils are derived from fragments of one IgG heavy chain, and one light chain, giving rise to a monoclonal and monotypic Ig staining pattern. Other heavy chains (such as IgA) are reported.
Here we present an intriguing case of AHL-type with both kappa and lambda light chain staining as well as multiple IgG subclass staining on direct immunofluorescence (DIF).
A 62-year-old Caucasian male presented to an outside institution with persistently rising serum creatinine and heavy proteinuria.
Acute on chronic kidney disease with nephrotic range proteinuria.
He had medical history of hypertension for the past 5 years and chronic renal insufficiency presumed to be due to hypertensive nephrosclerosis.
Blood pressure was up to 160/90 mmHg but there were no neurologic deficits or other specific findings such as rash or edema.
Baseline serum creatinine was stable at 1.7 mg/dL for 6 years until recent lab work revealed an elevation to 5.1 mg/dL and further increasing to 8.1 mg/dL within a span of a few months along with nephrotic-range proteinuria. Urinalysis showed greater than 300 mg/dL albumin, numerous red blood cells, and 5-10 white blood cells/high power field. Hepatitis B and hepatitis C serologies were negative. Other relevant laboratory data is shown in Tables 1 and 2.
| Test | Result | Reference ranges |
| Hemoglobin, g/dL | 8.4 | 13.8-17.2 |
| White blood cell count, /μL | 10000 | 4500-11000 |
| Platelet count, K/μL | 227000 | 150000-450000 |
| Serum creatinine, mg/dL | 8.1 | 0.72-1.25 |
| eGFR, mL/min/1.73 m2 | 7 | > 90 |
| Calcium (total), mg/dL | 8.0 | 8.4-10.2 |
| Phosphorus, mg/dL | 7.4 | 3.4-4.5 |
| Serum C3, mg/dL | 95 | 90-180 |
| Serum C4, mg/dL | 34 | 15-45 |
| Serum assays performed at outside hospital | Reference range and units (in our institution) | |
| Immunofixation, serum | Single spike-IgG lambda | Negative |
| Monoclonal protein 1 (mg/dL) | 900 | ≤ 0.0 |
| Monoclonal protein 2 (mg/dL) | Absent | ≤ 0.0 |
| Serum kappa free light chains (mg/L) | 37.7 | 3.9-26.0 |
| Serum lambda free light chains (mg/L) | 366.3 | 6.4-22.1 |
| Serum kappa:lambda ratio | 0.10 | 0.51-1.72 |
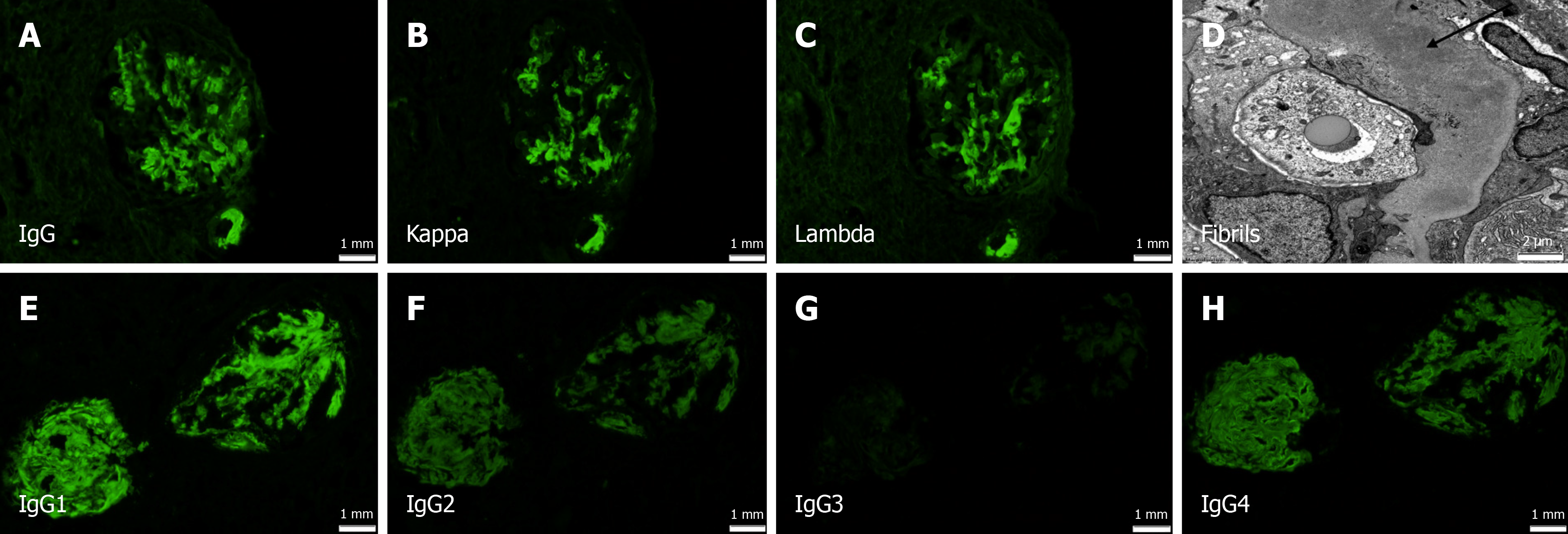
A renal biopsy was performed to elucidate the cause of his rapidly worsening renal function, proteinuria and hematuria. The biopsy showed a total of 30 glomeruli and 17 of them were sclerotic/solidified. Three glomeruli showed features of fibrocellular crescents. The remaining glomeruli showed focal mesangial expansion, non-argyrophilic on Jones methenamine silver stain. Interstitial fibrosis and tubular atrophy were moderately advanced involving 50%-60% of the renal cortex. DIF showed strong smudgy IgG, kappa and lambda staining in the glomeruli and vascular walls (Figure 1A-C). EM showed randomly arranged fibrils with a mean diameter of 10 nm (Figure 1D). Congo red stain was positive. IgG subclass staining showed strong IgG1, but also moderate IgG2 and IgG4 staining (polytypic pattern). IgG3 was negative (Figure 1E-H).
Immunostain for DNAJB9, performed at the Mayo Clinic, was negative. Serum protein electrophoresis (SPE) and serum immunofixation (SIfix), performed at the referring hospital, revealed a monoclonal IgG lambda spike. Flow cytometry on bone marrow showed 0.2% lambda-restricted plasma cells. MS was subsequently performed and the result was stated to be “possible AHL amyloid with IgG and lambda”.
AHL-type with IgG lambda
The patient was started on chemotherapy after the kidney biopsy diagnosis consisting of Cyclophosphamide, Bortezomib and dexamethasone, at the outside hospital and then referred to our institution for second opinion. The diagnosis of AHL amyloidosis was confirmed based on the existing biopsy and laboratory results. Subsequently a decision was made to add Daratumumab to the regimen.
The patient required dialysis shortly after the renal biopsy. He remains on dialysis upon 2-month follow-up.
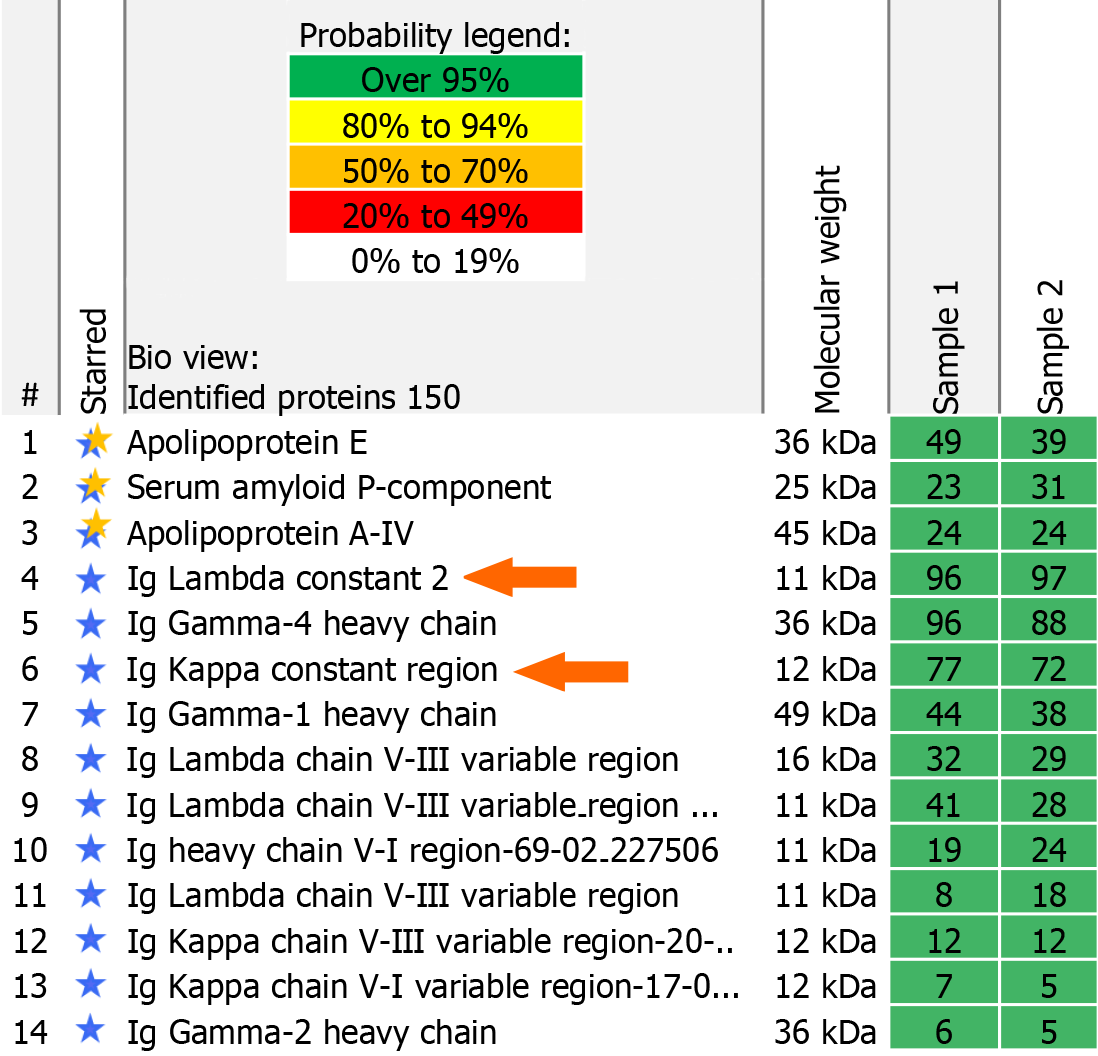
Renal AHL is rare comprising 7.3% of renal Ig-related amyloidosis cases[2]. To date, fewer than twenty cases of AHL have been reported. They demonstrate monoclonal staining pattern with one Ig heavy chain and one light chain on immunofluorescence. Although a few cases of IgA- and one case of IgM-containing AHL amyloid are described, the majority is made up of IgG heavy chain[2-4]. Conventionally, the diagnosis of renal AHL should be limited to cases that show strong equivalent staining for a single Ig heavy chain and a single light chain[2]. This stringent diagnostic criterion is essential to avoid over-diagnosing this rare entity. However, our exceptional case nicely demonstrates that even with a polyclonal IgG pattern on DIF, it could still be a monoclonal gammopathy-associated AHL amyloid. A more detailed review of the MS protein identification report (“scaffold”) was performed. Along with the common amyloid components (such as serum amyloid P component, apolipoprotein A4 and apolipoprotein E) and specific AHL amyloid components (high peptide counts for IgG heavy chain, lambda light chain), it also showed peptide counts for kappa light chains (Figure 2). This supported our tissue staining results on the kidney biopsy (which remained consistent on repetition). We want to highlight this diagnostic pitfall and review potential causes.
Fallacies in tissue staining can occur[5,6]. Eculizumab (composed of human and murine IgG and kappa chains) binds to tissue at the site of action and therefore can be seen on DIF stained tissue as positive staining for IgG and kappa[5]. However, this patient had not been receiving any targeted recombinant antibodies before the time of the biopsy, excluding this possibility. The reason in this case could be “non-specific trapping” of immunoreactants by the amyloid fibrils, or “contamination” of the amyloid deposit by abnormally high levels of other serum proteins (kappa light chains in this case)[7-9]. The trapped immunoreactants usually show lower intensity of staining compared to the true amyloid-forming protein (IgG and lambda light chain in this case). However, in this exceptional case, the trapped kappa light chains show equally strong staining intensity as the lambda, giving the deposits a polyclonal appearance. Accumulation of kappa light chains in serum due to limited glomerular filtration and increase in serum half-life of kappa light chains has been described in advanced chronic kidney disease[10]. Normally, kappa free light chain (FLC), which is a monomeric molecule (molecular weight 22.5 kDa), is cleared at a faster rate than the dimeric lambda FLC (molecular weight 45 kDa)[11]. Under circumstances with impaired renal function, the clearance of kappa light chains tends to be slower than usual. The alternative route of removal of FLC is through pinocytosis by the reticuloendothelial system[11]. This alternative route is not influenced by the molecular weight of FLC. Therefore, the serum half-lives as well as the clearance rate of kappa and lambda FLCs become comparable as renal function worsens[12]. This could be a reason for kappa light chain trapping and the non-specific staining for kappa in the amyloid deposits in this case. Such non-specific staining patterns can produce inconclusive and confusing results. This could be one of the reasons that immunostaining-based amyloid typing is fraught with difficulties, necessitating the use of MS which is considered the gold standard[4,7,8].
The other less likely possibility is the co-existence of two amyloid fibril proteins in the same patient[9], but this is unlikely. The first SPE and SIfix performed at the outside hospital prior to the kidney biopsy showed only a monoclonal IgG lambda spike in the serum.
Our case illustrates that the possibility of amyloidosis (particularly AHL) cannot be completely ruled out even if the immunofluorescence pattern is polyclonal with polytypic IgG staining. The importance of utilizing multiple diagnostic modalities, including special stains, immunohistochemistry, immunofluorescence, electron microscopy and even MS, in the interpretation of renal biopsies is re-emphasized. Finally, correlation with serum electrophoresis and immunofixation studies is critical, along with the knowledge of therapeutic targeted antibodies used in the management of these patients as these can potentially interfere with serum assays.
| 1. | Nasr SH, Vrana JA, Dasari S, Bridoux F, Fidler ME, Kaaki S, Quellard N, Rinsant A, Goujon JM, Sethi S, Fervenza FC, Cornell LD, Said SM, McPhail ED, Herrera Hernandez LP, Grande JP, Hogan MC, Lieske JC, Leung N, Kurtin PJ, Alexander MP. DNAJB9 Is a Specific Immunohistochemical Marker for Fibrillary Glomerulonephritis. Kidney Int Rep. 2018;3:56-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 2. | Nasr SH, Said SM, Valeri AM, Sethi S, Fidler ME, Cornell LD, Gertz MA, Dispenzieri A, Buadi FK, Vrana JA, Theis JD, Dogan A, Leung N. The diagnosis and characteristics of renal heavy-chain and heavy/light-chain amyloidosis and their comparison with renal light-chain amyloidosis. Kidney Int. 2013;83:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Safadi S, Saad A, Quint PS, Sethi S, Leung N, Kurtin P, Nasr SH. Disappearance of immunoglobulins from persistent renal amyloid deposits following stem cell transplantation for heavy-and light-chain amyloidosis. Nephrol Dial Transplant. 2015;30:1151-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Adachi M, Kitamura M, Muta K, Maekawa A, Uramatsu T, Tadokoro M, Funakoshi S, Hisano S, Kuwahara N, Shimizu A, Mukae H, Nishino T. IgM monoclonal gammopathy with heavy-and-light-chain amyloidosis resembling fibrillary glomerulonephritis determined by tandem mass spectrometry: a case report. BMC Nephrol. 2020;21:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Herlitz LC, Bomback AS, Markowitz GS, Stokes MB, Smith RN, Colvin RB, Appel GB, D'Agati VD. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol. 2012;23:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Murata K, McCash SI, Carroll B, Lesokhin AM, Hassoun H, Lendvai N, Korde NS, Mailankody S, Landau HJ, Koehne G, Chung DJ, Giralt SA, Ramanathan LV, Landgren O. Treatment of multiple myeloma with monoclonal antibodies and the dilemma of false positive M-spikes in peripheral blood. Clin Biochem. 2018;51:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Satoskar AA, Burdge K, Cowden DJ, Nadasdy GM, Hebert LA, Nadasdy T. Typing of amyloidosis in renal biopsies: diagnostic pitfalls. Arch Pathol Lab Med. 2007;131:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Chen J, Chen H, Zhou L, Liu D, Du F, Xiang H. Strong positive light chain immunostaining in a patient with transthyretin amyloidosis. Hematology. 2023;28:2244315. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Kebbel A, Röcken C. Immunohistochemical classification of amyloid in surgical pathology revisited. Am J Surg Pathol. 2006;30:673-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Parry HM, Pratt G, Hutchison CA. Monoclonal gammopathy of undetermined significance: an update for nephrologists. Adv Chronic Kidney Dis. 2012;19:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Yadav P, Leung N, Sanders PW, Cockwell P. The use of immunoglobulin light chain assays in the diagnosis of paraprotein-related kidney disease. Kidney Int. 2015;87:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Hutchison CA, Cockwell P, Harding S, Mead GP, Bradwell AR, Barnett AH. Quantitative assessment of serum and urinary polyclonal free light chains in patients with type II diabetes: an early marker of diabetic kidney disease? Expert Opin Ther Targets. 2008;12:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |