Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.3183
Revised: April 9, 2024
Accepted: April 22, 2024
Published online: June 16, 2024
Processing time: 126 Days and 11.6 Hours
Primary ciliary dyskinesia (PCD) is an inherited autosomal-recessive disorder of impaired mucociliary clearance characterized by chronic respiratory diseases, otolaryngological diseases, central nervous system abnormalities, reproductive system abnormalities, and cardiac function abnormalities. General anesthesia in these patients is associated with a higher incidence of respiratory complications than in patients without the disease.
A 16-year-old male patient was referred to the emergency room complaining of right ankle pain due to distal tibiofibular fracture. Three years prior, he had been diagnosed with PCD. At that time, he had experienced several episodes of pneumonia, sinusitis, and chronic middle ear infections, for which he underwent surgical interventions. At the current admission, he presented with cough and sputum but no other respiratory symptoms. A chest computed tomography scan revealed centrilobular ground-glass opacities in both lower lobes and a calcified nodule in the left lower lobe. For the surgical procedure and postoperative pain management, combined spinal-epidural anesthesia was employed. The patient’s postoperative pain score was measured by the numerical rating scale (NRS). On the day of surgery, his NRS was 5 points. By the second postoperative day, the NRS score had decreased to 2–3 points. The epidural catheter was removed on the fourth day following the operation. The patient was subsequently discharged no respiratory complications.
We performed combined spinal-epidural anesthesia in a patient with PCD. The patient experienced no additional respiratory complications and was discharged with a low NRS score for pain.
Core Tip: Primary ciliary dyskinesia (PCD) is an inherited autosomal-recessive disorder of impaired mucociliary clearance. General anesthesia in these patients is associated with a higher incidence of respiratory complications. We performed combined spinal-epidural anesthesia in a patient with PCD. The patient experienced no additional respiratory complications and was discharged with a low numerical rating scale score for pain.
- Citation: Park HJ, Kim YH, Yoon YJ, Cho SY. Regional anesthesia in a patient with primary ciliary dyskinesia: A case report. World J Clin Cases 2024; 12(17): 3183-3187
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/3183.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.3183
Primary ciliary dyskinesia (PCD) is a rare condition, with an estimated prevalence ranging from 1 in 4000 to 1 in 40000 individuals. It typically follows an autosomal-recessive inheritance pattern and is characterized by impaired mucociliary clearance[1]. PCD is associated with respiratory, otolaryngeal, central nervous system, reproductive, and cardiac abnormalities. Dysfunction of respiratory cilia leads to recurrent respiratory tract infections and bronchiectasis, which pose an increased risk during general anesthesia and increase the likelihood of respiratory complications[2-5].
In our case, we performed combined spinal-epidural anesthesia along with patient-controlled epidural anesthesia for postoperative pain management to mitigate the respiratory risks associated with general anesthesia in individuals with this condition.
A 16-year-old man (179 cm; 85 kg) presented with right ankle pain and a history of right distal tibiofibular fracture.
The patient had previously been diagnosed with PCD at other hospital and had experienced frequent episodes of respiratory and otolaryngological symptoms in 2020. Additionally, the patient had a history of surgical interventions for sinusitis and otitis media.
Cough and sputum were present, but there was no dyspnea. His initial vital signs were as follows: Blood pressure 100/60 mmHg, heart rate 68 beats/min, and body temperature 36.5 °C. No abnormalities were noted on laboratory examination.
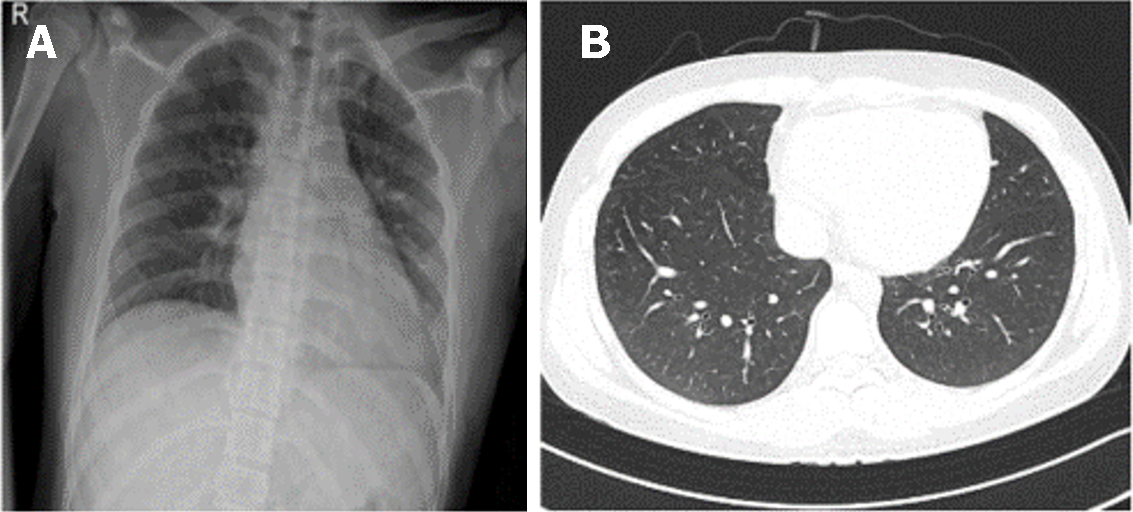
Initial chest X-ray yielded no specific findings (Figure 1A). Chest computed tomography showed centrilobular ground-glass opacities in both lower lobes and a calcified nodule in the left lower lobe (Figure 1B).
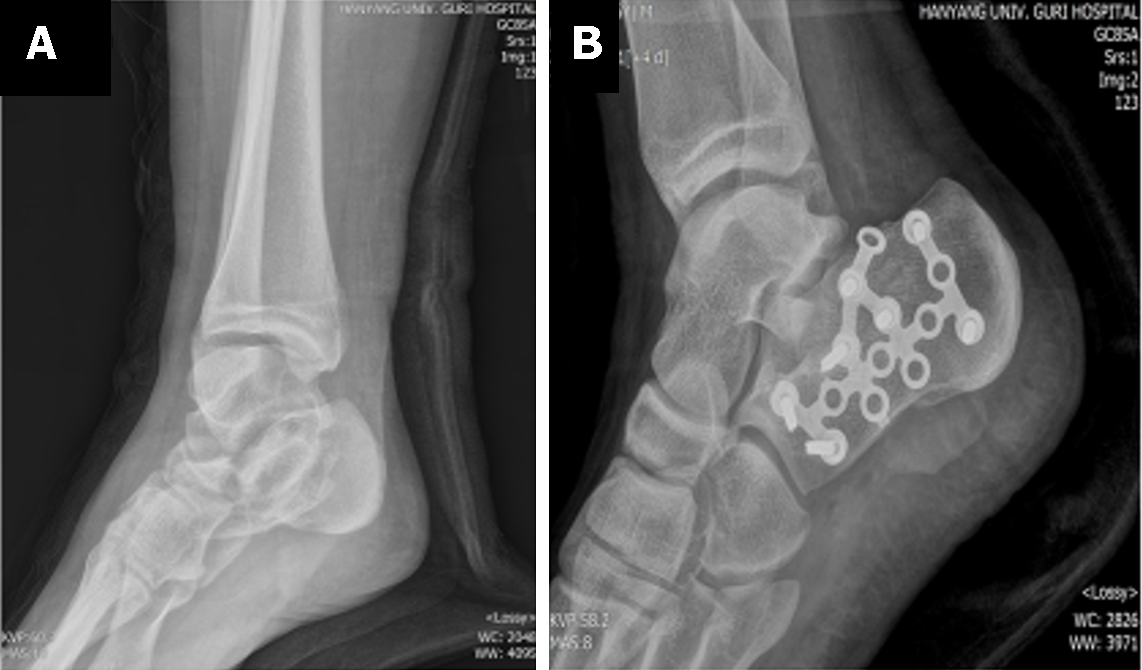
The final diagnosis was right comminuted calcaneus fracture in a patient with PCD (Figure 2A).
Combined spinal-epidural anesthesia was chosen to mitigate potential complications associated with general anesthesia, such as sputum accumulation and pneumonia. Upon arrival in the operating room, the patient presented with the following vital signs: Blood pressure 144/77 mmHg, heart rate 71 beats/min, peripheral oxygen saturation 99%, respiratory rate 20 rates/min, and body temperature 36.5 °C. Oxygen was administered at 3 L/min via a face mask. The patient was positioned in the right lateral decubitus position, and the lumbar third and fourth interbody spaces were sterilized with hexitanol. A local anesthetic, 2% lidocaine, was used to create a skin wheal. The epidural space was identified using the loss of resistance technique with a combined spinal-epidural anesthesia kit (CombiSpeed®, Ace Medical, Seoul, South Korea). Spinal anesthesia was executed using a 27G spinal needle and resulted in free flow of clear cerebrospinal fluid. After administering 14 mg of heavy marcaine, the spinal needle was removed. Subsequently, an epidural catheter was inserted through the epidural needle, and the patient was moved into the supine position. The level of spinal anesthesia was confirmed at the eighth thoracic level by assessing temperature loss using an alcohol swab. The operation was began, operation time was 100 min. The name of the operation was open reduction and internal fixation with 15 hole calcaneal plat and 7 cortical screws added by allogenous commercial chip bone graft (Figure 2B).
During the operation, the patient’s vital signs and oxygen saturation remained stable. The patient did not report any pain or respiratory symptoms. Postoperatively, patient-controlled epidural analgesia was administered via the epidural catheter. The analgesic mixture consisted of a total of 120 mL, comprising 20 mg ropivacaine, 1000 μg fentanyl, and normal saline. The drug infusion was regulated using an Automed 3200 pump set at an infusion rate of 2 mL/h. The system allowed an additional 2 mL to be administered on demand via a button, with a maximum limit of four doses per hour.
Postoperative pain was assessed using the numerical rating scale (NRS). On the first day after the operation, the NRS score ranged between 3 and 5; on the second day, it was between 2 and 3. The patient-controlled epidural analgesia pump and epidural catheter were removed on the fourth day following the operation.
There were no specific respiratory complications or epidural catheter-related complications from the time of the operation until discharge.
PCD is a congenital disorder characterized by structural and functional abnormalities of motile cilia that lead to impaired mucociliary clearance. It is predominantly an autosomal recessive genetic disorder[6]. From birth, PCD is characterized by recurrent and chronic infections in the upper and lower airways, attributed to impaired mucociliary clearance throughout the respiratory tract.
The prevalence of PCD is estimated at between 1:4000 and 1:40000, with potential variations in specific ethnic groups[7]. Timely and accurate diagnosis is crucial to prevent irreversible lung damage. Nitric oxide (NO) level in the nasal passages generally is lower in PCD patients, and measurement of NO is considered a valuable screening test in Europe and North America[8]. Additionally, assessment of ciliary ultrastructure using transmission electron microscopy has been regarded as a standard diagnostic method[9].
For respiratory management in patients with PCD, the primary objectives include improving lung function, preventing progression of the disease, and focusing on airway clearance therapies, complemented by aggressive antibiotic strategies. Airway clearance therapies might involve the use of bronchodilators, physical exercise, mucolytic agents, and various mechanical methods to assist in coughing[10]. These interventions collectively aim to optimize airway clearance and to maintain respiratory health in individuals with PCD.
Preoperative assessment may include evaluation of lung function, chest computed tomography, pulmonary function tests, and arterial blood gas analysis. Respiratory function should be optimized prior to surgery. While regional anesthesia methods might be ideal in some cases, general anesthesia is most common in PCD patients, particularly due to the frequent necessity of addressing thoracic and otolaryngological issues surgically[11]. When general anesthesia is considered, it is important to be aware of potential complications in this population, including anesthesia-related pulmonary aspiration, which can lead to pneumonia and respiratory infections. Additionally, risks such as respiratory failure, bronchospasm, atelectasis, and pulmonary edema should be considered[12,13].
Cheng et al[11] reported 99 cases of PCD, 85.4% of those patients was performed by general anesthesia and 14.6% of cases was performed by regional anesthesia. The intraoperative process was uneventful in most patients, and most patients recovered smoothly in postoperative course. But, Burduk et al[14] proposed that whenever possible, the use of regional or local anesthesia should be prioritized over general anesthesia due to the patient’s respiratory involvement. In this case, because the operation site was the calcaneus bone, regional anesthesia was performed.
When administering regional anesthesia, advantages include the patient’s effective clearance of airway secretions both during and after surgery. Additionally, there is minimal airway stimulation with regional anesthesia, resulting in reduced airway secretions and fewer anesthesia-related respiratory complications.
Because our patient was not undergoing thoracic or otolaryngological surgery and had some residual respiratory symptoms, it was decided that regional anesthesia would be the most suitable anesthetic approach. For postoperative pain management, selection of analgesics should involve short-acting opioids to avoid respiratory depression in PCD patients. In pediatric lower-limb surgery, epidural analgesia has been shown to be more effective than intravenous analgesia for postoperative pain control. Moreover, patient-controlled epidural analgesia in pediatric patients offers advantages over intravenous patient-controlled analgesia, such as a faster return to regular dietary intake and potentially expediting discharge times.
Potential complications associated with insertion of an epidural catheter include the risk of the catheter inadvertently entering the subdural or subarachnoid spaces, as well as the possibility of an accidental dural puncture. Additionally, complications such as nerve damage, subdural hematoma, meningitis, and epidural abscess may occur[15].
In this report, we present the case of a 16-year-old male patient with a history of respiratory and otolaryngological complications attributed to PCD. Lower limb surgery was conducted with combined spinal-epidural anesthesia, and anesthesia was successfully achieved without respiratory complications. Postoperative pain was efficiently managed with a patient-controlled epidural analgesia system. This case is presented in conjunction with a literature review.
| 1. | Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 822] [Article Influence: 16.8] [Reference Citation Analysis (34)] |
| 2. | Kennedy MP, Noone PG, Leigh MW, Zariwala MA, Minnix SL, Knowles MR, Molina PL. High-resolution CT of patients with primary ciliary dyskinesia. AJR Am J Roentgenol. 2007;188:1232-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Leigh MW, Ferkol TW, Davis SD, Lee HS, Rosenfeld M, Dell SD, Sagel SD, Milla C, Olivier KN, Sullivan KM, Zariwala MA, Pittman JE, Shapiro AJ, Carson JL, Krischer J, Hazucha MJ, Knowles MR. Clinical Features and Associated Likelihood of Primary Ciliary Dyskinesia in Children and Adolescents. Ann Am Thorac Soc. 2016;13:1305-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 4. | Knowles MR, Zariwala M, Leigh M. Primary Ciliary Dyskinesia. Clin Chest Med. 2016;37:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Morgan LC, Birman CS. The impact of Primary Ciliary Dyskinesia on the upper respiratory tract. Paediatr Respir Rev. 2016;18:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Kuehni CE, Lucas JS. Diagnosis of primary ciliary dyskinesia: summary of the ERS Task Force report. Breathe (Sheff). 2017;13:166-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | O'Callaghan C, Chetcuti P, Moya E. High prevalence of primary ciliary dyskinesia in a British Asian population. Arch Dis Child. 2010;95:51-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Walker WT, Jackson CL, Lackie PM, Hogg C, Lucas JS. Nitric oxide in primary ciliary dyskinesia. Eur Respir J. 2012;40:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Lucas JS, Burgess A, Mitchison HM, Moya E, Williamson M, Hogg C; National PCD Service, UK. Diagnosis and management of primary ciliary dyskinesia. Arch Dis Child. 2014;99:850-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 10. | Desai M, Weller PH, Spencer DA. Clinical benefit from nebulized human recombinant DNase in Kartagener's syndrome. Pediatr Pulmonol. 1995;20:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Cheng L, Dong Y, Liu S. Anesthetic Management of Patients With Kartagener Syndrome: A Systematic Review of 99 Cases. J Cardiothorac Vasc Anesth. 2023;37:1021-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 12. | Nason KS. Acute Intraoperative Pulmonary Aspiration. Thorac Surg Clin. 2015;25:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118:317-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 435] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 14. | Burduk PK, Wawrzyniak K, Kazmierczak W, Kusza K. Kartagener's syndrome--anaesthetic considerations for ENT surgery. Otolaryngol Pol. 2012;66:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Bai SJ, Koo BN, Kim JH, Doh PS, Kim KH, Shin YS. Comparison of continuous epidural and intravenous analgesia for postoperative pain control in pediatric lower extremity surgery. Yonsei Med J. 2004;45:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |